Purinergic Receptors P2X7 and P2X4 as Markers of Disease Progression in the rd10 Mouse Model of Inherited Retinal Dystrophy
Abstract
:1. Introduction
2. Results
2.1. Retinal Function in rd10 Mice
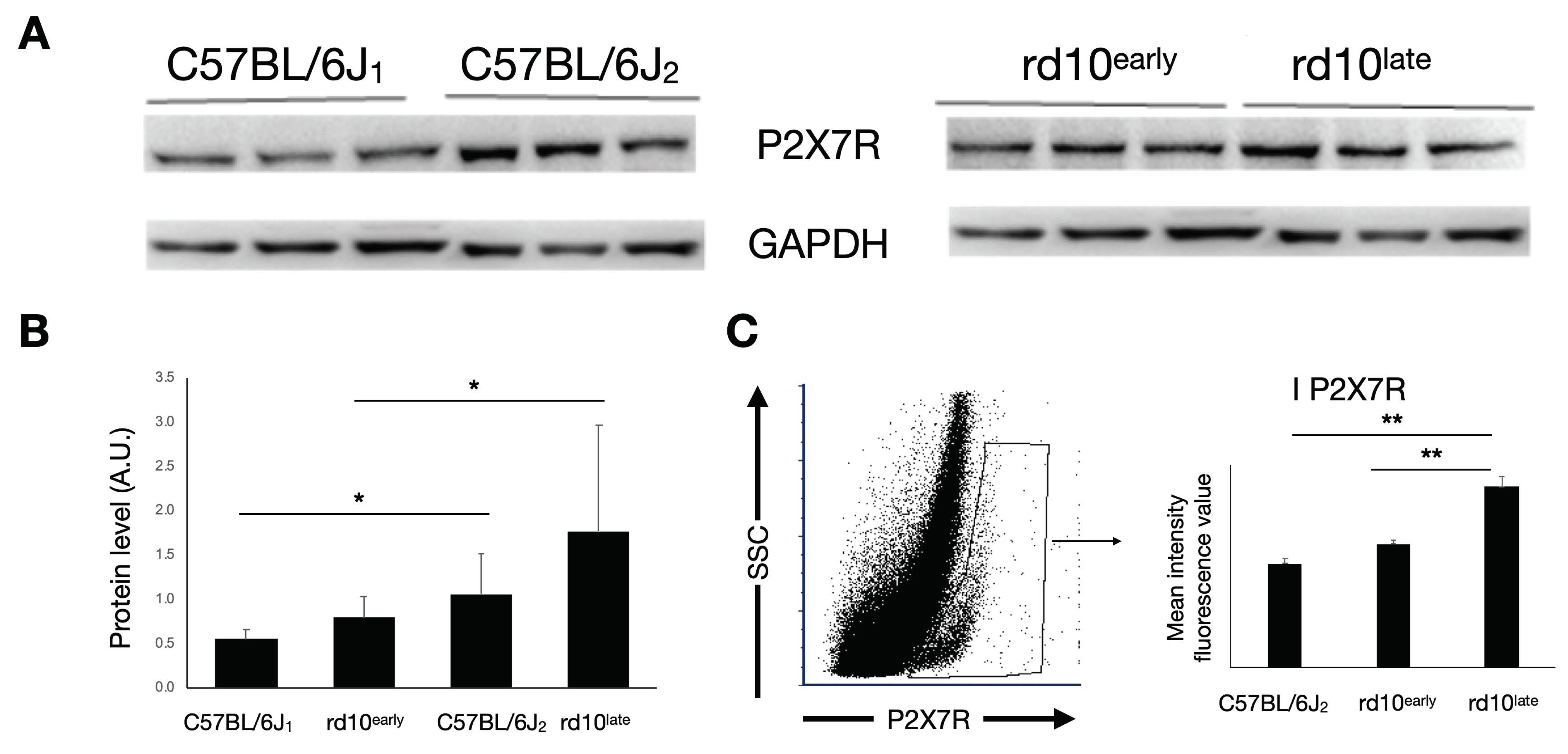
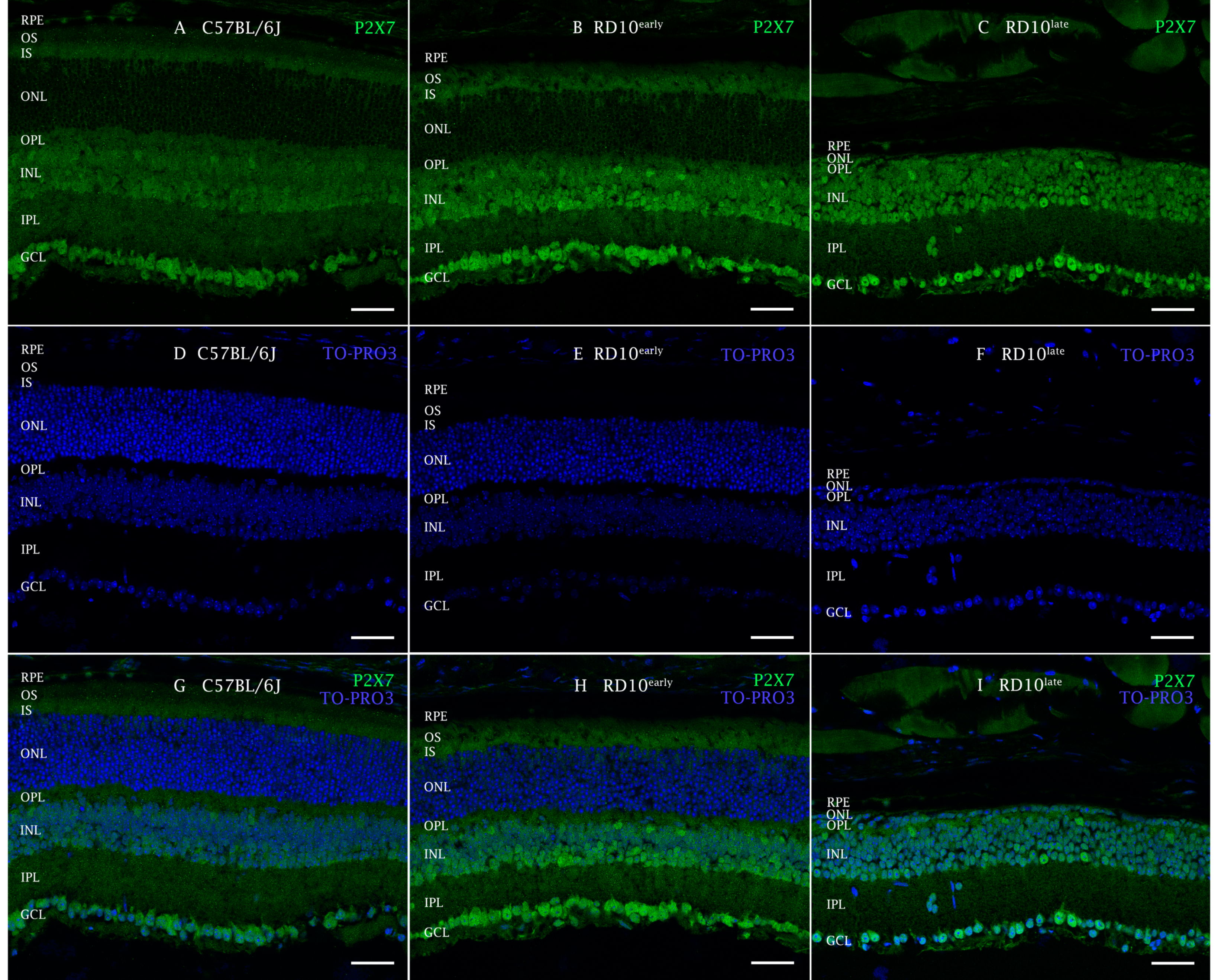
2.2. P2X7R Expression in rd10 Mice
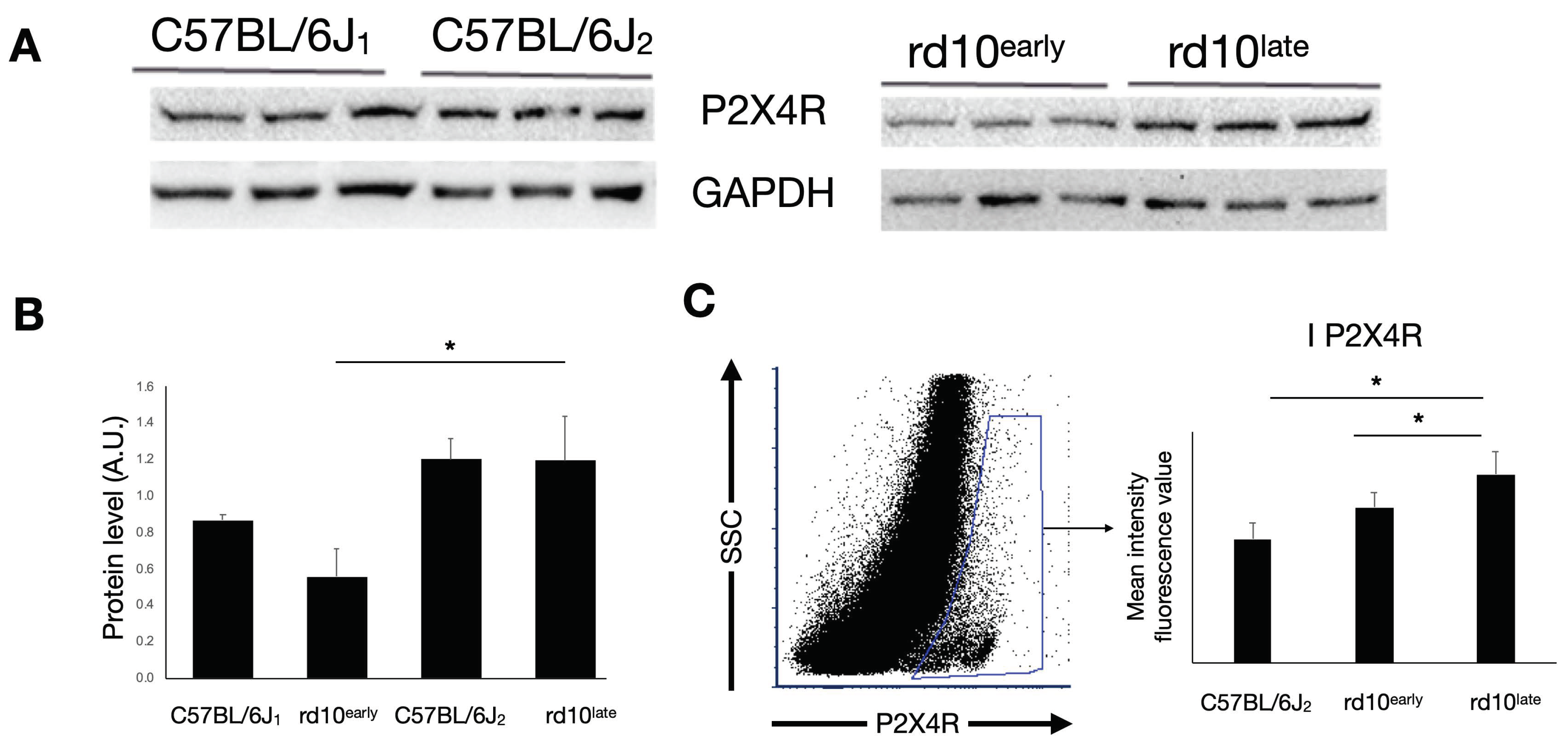
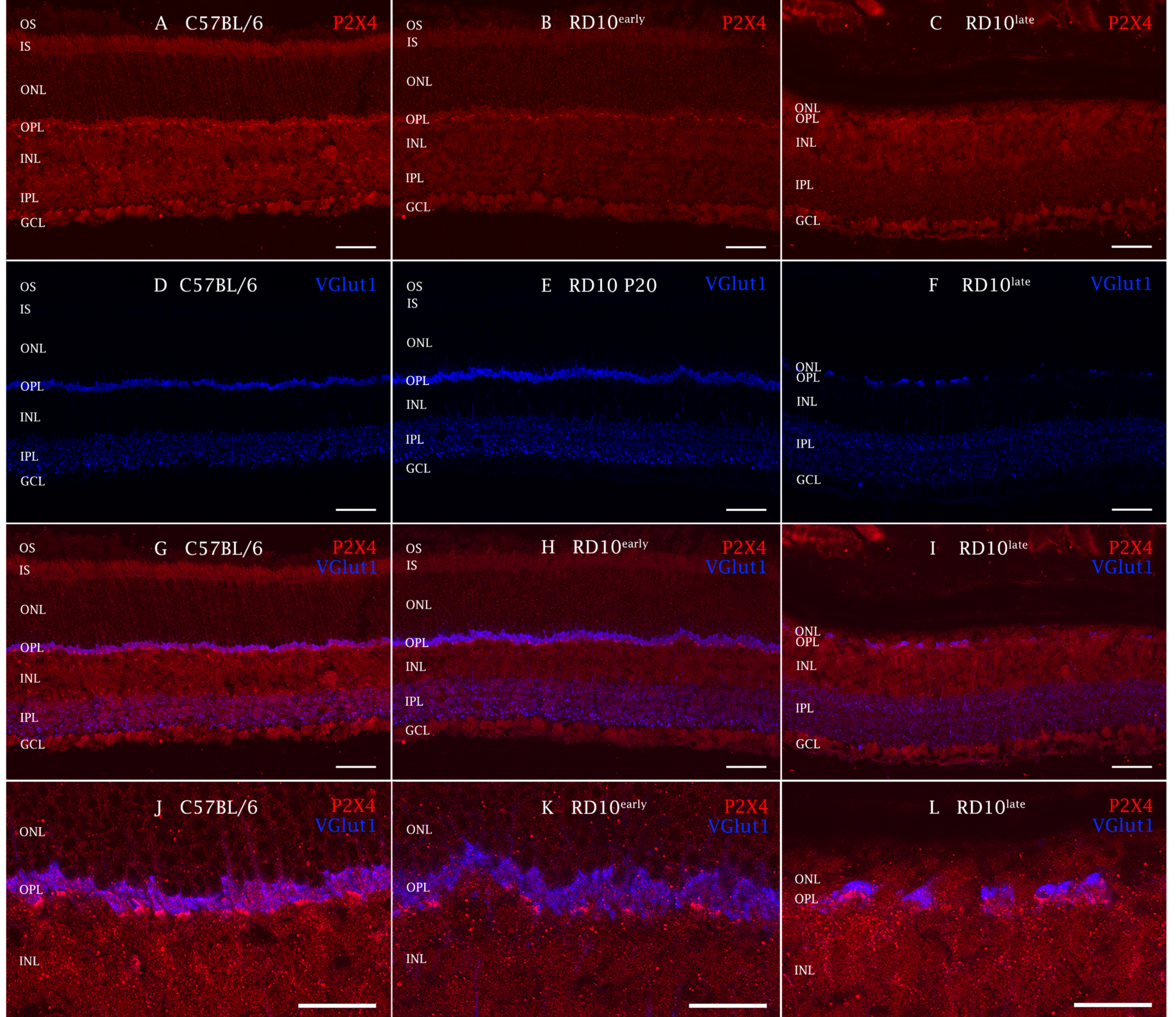
2.3. P2X4R Expression in rd10 Mice
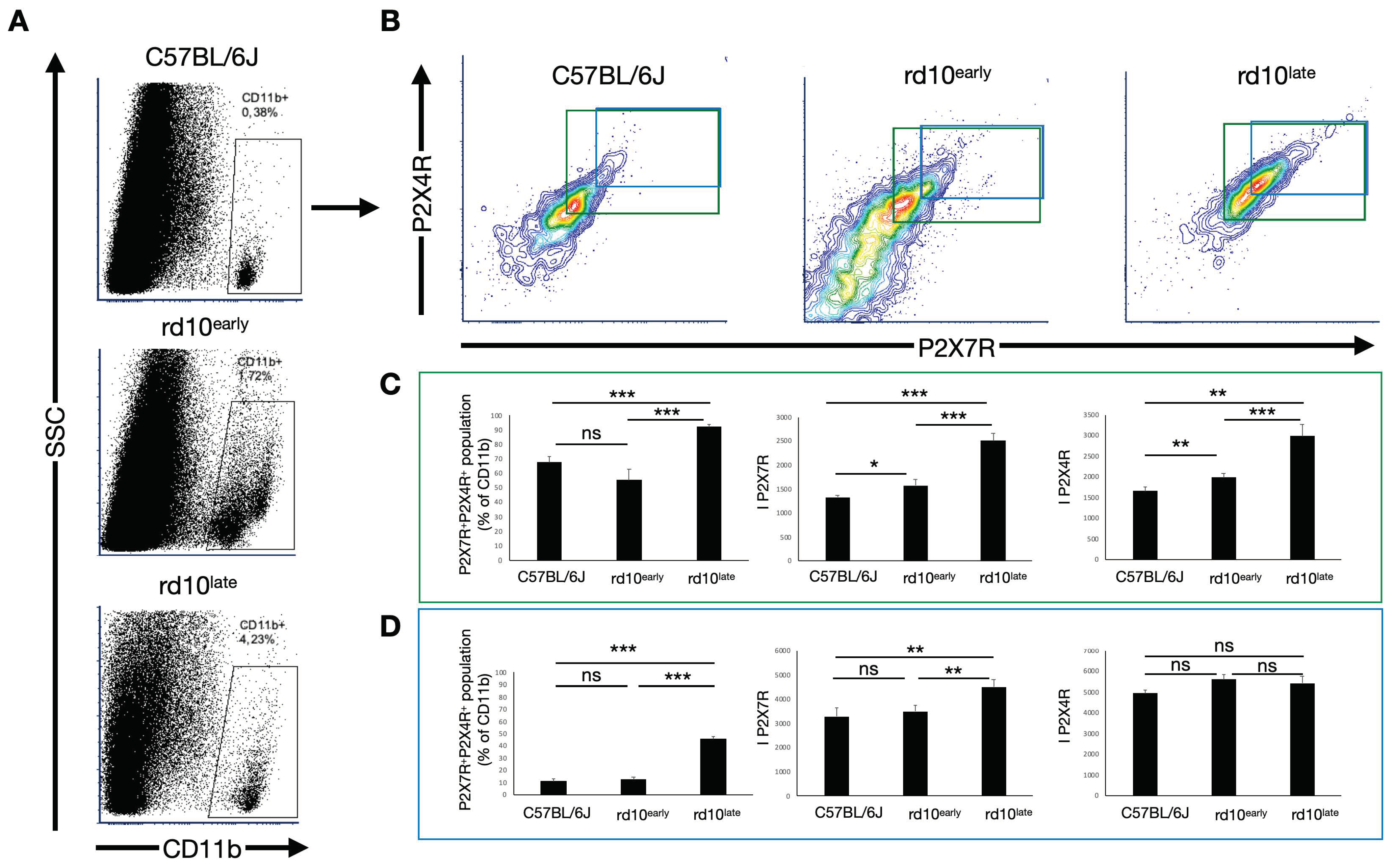
2.4. CD11b-Positive Cells in Degenerating Retinas Overexpress P2X7R but Not P2X4R
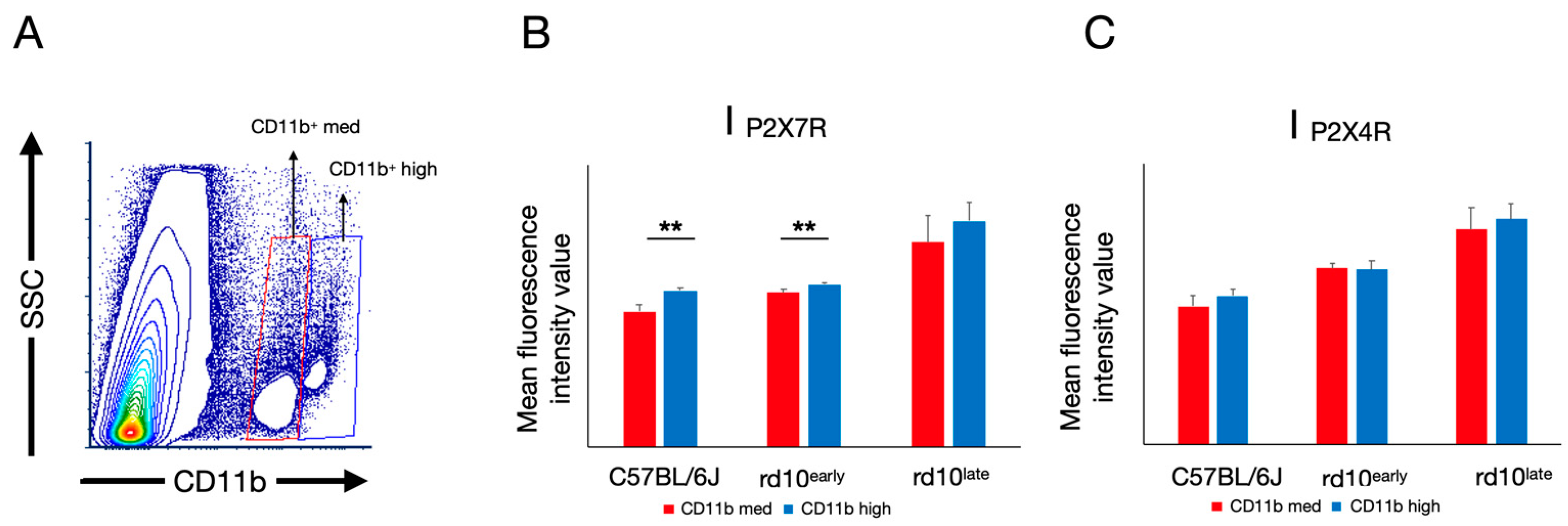
2.5. P2X7R and P2X4R Expression Levels Vary in Subsets of CD11b-Positive Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Electroretinography
4.3. Optomotor Test
4.4. Immunohistochemistry
4.5. Flow Cytometry
4.6. Western Blotting
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adinolfi, E.; Giuliani, A.L.; De Marchi, E.; Pegoraro, A.; Orioli, E.; Di Virgilio, F. The P2X7 receptor: A main player in inflammation. Biochem. Pharmacol. 2018, 151, 234–244. [Google Scholar] [CrossRef]
- Carver, K.A.; Lin, C.M.; Bowes Rickman, C.; Yang, D. Lack of the P2X7 receptor protects against AMD-like defects and microparticle accumulation in a chronic oxidative stress-induced mouse model of AMD. Biochem. Biophys. Res. Commun. 2017, 482, 81–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, S.; Das, S. P2X7 receptor as a key player in oxidative stress-driven cell fate in nonalcoholic steatohepatitis. Oxid. Med. Cell. Longev. 2015, 2015, 172493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savio, L.E.B.; de Andrade Mello, P.; da Silva, C.G.; Coutinho-Silva, R. The P2X7 Receptor in Inflammatory Diseases: Angel or Demon? Front. Pharmacol. 2018, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Sperlagh, B.; Illes, P. P2X7 receptor: An emerging target in central nervous system diseases. Trends. Pharmacol. Sci. 2014, 35, 537–547. [Google Scholar] [CrossRef]
- Calzaferri, F.; Ruiz-Ruiz, C.; Diego, A.M.G.; Pascual, R.; Méndez-López, I.; Cano-Abad, M.F.; Maneu, V.; Ríos, C.; Gandía, L.; García, A.G. The purinergic P2X7 receptor as a potential drug target to combat neuroinflammation in neurodegenerative diseases. Med. Res. Rev. 2020, 40, 2427–2465. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Mazziotta, C.; Lanzillotti, C.; Stefani, C.; Badiale, G.; Campione, G.; Martini, F.; Tognon, M. The Role of Purinergic P2X7 Receptor in Inflammation and Cancer: Novel Molecular Insights and Clinical Applications. Cancers 2022, 14, 1116. [Google Scholar] [CrossRef]
- Virgilio, F. P2X Receptors and Inflammation. Curr. Med. Chem. 2015, 22, 866–877. [Google Scholar] [CrossRef]
- Casas-Pruneda, G.; Reyes, J.P.; Perez-Flores, G.; Perez-Cornejo, P.; Arreola, J. Functional interactions between P2X4 and P2X7 receptors from mouse salivary epithelia. J. Physiol. 2009, 587, 2887–2901. [Google Scholar] [CrossRef]
- Craigie, E.; Birch, R.E.; Unwin, R.J.; Wildman, S.S. The relationship between P2X4 and P2X7: A physiologically important interaction? Front. Physiol. 2013, 4, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, M.; Prudic, K.; Pippel, A.; Klapperstuck, M.; Braam, U.; Muller, C.E.; Schmalzing, G.; Markwardt, F. Interaction of Purinergic P2X4 and P2X7 Receptor Subunits. Front. Pharmacol. 2017, 8, 860. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Masin, M.; Qureshi, O.S.; Murrell-Lagnado, R.D. Evidence for Functional P2X 4/P2X 7 Heteromeric Receptors. Mol. Pharmacol. 2007, 72, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Gao, J.; Niu, M.; Zhang, X.; Wang, J.; Xie, A. P2X4R Overexpression Upregulates Interleukin-6 and Exacerbates 6-OHDA-Induced Dopaminergic Degeneration in a Rat Model of PD. Front. Aging Neurosci. 2020, 12, 580068. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K. Overview for the study of P2 receptors: From P2 receptor history to neuropathic pain studies. J. Pharmacol. Sci. 2022, 149, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.J.; Baird, P.N.; Vessey, K.A.; Skarratt, K.K.; Fletcher, E.L.; Fuller, S.J.; Richardson, A.J.; Guymer, R.H.; Wiley, J.S. A rare functional haplotype of the P2RX4 and P2RX7 genes leads to loss of innate phagocytosis and confers increased risk of age-related macular degeneration. FASEB J. 2013, 27, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Yeung, D.; Kharidia, R.; Brown, S.C.; Gorecki, D.C. Enhanced expression of the P2X4 receptor in Duchenne muscular dystrophy correlates with macrophage invasion. Neurobiol. Dis. 2004, 15, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Casanovas, A.; Hernandez, S.; Tarabal, O.; Rossello, J.; Esquerda, J.E. Strong P2X4 purinergic receptor-like immunoreactivity is selectively associated with degenerating neurons in transgenic rodent models of amyotrophic lateral sclerosis. J. Comp. Neurol. 2008, 506, 75–92. [Google Scholar] [CrossRef]
- Sabado, J.; Casanovas, A.; Hernandez, S.; Piedrafita, L.; Hereu, M.; Esquerda, J.E. Immunodetection of disease-associated conformers of mutant cu/zn superoxide dismutase 1 selectively expressed in degenerating neurons in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2013, 72, 646–661. [Google Scholar] [CrossRef] [Green Version]
- Cieslak, M.; Roszek, K.; Wujak, M. Purinergic implication in amyotrophic lateral sclerosis-from pathological mechanisms to therapeutic perspectives. Purinergic Signal. 2019, 15, 1–15. [Google Scholar] [CrossRef]
- Suurväli, J.; Boudinot, P.; Kanellopoulos, J.; Rüütel Boudinot, S. P2X4: A fast and sensitive purinergic receptor. Biomed. J. 2017, 40, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Matyśniak, D.; Chumak, V.; Nowak, N.; Kukla, A.; Lehka, L.; Oslislok, M.; Pomorski, P. P2X7 receptor: The regulator of glioma tumor development and survival. Purinergic Signal. 2022, 18, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.; Aplin, F.P.; Jobling, A.I.; Phipps, J.A.; de Iongh, R.U.; Greferath, U.; Vessey, K.A.; Fletcher, E.L. Localization and Possible Function of P2X Receptors in Normal and Diseased Retinae. J. Ocul. Pharmacol. Ther. 2016, 32, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Puthussery, T.; Fletcher, E.L. P2 × 2 receptors on ganglion and amacrine cells in cone pathways of the rat retina. J. Comp. Neurol. 2006, 496, 595–609. [Google Scholar] [CrossRef]
- Vessey, K.A.; Fletcher, E.L. Rod and cone pathway signalling is altered in the P2X7 receptor knock out mouse. PLoS ONE 2012, 7, e29990. [Google Scholar] [CrossRef]
- Freitas, H.R.; Reis, R.A. Glutathione induces GABA release through P2X7R activation on Muller glia. Neurogenesis 2017, 4, e1283188. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, H.; Sugiyama, T.; Wu, D.M.; Kobayashi, M.; Yamanishi, S.; Katsumura, K.; Puro, D.G. ATP: A vasoactive signal in the pericyte-containing microvasculature of the rat retina. J. Physiol. 2003, 551, 787–799. [Google Scholar] [CrossRef]
- Pannicke, T.; Fischer, W.; Biedermann, B.; Schadlich, H.; Grosche, J.; Faude, F.; Wiedemann, P.; Allgaier, C.; Illes, P.; Burnstock, G.; et al. P2X7 receptors in Muller glial cells from the human retina. J. Neurosci. 2000, 20, 5965–5972. [Google Scholar] [CrossRef] [Green Version]
- Sanderson, J.; Dartt, D.A.; Trinkaus-Randall, V.; Pintor, J.; Civan, M.M.; Delamere, N.A.; Fletcher, E.L.; Salt, T.E.; Grosche, A.; Mitchell, C.H. Purines in the eye: Recent evidence for the physiological and pathological role of purines in the RPE, retinal neurons, astrocytes, Muller cells, lens, trabecular meshwork, cornea and lacrimal gland. Exp. Eye Res. 2014, 127, 270–279. [Google Scholar] [CrossRef]
- Wurm, A.; Pannicke, T.; Iandiev, I.; Francke, M.; Hollborn, M.; Wiedemann, P.; Reichenbach, A.; Osborne, N.N.; Bringmann, A. Purinergic signaling involved in Muller cell function in the mammalian retina. Prog. Retin. Eye Res. 2011, 30, 324–342. [Google Scholar] [CrossRef]
- Puthussery, T.; Fletcher, E.L. Synaptic localization of P2X7 receptors in the rat retina. J. Comp. Neurol. 2004, 472, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Wheeler-Schilling, T.H.; Marquordt, K.; Kohler, K.; Jabs, R.; Guenther, E. Expression of purinergic receptors in bipolar cells of the rat retina. Mol. Brain Res. 2000, 76, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Wheeler-Schilling, T.H.; Marquordt, K.; Kohler, K.; Guenther, E.; Jabs, R. Identification of purinergic receptors in retinal ganglion cells. Mol. Brain Res. 2001, 92, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Illes, P.; Khan, T.M.; Rubini, P. Neuronal P2X7 Receptors Revisited: Do They Really Exist? J. Neurosci. 2017, 37, 7049–7062. [Google Scholar] [CrossRef]
- Kaczmarek-Hajek, K.; Zhang, J.; Kopp, R.; Grosche, A.; Rissiek, B.; Saul, A.; Bruzzone, S.; Engel, T.; Jooss, T.; Krautloher, A.; et al. Re-evaluation of neuronal P2X7 expression using novel mouse models and a P2X7-specific nanobody. Elife 2018, 7, e36217. [Google Scholar] [CrossRef]
- Miras-Portugal, M.T.; Sebastian-Serrano, A.; de Diego Garcia, L.; Diaz-Hernandez, M. Neuronal P2X7 Receptor: Involvement in Neuronal Physiology and Pathology. J. Neurosci. 2017, 37, 7063–7072. [Google Scholar] [CrossRef] [Green Version]
- Ho, T.; Vessey, K.A.; Fletcher, E.L. Immunolocalization of the P2X4 receptor on neurons and glia in the mammalian retina. Neuroscience 2014, 277, 55–71. [Google Scholar] [CrossRef]
- Sugiyama, T.; Oku, H.; Shibata, M.; Fukuhara, M.; Yoshida, H.; Ikeda, T. Involvement of P2X7 receptors in the hypoxia-induced death of rat retinal neurons. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3236–3243. [Google Scholar] [CrossRef] [Green Version]
- Xue, B.; Xie, Y.; Xue, Y.; Hu, N.; Zhang, G.; Guan, H.; Ji, M. Involvement of P2X7 receptors in retinal ganglion cell apoptosis induced by activated Muller cells. Exp. Eye Res. 2016, 153, 42–50. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Laties, A.M.; Mitchell, C.H. Stimulation of P2X7 receptors elevates Ca2+ and kills retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2183–2191. [Google Scholar] [CrossRef]
- Reichenbach, A.; Bringmann, A. Purinergic signaling in retinal degeneration and regeneration. Neuropharmacology 2016, 104, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Hu, Y.; Zhou, L.; Cheng, X. P2X7 receptor antagonist protects retinal ganglion cells by inhibiting microglial activation in a rat chronic ocular hypertension model. Mol. Med. Rep. 2018, 17, 2289–2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monif, M.; Burnstock, G.; Williams, D.A. Microglia: Proliferation and activation driven by the P2X7 receptor. Int. J. Biochem. Cell Biol. 2010, 42, 1753–1756. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, N.; Fernandez-Sanchez, L.; Campello, L.; Maneu, V.; De la Villa, P.; Lax, P.; Pinilla, I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog. Retin. Eye Res. 2014, 43, 17–75. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-X.; Zhao, G.-L.; Hu, X.; Zhou, H.; Li, S.-Y.; Li, F.; Miao, Y.; Lei, B.; Wang, Z. P2X7/P2X4 Receptors Mediate Proliferation and Migration of Retinal Microglia in Experimental Glaucoma in Mice. Neurosci. Bull. 2022, 38, 901–915. [Google Scholar] [CrossRef] [PubMed]
- Pérez de Lara, M.J.; Avilés-Trigueros, M.; Guzmán-Aránguez, A.; Valiente-Soriano, F.J.; de la Villa, P.; Vidal-Sanz, M.; Pintor, J. Potential role of P2X7 receptor in neurodegenerative processes in a murine model of glaucoma. Brain Res. Bull. 2019, 150, 61–74. [Google Scholar] [CrossRef]
- Yang, D.; Chen, J. The P2X7 Receptor in AMD. Austin J. Clin. Ophthalmol. 2014, 1, 1012. [Google Scholar]
- Tassetto, M.; Scialdone, A.; Solini, A.; Di Virgilio, F. The P2X7 Receptor: A Promising Pharmacological Target in Diabetic Retinopathy. Int. J. Mol. Sci. 2021, 22, 7110. [Google Scholar] [CrossRef]
- Sugiyama, T. Role of P2X7 receptors in the development of diabetic retinopathy. World J. Diabetes 2014, 5, 141–145. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kobayashi, M.; Kawamura, H.; Li, Q.; Puro, D.G. Enhancement of P2X(7)-induced pore formation and apoptosis: An early effect of diabetes on the retinal microvasculature. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1026–1032. [Google Scholar]
- Maneu, V.; Noailles, A.; Megias, J.; Gomez-Vicente, V.; Carpena, N.; Gil, M.L.; Gozalbo, D.; Cuenca, N. Retinal microglia are activated by systemic fungal infection. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3578–3585. [Google Scholar] [CrossRef] [PubMed]
- Maneu, V.; Noailles, A.; Gómez-Vicente, V.; Carpena, N.; Cuenca, N.; Gil, M.L.; Gozalbo, D. Immunosuppression, peripheral inflammation and invasive infection from endogenous gut microbiota activate retinal microglia in mouse models. Microbiol. Immunol. 2016, 60, 617–625. [Google Scholar] [CrossRef]
- Noailles, A.; Maneu, V.; Campello, L.; Gómez-Vicente, V.; Lax, P.; Cuenca, N. Persistent inflammatory state after photoreceptor loss in an animal model of retinal degeneration. Sci. Rep. 2016, 6, 33356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noailles, A.; Maneu, V.; Campello, L.; Lax, P.; Cuenca, N. Systemic inflammation induced by lipopolysaccharide aggravates inherited retinal dystrophy. Cell Death Dis. 2018, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Franke, H.; Klimke, K.; Brinckmann, U.; Grosche, J.; Francke, M.; Sperlagh, B.; Reichenbach, A.; Liebert, U.G.; Illes, P. P2X(7) receptor-mRNA and -protein in the mouse retina; changes during retinal degeneration in BALBCrds mice. Neurochem. Int. 2005, 47, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Perez de Lara, M.J.; Guzman-Aranguez, A.; de la Villa, P.; Diaz-Hernandez, J.I.; Miras-Portugal, M.T.; Pintor, J. Increased levels of extracellular ATP in glaucomatous retinas: Possible role of the vesicular nucleotide transporter during the development of the pathology. Mol. Vis. 2015, 21, 1060–1070. [Google Scholar]
- Yang, D. Targeting the P2X7 Receptor in Age-Related Macular Degeneration. Vision 2017, 1, 11. [Google Scholar] [CrossRef] [Green Version]
- Zabala, A.; Vazquez-Villoldo, N.; Rissiek, B.; Gejo, J.; Martin, A.; Palomino, A.; Perez-Samartín, A.; Pulagam, K.R.; Lukowiak, M.; Capetillo-Zarate, E.; et al. P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis. EMBO Mol. Med. 2018, 10, e8743. [Google Scholar] [CrossRef]
- Chen, H.; Xia, Q.; Feng, X.; Cao, F.; Yu, H.; Song, Y.; Ni, X. Effect of P2X4R on airway inflammation and airway remodeling in allergic airway challenge in mice. Mol. Med. Rep. 2016, 13, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Duan, M.; Steinfort, D.P.; Smallwood, D.; Hew, M.; Chen, W.; Ernst, M.; Irving, L.B.; Anderson, G.P.; Hibbs, M.L. CD11b immunophenotyping identifies inflammatory profiles in the mouse and human lungs. Mucosal. Immunol. 2016, 9, 550–563. [Google Scholar] [CrossRef] [Green Version]
- Kutsyr, O.; Sánchez-Sáez, X.; Martínez-Gil, N.; de Juan, E.; Lax, P.; Maneu, V.; Cuenca, N. Gradual increase in environmental light intensity induces oxidative stress and inflammation and accelerates retinal neurodegeneration. Investig. Ophthalmol. Vis. Sci. 2020, 61, 1. [Google Scholar] [CrossRef] [PubMed]
- Campello, L.; Kutsyr, O.; Noailles, A.; Michalska, P.; Fernandez-Sanchez, L.; Martinez-Gil, N.; Ortuno-Lizaran, I.; Sanchez-Saez, X.; de Juan, E.; Lax, P.; et al. New Nrf2-Inducer Compound ITH12674 Slows the Progression of Retinitis Pigmentosa in the Mouse Model rd10. Cell Physiol. Biochem. 2020, 54, 142–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, W.; Rubini, P.; Tang, Y.; Engel, T.; Illes, P. Inherent P2X7 Receptors Regulate Macrophage Functions during Inflammatory Diseases. Int. J. Mol. Sci. 2021, 23, 232. [Google Scholar] [CrossRef] [PubMed]
- Gudipaty, L.; Humphreys, B.D.; Buell, G.; Dubyak, G.R. Regulation of P2X 7 nucleotide receptor function in human monocytes by extracellular ions and receptor density. Am. J. Physiol. Physiol. 2001, 280, C943–C953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.; Roubeix, C.; Sennlaub, F.; Saban, D.R. Microglia versus Monocytes: Distinct Roles in Degenerative Diseases of the Retina. Trends. Neurosci. 2020, 43, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Funatsu, J.; Murakami, Y.; Shimokawa, S.; Nakatake, S.; Fujiwara, K.; Okita, A.; Fukushima, M.; Shibata, K.; Yoshida, N.; Koyanagi, Y.; et al. Circulating inflammatory monocytes oppose microglia and contribute to cone cell death in retinitis pigmentosa. PNAS Nexus 2022, 1, pgac003. [Google Scholar] [CrossRef]
- Anccasi, R.M.; Ornelas, I.M.; Cossenza, M.; Persechini, P.M.; Ventura, A.L. ATP induces the death of developing avian retinal neurons in culture via activation of P2X7 and glutamate receptors. Purinergic Signal. 2013, 9, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Kakurai, K.; Sugiyama, T.; Kurimoto, T.; Oku, H.; Ikeda, T. Involvement of P2X(7) receptors in retinal ganglion cell death after optic nerve crush injury in rats. Neurosci. Lett. 2013, 534, 237–241. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Giurdanella, G.; Di Paola, L.; Leggio, G.M.; Drago, F.; Salomone, S.; Bucolo, C. P2X7 receptor antagonism: Implications in diabetic retinopathy. Biochem. Pharmacol. 2017, 138, 130–139. [Google Scholar] [CrossRef]
- Puthussery, T.; Fletcher, E. Extracellular ATP induces retinal photoreceptor apoptosis through activation of purinoceptors in rodents. J. Comp. Neurol. 2009, 513, 430–440. [Google Scholar] [CrossRef]
- Glass, G. Cardiovascular combinations. Nat. Rev. Drug Discov. 2004, 3, 731–732. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, R.W.; Brockway-Lunardi, L.M.; Bonk, D.T.; Dohoney, K.M.; Doroshow, J.H.; Meech, S.J.; Ratain, M.J.; Topalian, S.L.; Pardoll, D.M. Opportunities and Challenges in the Development of Experimental Drug Combinations for Cancer. JNCI J. Natl. Cancer Inst. 2011, 103, 1222–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, H.S. Advair: Combination treatment with fluticasone propionate/salmeterol in the treatment of asthma. J. Allergy Clin. Immunol. 2001, 107, 397–416. [Google Scholar] [CrossRef] [PubMed]
- Larder, B.A.; Kemp, S.D.; Harrigan, P.R. Potential Mechanism for Sustained Antiretroviral Efficacy of AZT-3TC Combination Therapy. Science 1995, 269, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Lax, P.; Otalora, B.B.; Esquiva, G.; Rol Mde, L.; Madrid, J.A.; Cuenca, N. Circadian dysfunction in P23H rhodopsin transgenic rats: Effects of exogenous melatonin. J. Pineal Res. 2011, 50, 183–191. [Google Scholar] [CrossRef]
- Noailles, A.; Fernández-Sánchez, L.; Lax, P.; Cuenca, N. Microglia activation in a model of retinal degeneration and TUDCA neuroprotective effects. J. Neuroinflamm. 2014, 11, 186. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Gil, N.; Kutsyr, O.; Noailles, A.; Fernández-Sánchez, L.; Vidal, L.; Sánchez-Sáez, X.; Sánchez-Castillo, C.; Lax, P.; Cuenca, N.; García, A.G.; et al. Purinergic Receptors P2X7 and P2X4 as Markers of Disease Progression in the rd10 Mouse Model of Inherited Retinal Dystrophy. Int. J. Mol. Sci. 2022, 23, 14758. https://doi.org/10.3390/ijms232314758
Martínez-Gil N, Kutsyr O, Noailles A, Fernández-Sánchez L, Vidal L, Sánchez-Sáez X, Sánchez-Castillo C, Lax P, Cuenca N, García AG, et al. Purinergic Receptors P2X7 and P2X4 as Markers of Disease Progression in the rd10 Mouse Model of Inherited Retinal Dystrophy. International Journal of Molecular Sciences. 2022; 23(23):14758. https://doi.org/10.3390/ijms232314758
Chicago/Turabian StyleMartínez-Gil, Natalia, Oksana Kutsyr, Agustina Noailles, Laura Fernández-Sánchez, Lorena Vidal, Xavier Sánchez-Sáez, Carla Sánchez-Castillo, Pedro Lax, Nicolás Cuenca, Antonio G. García, and et al. 2022. "Purinergic Receptors P2X7 and P2X4 as Markers of Disease Progression in the rd10 Mouse Model of Inherited Retinal Dystrophy" International Journal of Molecular Sciences 23, no. 23: 14758. https://doi.org/10.3390/ijms232314758





