Peptide Modification Diminishes HLA Class II-restricted CD4+ T Cell Recognition of Prostate Cancer Cells
Abstract
:1. Introduction
2. Results
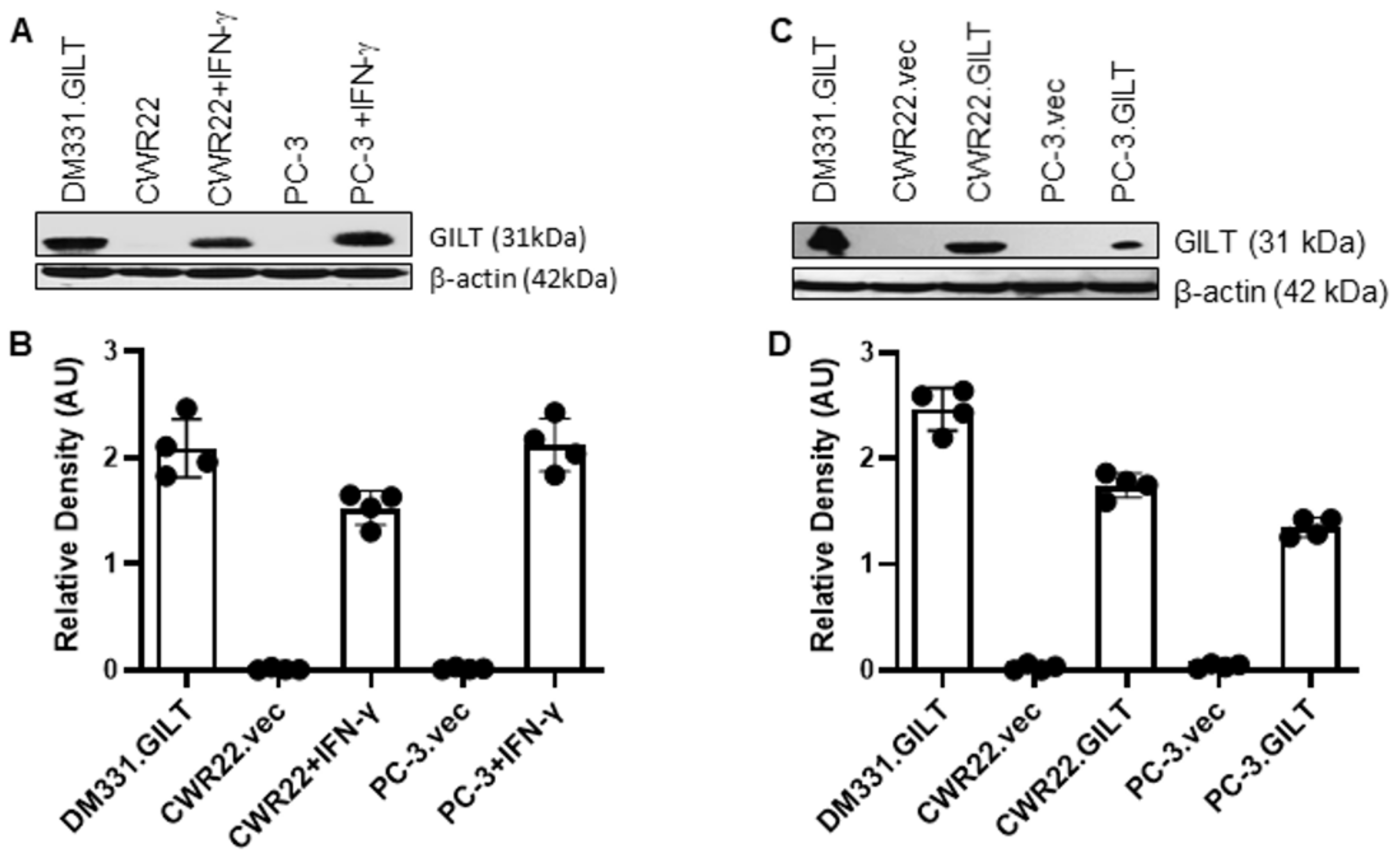
2.1. IFN-γ Treatment Induces GILT in Human Prostate Cancer Cells
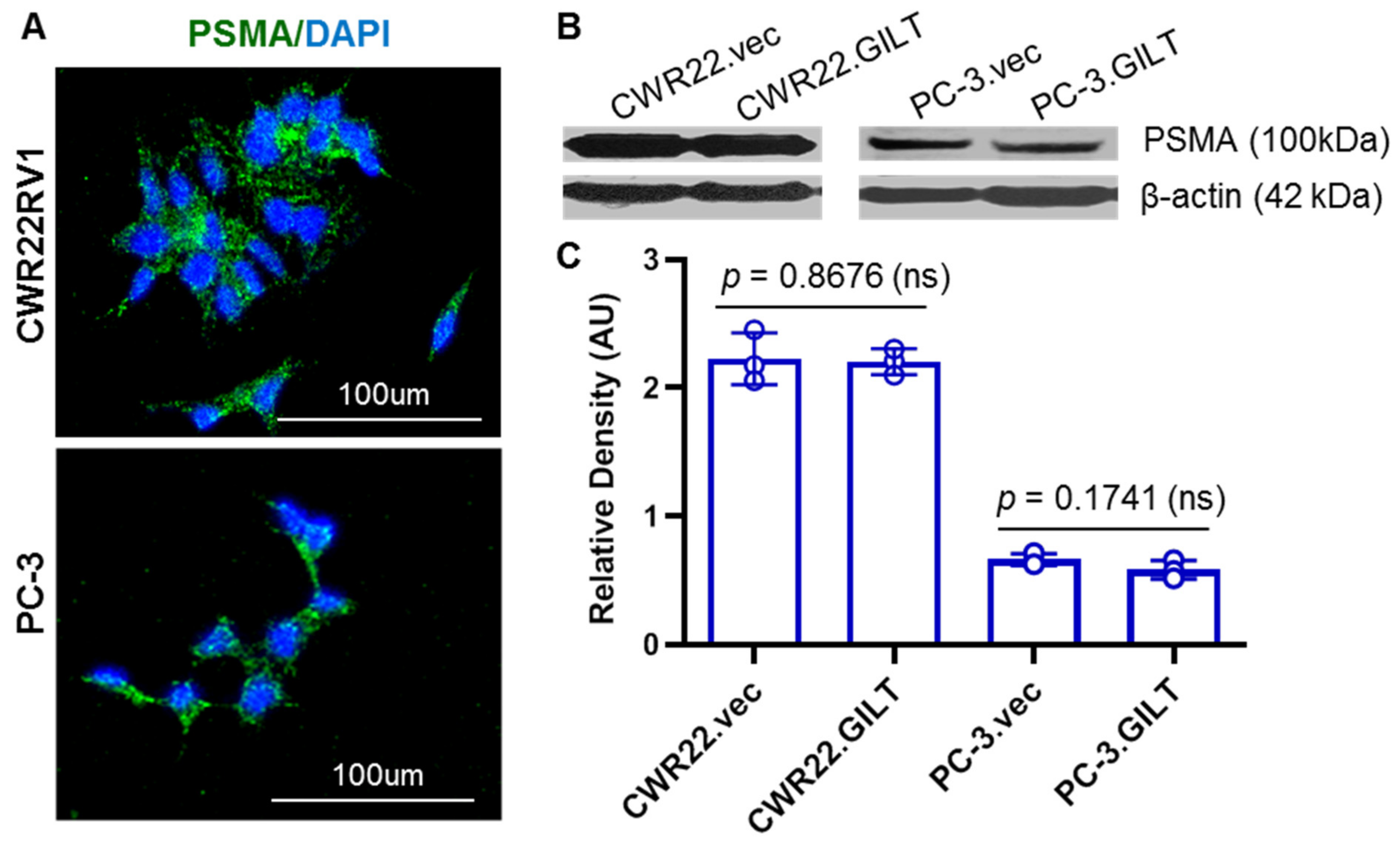
2.2. GILT Expression Did Not Alter PSMA Levels in Prostate Cancer Cells
2.3. Prostate Cancer Cell EHLA Class II Protein and GILT Insertion Did Not Markedly Alter HLA Protein Expression on the Cell Surface of CWR22 and PC-3 Cells
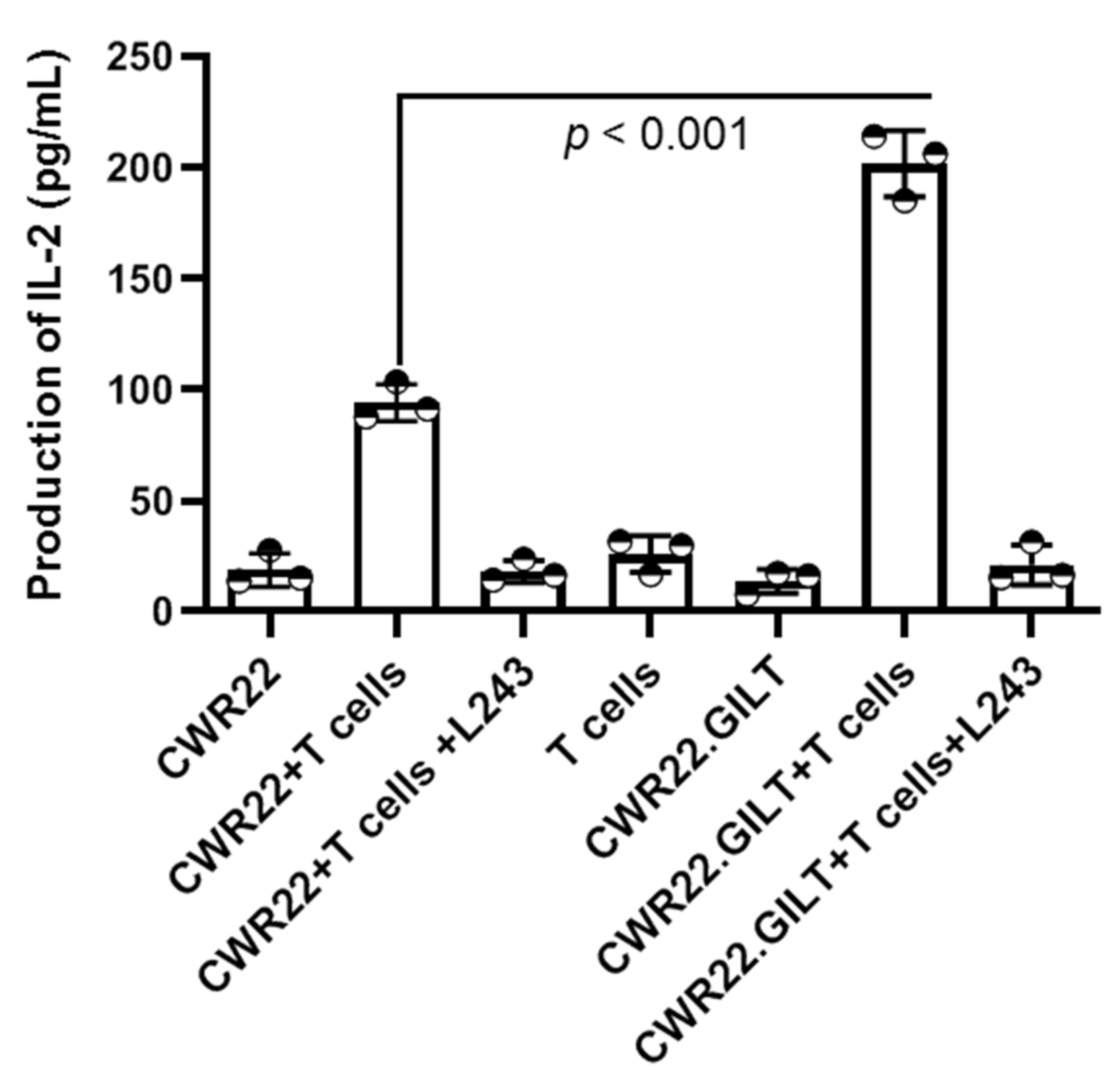
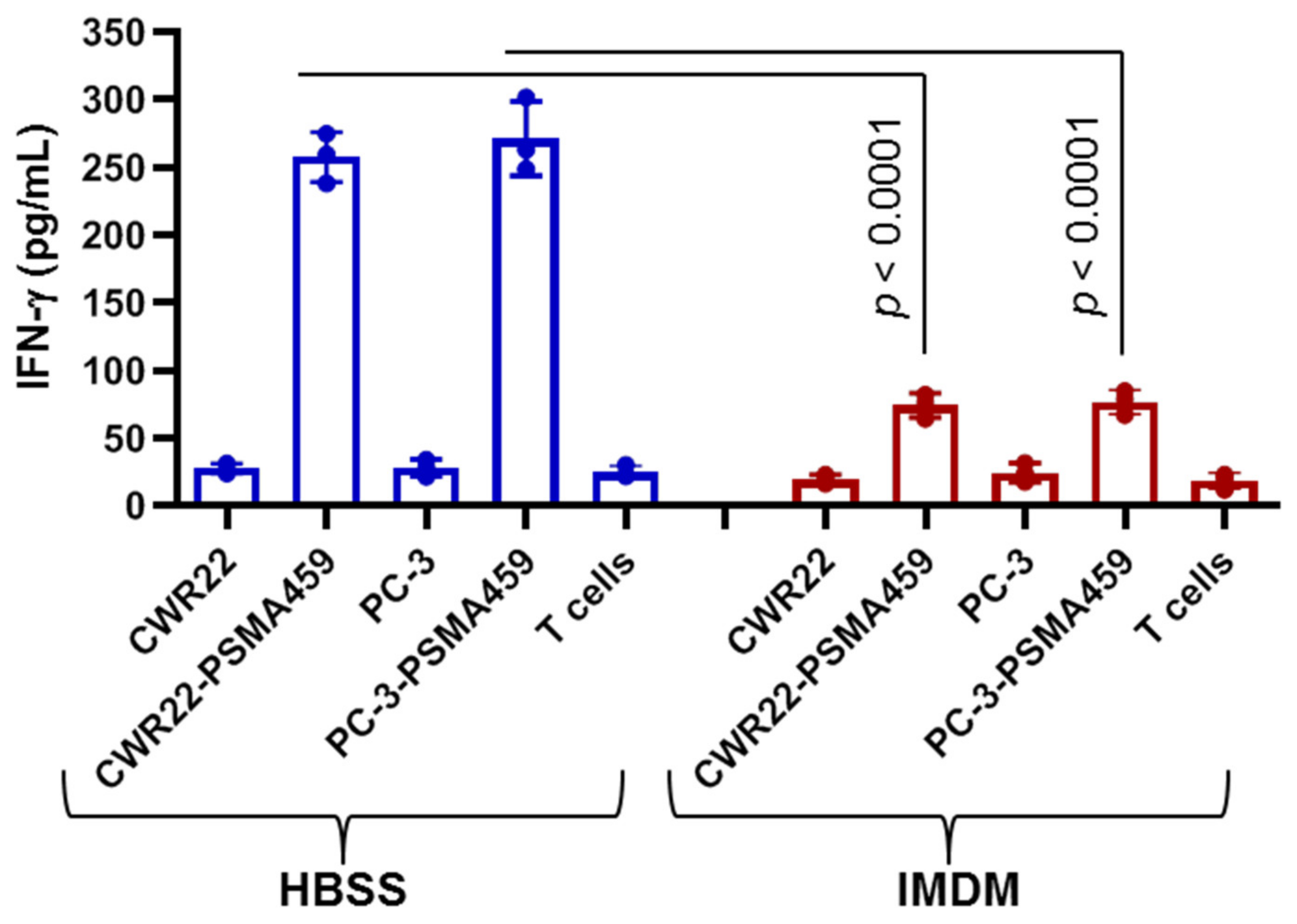
2.4. GILT Expression Enhances the HLA Class II Mediated Antigen Presentation in PSMA-Expressing Prostate Cancer Cells
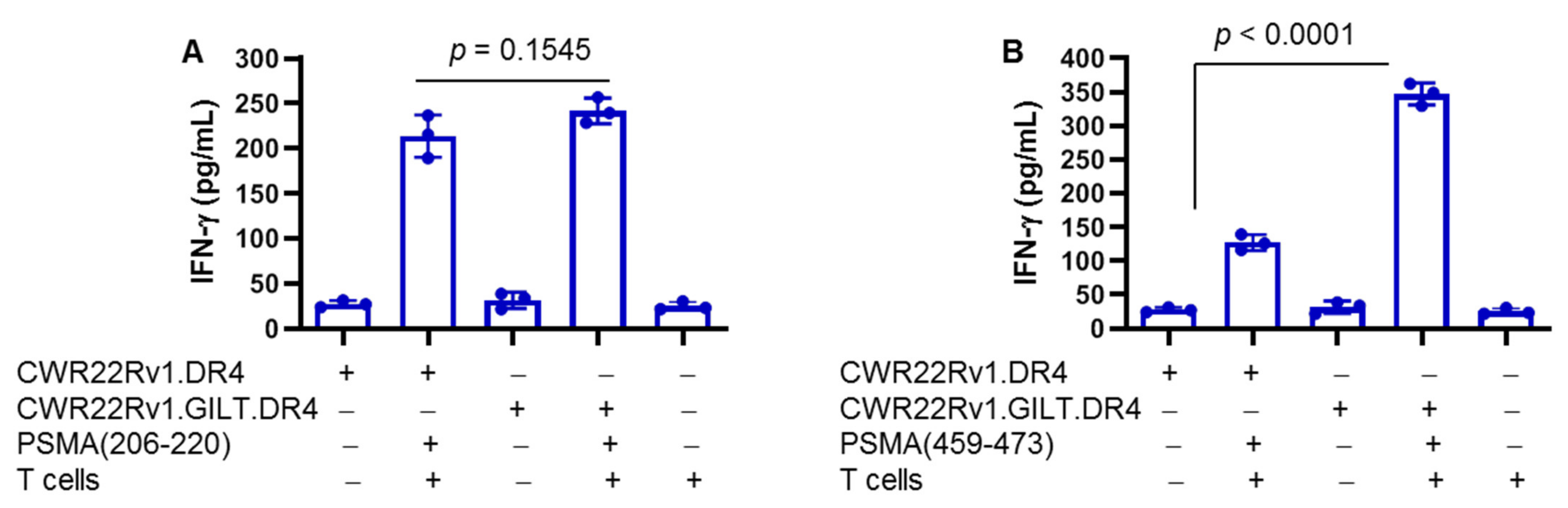
2.5. Differential Presentation of PSMA Peptides to CD4+ T Cells: Role of GILT in Enhanced Presentation
2.6. Immunodominant PSMA459 Peptide Contains a Cysteine Residue Which May Become Oxidized and Disrupt Peptide Presentation by CWR22 Cells
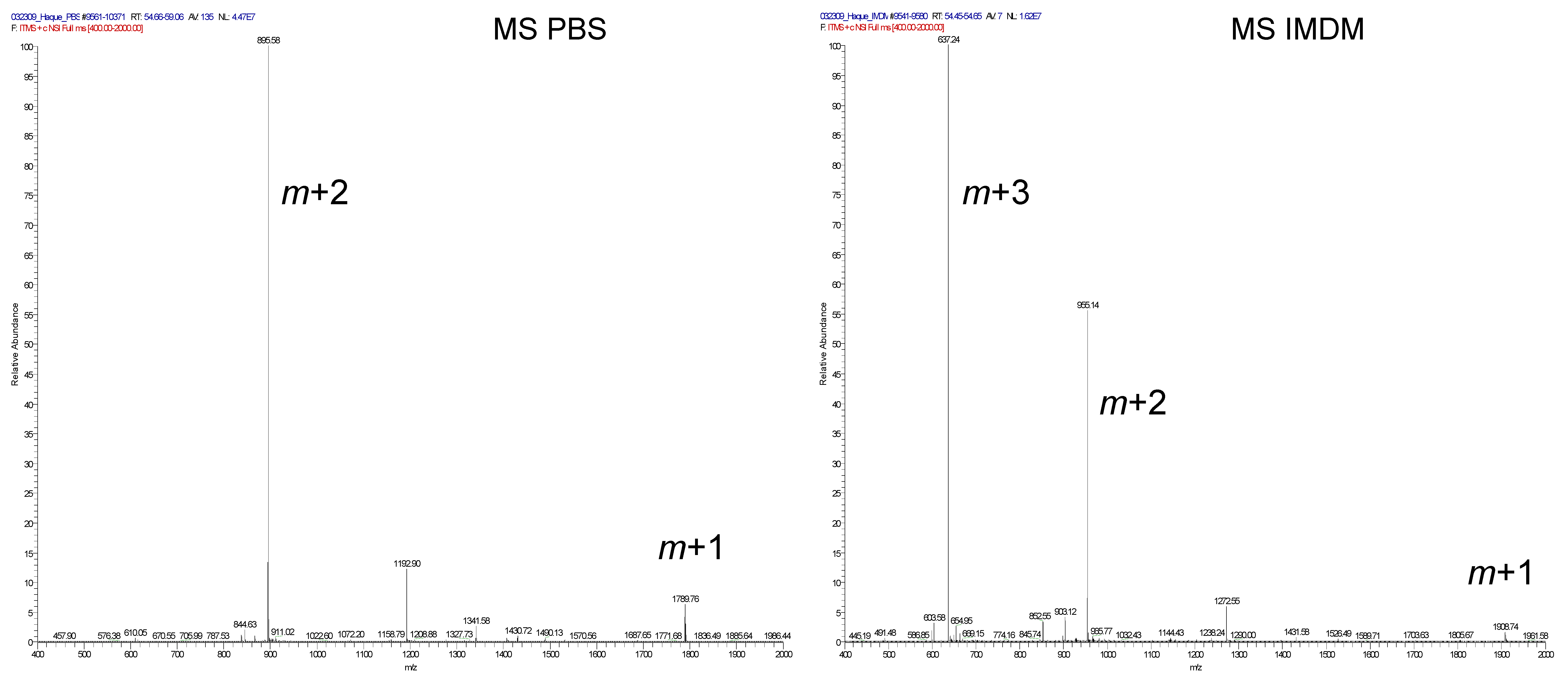
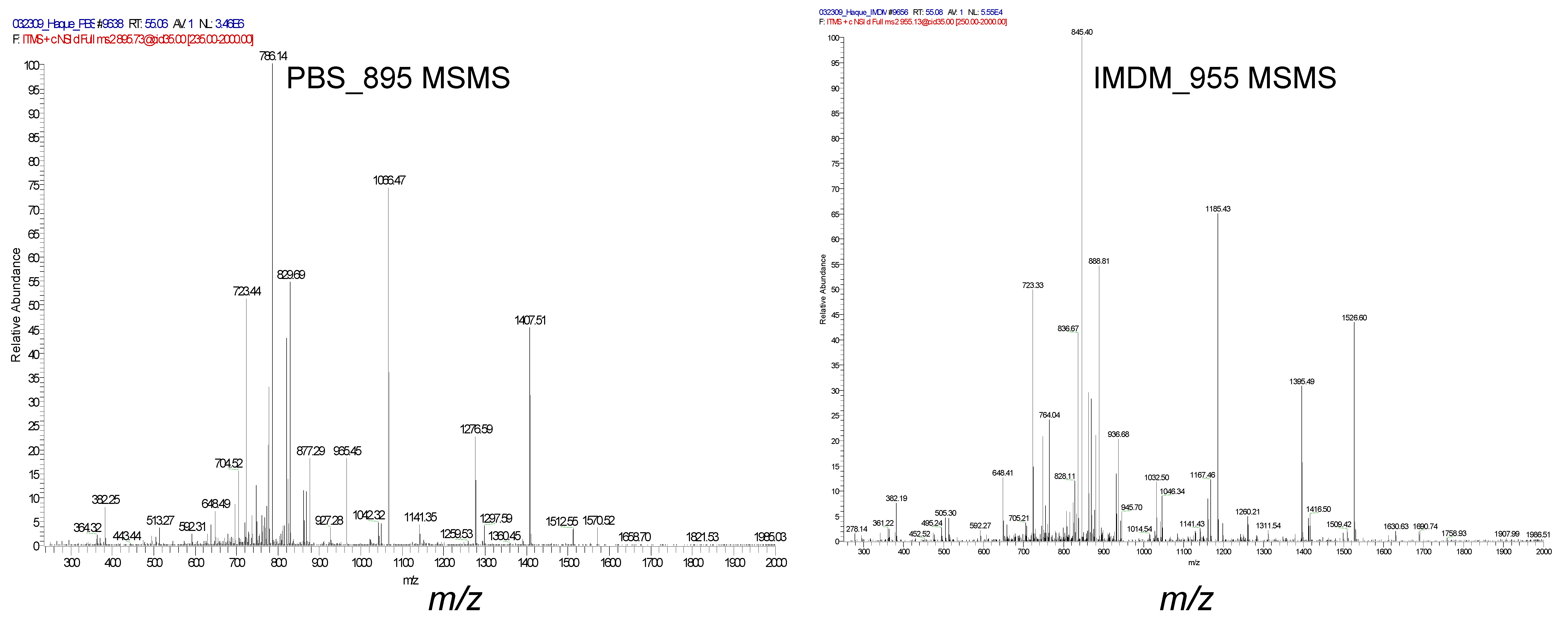
2.7. Immunodominant PSMA459 Peptide Becomes Cysteinylated under a Normal Physiological Concentration of Cysteine in Media
3. Discussion
4. Methods and Materials
4.1. Cell Lines and Culture Conditions
4.2. Cell Transduction and Transfection
4.3. Peptides
4.4. Antibodies
4.5. Flow Cytometry
4.6. Western Blot Analysis
4.7. Confocal Microscopy
4.8. Sample Preparation for LC MS/MS
4.9. Mass Spectrometry
4.10. Antigen Presentation Assays
4.11. Enzyme-Linked Immunosorbent Assay
4.12. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HLA | Human leukocyte antigen |
| PSMA | Prostate-specific membrane antigen |
| GILT | Gamma–interferon inducible lysosomal thiol reductase |
| IMDM | Iscove’s Modified Dulbecco’s Medium |
| IFN-γ | Interferon-gamma |
| PBMC | Peripheral blood mononuclear cells |
| DTT | Dithiothreitol |
| ELISA | Enzyme-linked immunosorbent assay |
| LC | Liquid chromatography |
| MS/MS | Mass spectrometry |
| AU | Arbitrary units |
| ANOVA | Analysis of variance |
| STDEV | Standard deviation |
References
- ACS. Cancer Facts & Figures 2010; American Cancer Society: Atlanta, GA, USA, 2010; p. 4. [Google Scholar]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Simmons, M.N.; Berglund, R.K.; Jones, J.S. A practical guide to prostate cancer diagnosis and management. Cleve Clin. J. Med. 2011, 78, 321–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonarakis, E.S.; Armstrong, A.J. Emerging therapeutic approaches in the management of metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2011, 14, 206–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asmane, I.; Ceraline, J.; Duclos, B.; Rob, L.; Litique, V.; Barthelemy, P.; Bergerat, J.P.; Dufour, P.; Kurtz, J.E. New Strategies for Medical Management of Castration-Resistant Prostate Cancer. Oncology 2011, 80, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Crook, J. The role of brachytherapy in the definitive management of prostate cancer. Cancer Radiother. 2011, 15, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Anassi, E.; Ndefo, U.A. Sipuleucel-T (provenge) injection: The first immunotherapy agent (vaccine) for hormone-refractory prostate cancer. Pharm. Ther. 2011, 36, 197–202. [Google Scholar]
- Cheever, M.A.; Higano, C. PROVENGE (Sipuleucel-T) in Prostate Cancer: The First FDA Approved Therapeutic Cancer Vaccine. Clin. Cancer Res. 2011, 17, 3520–3526. [Google Scholar] [CrossRef] [Green Version]
- Karwacki, J.; Kielbik, A.; Szlasa, W.; Sauer, N.; Kowalczyk, K.; Krajewski, W.; Saczko, J.; Kulbacka, J.; Szydelko, T.; Malkiewicz, B. Boosting the Immune Response-Combining Local and Immune Therapy for Prostate Cancer Treatment. Cells 2022, 11, 2793. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.X.; Kwak, L.; Hamid, A.; He, M.; Sweeney, C.; Flanders, S.C.; Harmon, M.; Choudhury, A.D. Outcomes in men with metastatic castration-resistant prostate cancer who received sipuleucel-T and no immediate subsequent therapy: Experience at Dana Farber and in the PROCEED Registry. Prostate Cancer Prostatic Dis. 2022, 25, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.; Zhang, L.; Subudhi, S.; Chen, B.; Marquez, J.; Liu, E.V.; Allaire, K.; Cheung, A.; Ng, S.; Nguyen, C.; et al. Pre-existing immune status associated with response to combination of sipuleucel-T and ipilimumab in patients with metastatic castration-resistant prostate cancer. J. Immunother. Cancer 2021, 9, e002254. [Google Scholar] [CrossRef]
- Rice, M.A.; Malhotra, S.V.; Stoyanova, T. Second-Generation Antiandrogens: From Discovery to Standard of Care in Castration Resistant Prostate Cancer. Front. Oncol. 2019, 9, 801. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, S.I.M.; Ju, X.; Horvath, L.G.; Clark, G.J. Moving on From Sipuleucel-T: New Dendritic Cell Vaccine Strategies for Prostate Cancer. Front. Immunol. 2021, 12, 641307. [Google Scholar] [CrossRef]
- Caram, M.E.V.; Ross, R.; Lin, P.; Mukherjee, B. Factors Associated with Use of Sipuleucel-T to Treat Patients With Advanced Prostate Cancer. JAMA Netw. Open 2019, 2, e192589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, B.L. Immunotherapy in treatment of metastatic prostate cancer: An approach to circumvent immunosuppressive tumor microenvironment. Prostate 2021, 81, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Neeley, Y.C.; McDonagh, K.T.; Overwijk, W.W.; Restifo, N.P.; Sanda, M.G. Antigen-specific tumor vaccine efficacy in vivo against prostate cancer with low class I MHC requires competent class II MHC. Prostate 2002, 53, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doonan, B.P.; Haque, A. Prostate Cancer Immunotherapy: Exploiting the HLA Class II Pathway in Vaccine Design. J. Clin. Cell. Immunol. 2015, 6, 351. [Google Scholar] [CrossRef]
- Johnson, B.M.; Radwan, F.F.Y.; Hossain, A.; Doonan, B.P.; Hathaway-Schrader, J.D.; God, J.M.; Voelkel-Johnson, C.V.; Banik, N.L.; Reddy, S.V.; Haque, A. Endoplasmic reticulum stress, autophagic and apoptotic cell death, and immune activation by a natural triterpenoid in human prostate cancer cells. J. Cell. Biochem. 2019, 120, 6264–6276. [Google Scholar] [CrossRef]
- Klebanoff, C.A.; Gattinoni, L.; Restifo, N.P. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol. Rev. 2006, 211, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Knutson, K.L.; Disis, M.L. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol. Immunother. 2005, 54, 721–728. [Google Scholar] [CrossRef]
- Doonan, B.P.; Haque, A. HLA Class II Antigen Presentation in Prostate Cancer Cells: A Novel Approach to Prostate Tumor Immunotherapy. Open Cancer Immunol. J. 2010, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.M.; Doonan, B.P.; Radwan, F.F.; Haque, A. Ganoderic Acid DM: An Alternative Agent for the Treatment of Advanced Prostate Cancer. Open Prostate Cancer J. 2010, 3, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Norton, D.L.; Haque, A. Insights into the Role of GILT in HLA Class II Antigen Processing and Presentation by Melanoma. J. Oncol. 2009, 2009, 142959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.A.; Srivastava, M.K.; Bosch, J.J.; Clements, V.K.; Ksander, B.R.; Ostrand-Rosenberg, S. The absence of invariant chain in MHC II cancer vaccines enhances the activation of tumor-reactive type 1 CD4+ T lymphocytes. Cancer Immunol. Immunother. CII 2008, 57, 389–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, A.; Blum, J.S. New insights in antigen processing and epitope selection: Development of novel immunotherapeutic strategies for cancer, autoimmunity and infectious diseases. J. Biol. Regul. Homeost. Agents 2005, 19, 93–104. [Google Scholar]
- Hillman, G.G.; Kallinteris, N.L.; Lu, X.; Wang, Y.; Wright, J.L.; Li, Y.; Wu, S.; Forman, J.D.; Gulfo, J.V.; Humphreys, R.E.; et al. Turning tumor cells in situ into T-helper cell-stimulating, MHC class II tumor epitope-presenters: Immuno-curing and immuno-consolidation. Cancer Treat. Rev. 2004, 30, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Nanda, N.K.; Birch, L.; Greenberg, N.M.; Prins, G.S. MHC class I and class II molecules are expressed in both human and mouse prostate tumor microenvironment. Prostate 2006, 66, 1275–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, M.; Reichardt, W.; Koerner, J.; Groettrup, M.J. Coencapsulation of tumor lysate and CpG-ODN in PLGA-microspheres enables successful immunotherapy of prostate carcinoma in TRAMP mice. Control Release 2012, 162, 159–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younger, A.R.; Amria, S.; Jeffrey, W.A.; Mahdy, A.E.; Goldstein, O.G.; Norris, J.S.; Haque, A. HLA class II antigen presentation by prostate cancer cells. Prostate Cancer Prostatic Dis. 2008, 11, 334–341. [Google Scholar] [CrossRef]
- Saha, B.; Jyothi Prasanna, S.; Chandrasekar, B.; Nandi, D. Gene modulation and immunoregulatory roles of interferon gamma. Cytokine 2010, 50, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, B.; Phan, U.T.; Geuze, H.J.; Cresswell, P. Enzymatic reduction of disulfide bonds in lysosomes: Characterization of a gamma-interferon-inducible lysosomal thiol reductase (GILT). Proc. Natl. Acad. Sci. USA 2000, 97, 745–750. [Google Scholar] [CrossRef] [Green Version]
- Hathaway-Schrader, J.D.; Norton, D.; Hastings, K.; Doonan, B.P.; Fritz, S.T.; Bethard, J.R.; Blum, J.S.; Haque, A. GILT Expression in Human Melanoma Cells Enhances Generation of Antigenic Peptides for HLA Class II-Mediated Immune Recognition. Int. J. Mol. Sci. 2022, 23, 1066. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, O.G.; Hajiaghamohseni, L.M.; Amria, S.; Sundaram, K.; Reddy, S.V.; Haque, A. Gamma-IFN-inducible-lysosomal thiol reductase modulates acidic proteases and HLA class II antigen processing in melanoma. Cancer Immunol. Immunother. CII 2008, 57, 1461–1470. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.A.; Hawes, J.W.; Blum, J.S. Cysteinylation of MHC class II ligands: Peptide endocytosis and reduction within APC influences T cell recognition. J. Immunol. 2001, 166, 4543–4551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Haque, M.A.; Blum, J.S. Role of disulfide bonds in regulating antigen processing and epitope selection. J. Immunol. 2002, 169, 2444–2450. [Google Scholar] [CrossRef] [Green Version]
- Hope, T.A.; Aggarwal, R.; Chee, B.; Tao, D.; Greene, K.L.; Cooperberg, M.R.; Feng, F.; Chang, A.; Ryan, C.J.; Small, E.J.; et al. Impact of (68)Ga-PSMA-11 PET on Management in Patients with Biochemically Recurrent Prostate Cancer. J. Nucl. Med. 2017, 58, 1956–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.S. Overview of prostate-specific membrane antigen. Rev. Urol. 2004, 6 (Suppl. S10), S13–S18. [Google Scholar] [PubMed]
- Bouchelouche, K.; Choyke, P.L.; Capala, J. Prostate specific membrane antigen- a target for imaging and therapy with radionuclides. Discov. Med. 2010, 9, 55–61. [Google Scholar] [PubMed]
- Hupe, M.C.; Philippi, C.; Roth, D.; Kumpers, C.; Ribbat-Idel, J.; Becker, F.; Joerg, V.; Duensing, S.; Lubczyk, V.H.; Kirfel, J.; et al. Expression of Prostate-Specific Membrane Antigen (PSMA) on Biopsies Is an Independent Risk Stratifier of Prostate Cancer Patients at Time of Initial Diagnosis. Front. Oncol. 2018, 8, 623. [Google Scholar] [CrossRef] [PubMed]
- Schroers, R.; Shen, L.; Rollins, L.; Xiao, Z.; Sonderstrup, G.; Slawin, K.; Huang, X.F.; Chen, S.Y. Identification of MHC class II-restricted T-cell epitopes in prostate-specific membrane antigen. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 3260–3271. [Google Scholar]
- Pathak, S.S.; Blum, J.S. Endocytic recycling is required for the presentation of an exogenous peptide via MHC class II molecules. Traffic 2000, 1, 561–569. [Google Scholar] [CrossRef]
- Haque, A.; Hajiaghamohseni, L.M.; Li, P.; Toomy, K.; Blum, J.S. Invariant chain modulates HLA class II protein recycling and peptide presentation in nonprofessional antigen presenting cells. Cell. Immunol. 2007, 249, 20–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmehrath, A.O.; Afifi, A.M.; Al-Husseini, M.J.; Saad, A.M.; Wilson, N.; Shohdy, K.S.; Pilie, P.; Sonbol, M.B.; Alhalabi, O. Causes of Death Among Patients With Metastatic Prostate Cancer in the US From 2000 to 2016. JAMA Netw. Open 2021, 4, e2119568. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Wurnschimmel, C.; Nocera, L.; Colla Ruvolo, C.; Tian, Z.; Shariat, S.F.; Saad, F.; Briganti, A.; Tilki, D.; Graefen, M.; et al. Overall Survival After Systemic Treatment in High-volume Versus Low-volume Metastatic Hormone-sensitive Prostate Cancer: Systematic Review and Network Meta-analysis. Eur. Urol. Focus 2022, 8, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Graf, R.P.; Fisher, V.; Weberpals, J.; Gjoerup, O.; Tierno, M.B.; Huang, R.S.P.; Sayegh, N.; Lin, D.I.; Raskina, K.; Schrock, A.B.; et al. Comparative Effectiveness of Immune Checkpoint Inhibitors vs Chemotherapy by Tumor Mutational Burden in Metastatic Castration-Resistant Prostate Cancer. JAMA Netw. Open 2022, 5, e225394. [Google Scholar] [CrossRef]
- Brower, V. Approval of provenge seen as first step for cancer treatment vaccines. J. Natl. Cancer Inst. 2010, 102, 1108–1110. [Google Scholar] [CrossRef] [Green Version]
- Klyushnenkova, E.N.; Kouiavskaia, D.V.; Berard, C.A.; Alexander, R.B. Cutting edge: Permissive MHC class II allele changes the pattern of antitumor immune response resulting in failure of tumor rejection. J. Immunol. 2009, 182, 1242–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrand-Rosenberg, S. CD4+ T lymphocytes: A critical component of antitumor immunity. Cancer Investig. 2005, 23, 413–419. [Google Scholar]
- Rosenberg, S.A.; Yang, J.C.; Restifo, N.P. Cancer immunotherapy: Moving beyond current vaccines. Nat. Med. 2004, 10, 909–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Zhang, H.; Zhao, J. The role of CD4 T cell help for CD8 CTL activation. Biochem. Biophys. Res. Commun. 2009, 384, 405–408. [Google Scholar] [CrossRef]
- Gerloni, M.; Zanetti, M. CD4 T cells in tumor immunity. Springer Semin. Immunopathol. 2005, 27, 37–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, M.A.; Li, P.; Jackson, S.K.; Zarour, H.M.; Hawes, J.W.; Phan, U.T.; Maric, M.; Cresswell, P.; Blum, J.S. Absence of gamma-interferon-inducible lysosomal thiol reductase in melanomas disrupts T cell recognition of select immunodominant epitopes. J. Exp. Med. 2002, 195, 1267–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar]
- Giraudet, A.L.; Kryza, D.; Hofman, M.; Moreau, A.; Fizazi, K.; Flechon, A.; Hicks, R.J.; Tran, B. PSMA targeting in metastatic castration-resistant prostate cancer: Where are we and where are we going? Ther. Adv. Med. Oncol. 2021, 13, 17588359211053898. [Google Scholar] [CrossRef] [PubMed]
- Pomykala, K.L.; Czernin, J.; Grogan, T.R.; Armstrong, W.R.; Williams, J.; Calais, J. Total-Body (68)Ga-PSMA-11 PET/CT for Bone Metastasis Detection in Prostate Cancer Patients: Potential Impact on Bone Scan Guidelines. J. Nucl. Med. 2020, 61, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Elsasser-Beile, U.; Buhler, P.; Wolf, P. Targeted therapies for prostate cancer against the prostate specific membrane antigen. Curr. Drug Targets 2009, 10, 118–125. [Google Scholar] [CrossRef]
- Kuratsukuri, K.; Sone, T.; Wang, C.Y.; Nishisaka, N.; Jones, R.F.; Haas, G.P. Inhibition of prostate-specific membrane antigen (PSMA)-positive tumor growth by vaccination with either full-length or the C-terminal end of PSMA. Int. J. Cancer 2002, 102, 244–249. [Google Scholar] [CrossRef]
- Slovin, S.F. Targeting novel antigens for prostate cancer treatment: Focus on prostate-specific membrane antigen. Expert Opin. Ther. Targets 2005, 9, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Fishman, M. A changing world for DCvax: A PSMA loaded autologous dendritic cell vaccine for prostate cancer. Expert Opin. Biol. Ther. 2009, 9, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.; Peterson, A.C.; Rini, B.I.; Harlin, H.; Gajewski, T.F.; Stadler, W.M. The HLA-A2-restricted PSMA peptide LLHETDSAV is poorly immunogenic in patients with metastatic prostate cancer. Prostate 2009, 69, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Hiraiwa, A.; Yamanaka, K.; Kwok, W.W.; Mickelson, E.M.; Masewicz, S.; Hansen, J.A.; Radka, S.F.; Nepom, G.T. Structural requirements for recognition of the HLA-Dw14 class II epitope: A key HLA determinant associated with rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 1990, 87, 8051–8055. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, P.W.; Haque, A.; Klemsz, M.J.; Kaplan, M.H.; Blum, J.S. Cutting edge: Induction of the antigen-processing enzyme IFN-gamma-inducible lysosomal thiol reductase in melanoma cells Is STAT1-dependent but CIITA-independent. J. Immunol. 2004, 173, 731–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lich, J.D.; Elliott, J.F.; Blum, J.S. Cytoplasmic processing is a prerequisite for presentation of an endogenous antigen by major histocompatibility complex class II proteins. J. Exp. Med. 2000, 191, 1513–1524. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doonan, B.P.; Amria, S.; Bethard, J.R.; Banik, N.L.; Hathaway-Schrader, J.D.; Haque, A. Peptide Modification Diminishes HLA Class II-restricted CD4+ T Cell Recognition of Prostate Cancer Cells. Int. J. Mol. Sci. 2022, 23, 15234. https://doi.org/10.3390/ijms232315234
Doonan BP, Amria S, Bethard JR, Banik NL, Hathaway-Schrader JD, Haque A. Peptide Modification Diminishes HLA Class II-restricted CD4+ T Cell Recognition of Prostate Cancer Cells. International Journal of Molecular Sciences. 2022; 23(23):15234. https://doi.org/10.3390/ijms232315234
Chicago/Turabian StyleDoonan, Bently P., Shereen Amria, Jennifer R. Bethard, Narendra L. Banik, Jessica D. Hathaway-Schrader, and Azizul Haque. 2022. "Peptide Modification Diminishes HLA Class II-restricted CD4+ T Cell Recognition of Prostate Cancer Cells" International Journal of Molecular Sciences 23, no. 23: 15234. https://doi.org/10.3390/ijms232315234






