Identification of Important Factors Causing Developmental Arrest in Cloned Pig Embryos by Embryo Biopsy Combined with Microproteomics
Abstract
:1. Introduction
2. Results
2.1. Tracing the Developmental Fates of Different SCNT Embryos by Embryo Biopsy
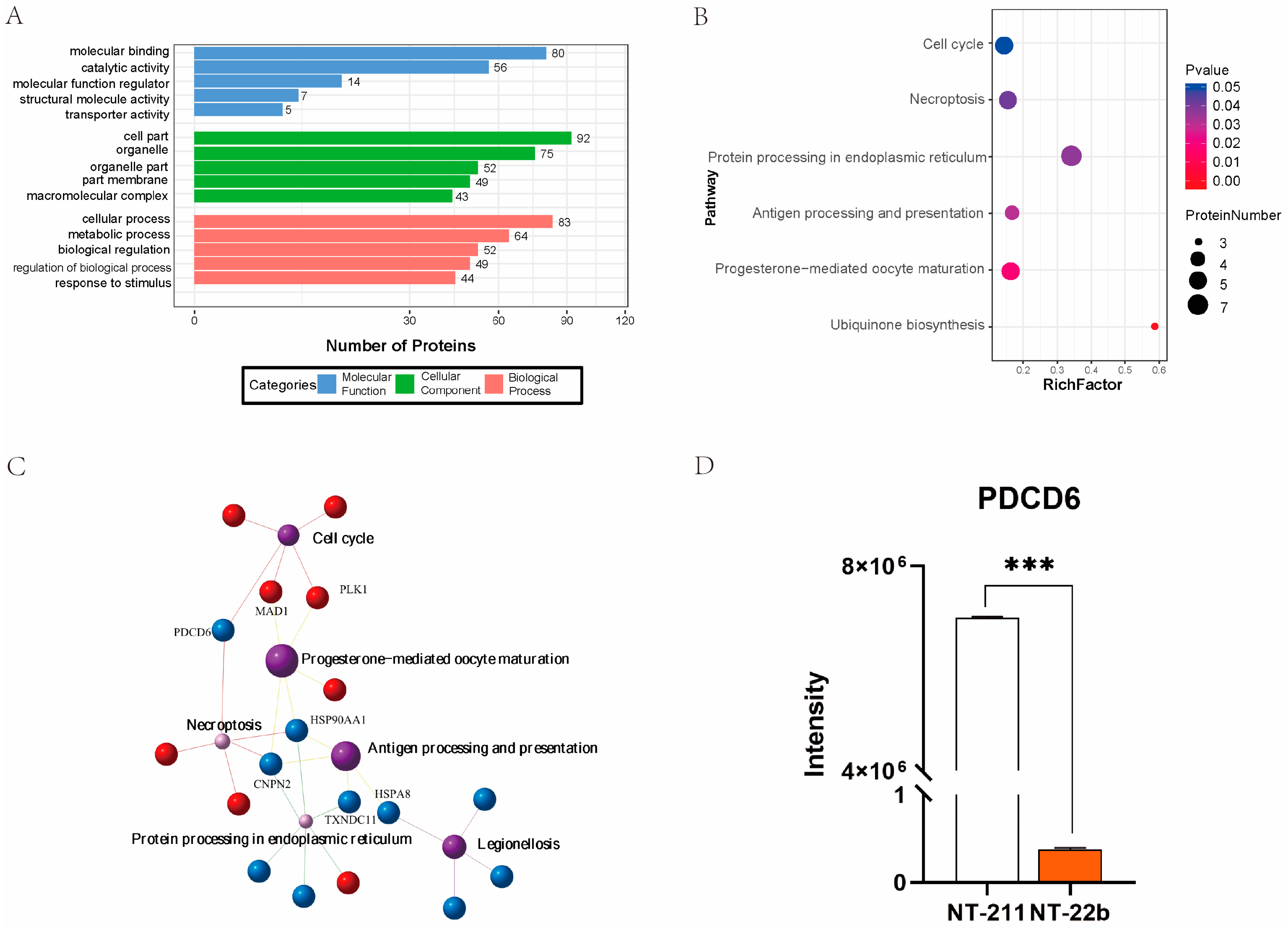
2.2. Identification of Potential Factors That Regulate the Developmental Fates of Cloned Porcine Embryos
2.3. Microinjection of PDCD6 siRNA Significantly Improved the Developmental Efficiency of Porcine SCNT Embryos
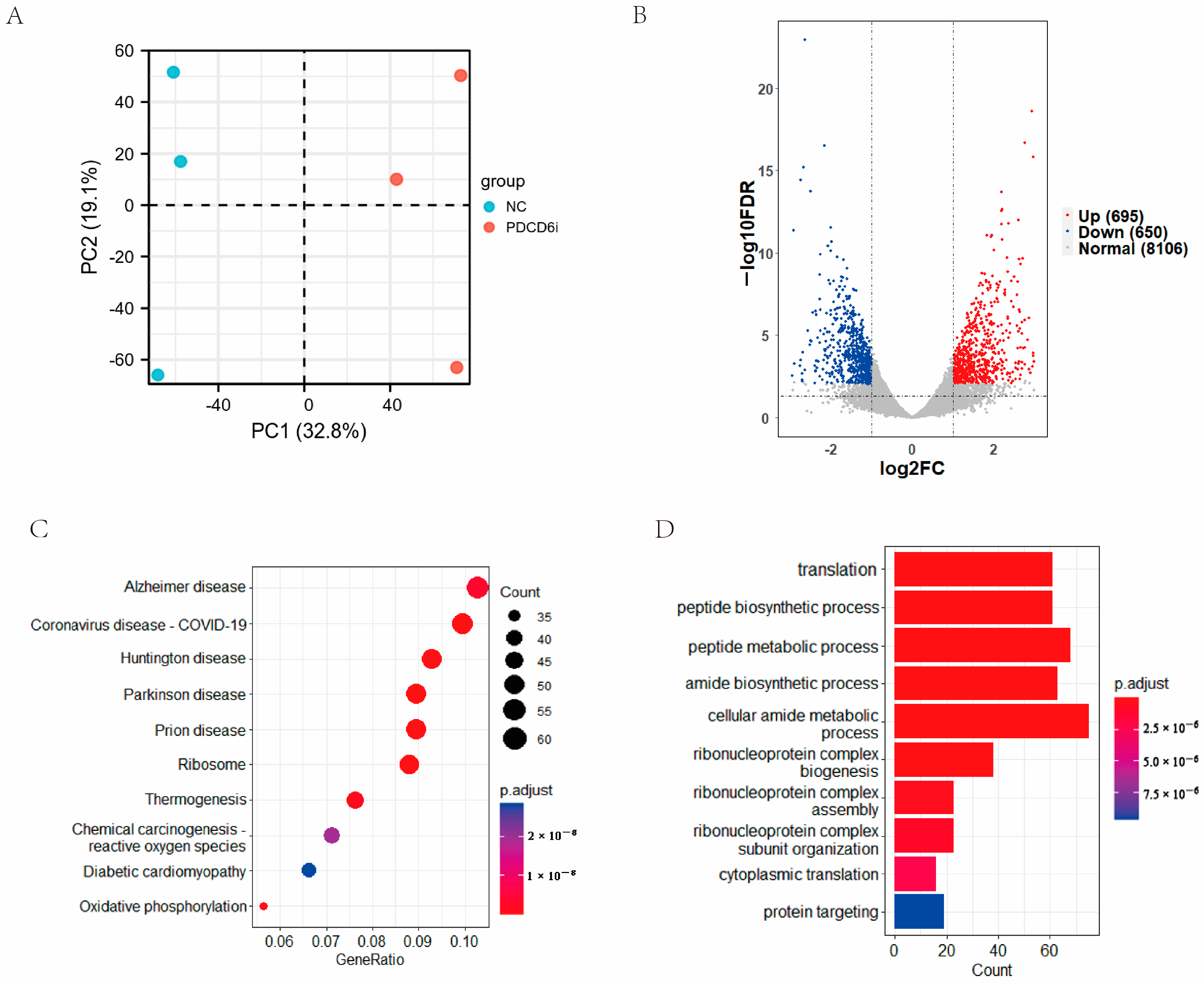
2.4. Effects of PDCD6 siRNA Microinjection on the Transcriptome of Injected SCNT Embryos at the Two-Cell Stage
2.5. PDCD6 siRNA Microinjection Inhibited the Expression of Pro-Apoptotic Genes in Injected Cloned Embryos
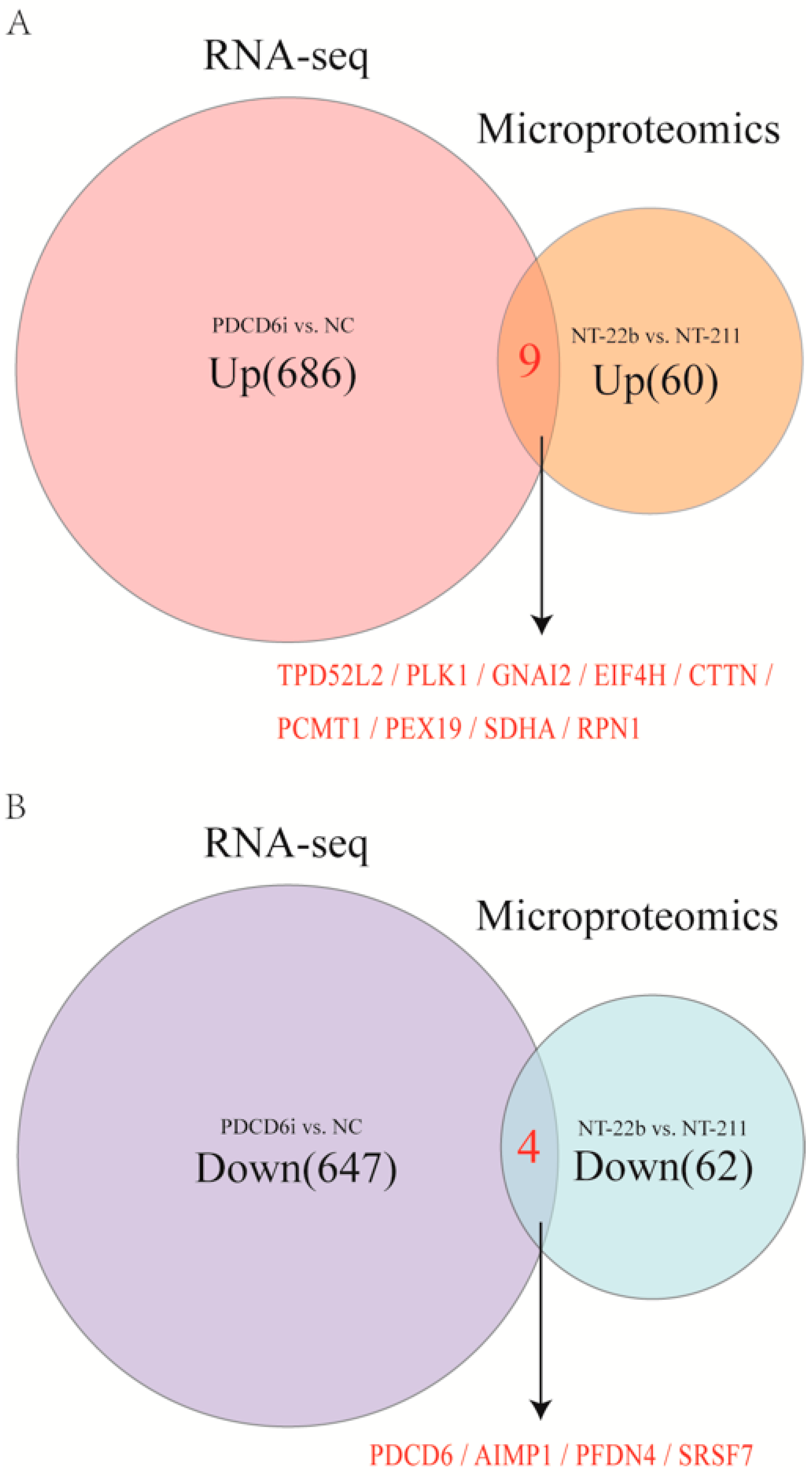
2.6. Intersection Analysis of the Microproteome and Transcriptome Results Identified Potential Factors That Regulate Cloned Porcine Embryo Development
2.7. PLK1 mRNA Injection Significantly Increased the Cleavage Rate of Cloned Porcine Embryos
3. Discussion
4. Materials and Methods
4.1. In Vitro Oocyte Maturation
4.2. Cell Culture
4.3. SCNT
4.4. Blastomere Isolation from Cloned Embryos
4.5. Microproteomic Analysis
4.6. PDCD6 siRNA Design, Synthesis, and Transfection
- siRNA-155 (forward): 5′-GCUUCCUGUGGAACGUCUUTT-3′;
- siRNA-155 (reverse): 5′-AAGACGUUCCACAGGAAGCTT-3′;
- siRNA-204 (forward): 5′-GAUAUCGGACAACGAGCUUTT-3′;
- siRNA-204 (reverse): 5′-AAGCUCGUUGUCCGAUAUCTT-3′;
- siRNA-314 (forward): 5′-GCGUGAAUUUCAGCGAGUUTT-3′;
- siRNA-314 (reverse): 5′-AACUCGCUGAAAUUCACGCTT-3′;
- NC-siRNA (forward): 5′-UUCUCCGAACGUGUCACGUTT-3′;
- NC-siRNA (reverse): 5′-ACGUGACACGUUCGGAGAATT-3′.
4.7. PLK1 mRNA Synthesis
4.8. Microinjection
4.9. Transcriptome Sequencing
4.10. qPCR Analysis
- PDCD6 (forward): 5′-AAGACAGGAGCGGCGTGATAT-3′;
- PDCD6 (reverse): 5′-CGTTCTGCCAGTCGGTGATG-3′;
- CASP3 (forward): 5′-GGACTGCTGTAGAACTCTAACTGG-3′;
- CASP3 (reverse): 5′-CAAGAAGTCTGCCTCAACTGGTAT-3′;
- BCL2 (forward): 5′-GTGTGTGGAGAGCGTCAACC-3′;
- BCL2 (reverse): 5′-CCTTCAGAGACAGCCAGGAGAA-3′;
- BAX (forward): 5′-TCTACCAAGAAGTTGAGCGAGTGT-3′;
- BAX (reverse): 5′-CCAGTTGAAGTTGCCGTCAGC-3′;
- CYCS (forward): 5′-CTGCGAGTGGTGGCTTGTCT-3′;
- CYCS (reverse): 5′-CAGTCTTGTGTTTGCCTCCCTTT-3′;
- APAF1 (forward): 5′-CGACTGGAGATGACAACGGAGAA-3′;
- APAF1 (reverse): 5′-ACTAAGACTGGAGCACACGAATGA-3′;
- β-actin (forward): 5′-CCACGAGACCACCTTCAACTC-3′;
- β-actin (reverse): 5′-TGATCTCCTTCTGCATCCTGT-3′;
4.11. Immunofluorescence
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| APAF1 | apoptotic protease activating factor 1 |
| BCL-2 | B-cell lymphoma 2 |
| CASP3 | Caspase-3 |
| cDNA | Complementary DNA |
| CHIP-seq | Chromatin immunoprecipitation sequence |
| COCs | Cumulus oocyte complexes |
| CYCS | Cytochrome |
| DEGs | Differentially expressed genes |
| DEPs | Differentially expressed proteins |
| DNA | Deoxyribonucleic acid |
| DPBS | Cumulus oocyte complexes |
| DPBS | Dulbecco’s phosphate-buffered saline |
| FPKM | Fragments per kilobase of exon model per million mapped fragments |
| GO | Gene ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| mRNA | Messenger RNA |
| PCA | Principal component analysis |
| PDCD6 | Programmed cell death protein 6 |
| PDCD6i | PDCD6 siRNA |
| PLK1 | Polo-like kinase 1 |
| PZM-3 | Porcine zygote medium 3 |
| qPCR | Quantitative real-time PCR |
| RNA | Ribonucleic acid |
| SCNT | Somatic cell nuclear |
| siRNA | Small interfering RNA |
| μL | Microlitre |
| μM | Micromole/L |
References
- Loi, P.; Ptak, G.; Barboni, B.; Fulka, J.J.; Cappai, P.; Clinton, M. Genetic rescue of an endangered mammal by cross-species nuclear transfer using post-mortem somatic cells. Nat. Biotechnol. 2001, 19, 962–964. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shi, J.; Liu, D.; Zhou, R.; Zeng, H.; Zhou, X.; Mai, R.; Zeng, S.; Luo, L.; Yu, W.; et al. Effects of donor fibroblast cell type and transferred cloned embryo number on the efficiency of pig cloning. Cell Reprogram. 2013, 15, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tachibana, M.; Amato, P.; Sparman, M.; Gutierrez, N.M.; Tippner-Hedges, R.; Ma, H.; Kang, E.; Fulati, A.; Lee, H.S.; Sritanaudomchai, H.; et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell 2013, 153, 1228–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Li, Z.; Yang, H.; Liu, D.; Cai, G.; Li, G.; Mo, J.; Wang, D.; Zhong, C.; Wang, H.; et al. Novel transgenic pigs with enhanced growth and reduced environmental impact. eLife 2018, 7, e34286. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Liao, S.; Kuang, Z.; Zhu, Q.; Wei, H.; Shi, J.; Zheng, E.; Xu, Z.; Huang, S.; Hong, L.; et al. Genetically Engineered Pigs as Efficient Salivary Gland Bioreactors for Production of Therapeutically Valuable Human Nerve Growth Factor. Cells 2022, 11, 2378. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, Y.; Wiriyahdamrong, T.; Yuan, Z.; Qing, Y.; Li, H.; Xu, K.; Guo, J.; Jia, B.; Zhang, X.; et al. Improved production of GTKO/hCD55/hCD59 triple-gene-modified Diannan miniature pigs for xenotransplantation by recloning. Transgenic. Res. 2020, 29, 369–379. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Lovendahl, P.; Schmidt, M.; Larsen, K.; Callesen, H. In vitro manipulation techniques of porcine embryos: A meta-analysis related to transfers, pregnancies and piglets. Reprod. Fertil. Dev. 2015, 27, 429–439. [Google Scholar] [CrossRef]
- Matoba, S.; Zhang, Y. Somatic Cell Nuclear Transfer Reprogramming: Mechanisms and Applications. Cell Stem Cell 2018, 23, 471–485. [Google Scholar] [CrossRef] [Green Version]
- Thuan, N.V.; Kishigami, S.; Wakayama, T. How to improve the success rate of mouse cloning technology. J. Reprod. Dev. 2010, 56, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Malin, K.; Witkowska-Pilaszewicz, O.; Papis, K. The many problems of somatic cell nuclear transfer in reproductive cloning of mammals. Theriogenology 2022, 189, 246–254. [Google Scholar] [CrossRef]
- Srirattana, K.; Kaneda, M.; Parnpai, R. Strategies to Improve the Efficiency of Somatic Cell Nuclear Transfer. Int. J. Mol. Sci. 2022, 23, 1969. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Kohda, T.; Sugimoto, M.; Sado, T.; Ogonuki, N.; Matoba, S.; Shiura, H.; Ikeda, R.; Mochida, K.; Fujii, T.; et al. Impeding Xist expression from the active X chromosome improves mouse somatic cell nuclear transfer. Science 2010, 330, 496–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matoba, S.; Liu, Y.; Lu, F.; Iwabuchi, K.A.; Shen, L.; Inoue, A.; Zhang, Y. Embryonic development following somatic cell nuclear transfer impeded by persisting histone methylation. Cell 2014, 159, 884–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.Y.; Li, Z.K.; Wang, L.B.; Liu, C.; Sun, X.H.; Feng, G.H.; Wang, J.Q.; Li, Y.F.; Qiao, L.Y.; Nie, H.; et al. Overcoming Intrinsic H3K27me3 Imprinting Barriers Improves Post-implantation Development after Somatic Cell Nuclear Transfer. Cell Stem Cell 2020, 27, 315–325. [Google Scholar] [CrossRef]
- Liu, W.; Liu, X.; Wang, C.; Gao, Y.; Gao, R.; Kou, X.; Zhao, Y.; Li, J.; Wu, Y.; Xiu, W.; et al. Identification of key factors conquering developmental arrest of somatic cell cloned embryos by combining embryo biopsy and single-cell sequencing. Cell Discov. 2016, 2, 16010. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Wang, C.; Gao, Y.; Xiu, W.; Chen, J.; Kou, X.; Zhao, Y.; Liao, Y.; Bai, D.; Qiao, Z.; et al. Inhibition of Aberrant DNA Re-methylation Improves Post-implantation Development of Somatic Cell Nuclear Transfer Embryos. Cell Stem Cell 2018, 23, 426–435. [Google Scholar] [CrossRef] [Green Version]
- Hadley, K.C.; Rakhit, R.; Guo, H.; Sun, Y.; Jonkman, J.E.; McLaurin, J.; Hazrati, L.N.; Emili, A.; Chakrabartty, A. Determining composition of micron-scale protein deposits in neurodegenerative disease by spatially targeted optical microproteomics. eLife 2015, 4, e09579. [Google Scholar] [CrossRef]
- Longuespee, R.; Alberts, D.; Pottier, C.; Smargiasso, N.; Mazzucchelli, G.; Baiwir, D.; Kriegsmann, M.; Herfs, M.; Kriegsmann, J.; Delvenne, P.; et al. A laser microdissection-based workflow for FFPE tissue microproteomics: Important considerations for small sample processing. Methods 2016, 104, 154–162. [Google Scholar] [CrossRef]
- Delcourt, V.; Franck, J.; Leblanc, E.; Narducci, F.; Robin, Y.M.; Gimeno, J.P.; Quanico, J.; Wisztorski, M.; Kobeissy, F.; Jacques, J.F.; et al. Combined Mass Spectrometry Imaging and Top-down Microproteomics Reveals Evidence of a Hidden Proteome in Ovarian Cancer. EBioMedicine 2017, 21, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Bensaddek, D.; Narayan, V.; Nicolas, A.; Murillo, A.B.; Gartner, A.; Kenyon, C.J.; Lamond, A.I. Micro-proteomics with iterative data analysis: Proteome analysis in C. elegans at the single worm level. Proteomics 2016, 16, 381–392. [Google Scholar] [CrossRef]
- He, X.; Tan, C.; Li, Z.; Zhao, C.; Shi, J.; Zhou, R.; Wang, X.; Jiang, G.; Cai, G.; Liu, D.; et al. Characterization and comparative analyses of transcriptomes of cloned and in vivo fertilized porcine pre-implantation embryos. Biol. Open 2019, 8, bio039917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasquale, V.; Emanuela, L.; Luciano, D.A. Interfering with Apoptosis: Ca$^{2+}$ -Binding Protein ALG-2 and Alzheimer’s Disease Gene ALG-3. Science 1996, 271, 521–525. Available online: http://www.jstor.org/stable/2890160 (accessed on 30 June 2021).
- Rao, R.V.; Poksay, K.S.; Castro-Obregon, S.; Schilling, B.; Row, R.H.; Del, R.G.; Gibson, B.W.; Ellerby, H.M.; Bredesen, D.E. Molecular components of a cell death pathway activated by endoplasmic reticulum stress. J. Biol. Chem. 2004, 279, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Tarabykina, S.; Mollerup, J.; Winding, P.; Berchtold, M.W. ALG-2, a multifunctional calcium binding protein? Front. Biosci. 2004, 9, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.R.; Cordonier, H.; Czyba, J.C.; Guerin, J.F. Apoptosis in preimplantation mammalian embryo and genetics. Ital. J. Anat. Embryol. 2001, 106, 101–108. [Google Scholar] [PubMed]
- Hao, Y.; Lai, L.; Mao, J.; Im, G.S.; Bonk, A.; Prather, R.S. Apoptosis and in vitro development of preimplantation porcine embryos derived in vitro or by nuclear transfer. Biol. Reprod. 2003, 69, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Wang, Y.; Xing, X.; Zhang, L.; Sun, H.; Zhang, Y. Melatonin significantly improves the developmental competence of bovine somatic cell nuclear transfer embryos. J. Pineal. Res. 2015, 59, 455–468. [Google Scholar] [CrossRef]
- Chang, H.Y.; Xie, R.X.; Zhang, L.; Fu, L.Z.; Zhang, C.T.; Chen, H.H.; Wang, Z.Q.; Zhang, Y.; Quan, F.S. Overexpression of miR-101-2 in donor cells improves the early development of Holstein cow somatic cell nuclear transfer embryos. J. Dairy Sci. 2019, 102, 4662–4673. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Rho, S.B.; Chun, T. Programmed cell death 6 (PDCD6) protein interacts with death-associated protein kinase 1 (DAPk1): Additive effect on apoptosis via caspase-3 dependent pathway. Biotechnol. Lett. 2005, 27, 1011–1015. [Google Scholar] [CrossRef]
- Suzuki, K.; Dashzeveg, N.; Lu, Z.G.; Taira, N.; Miki, Y.; Yoshida, K. Programmed cell death 6, a novel p53-responsive gene, targets to the nucleus in the apoptotic response to DNA damage. Cancer Sci. 2012, 103, 1788–1794. [Google Scholar] [CrossRef]
- Baran, V.; Brzakova, A.; Rehak, P.; Kovarikova, V.; Solc, P. PLK1 regulates spindle formation kinetics and APC/C activation in mouse zygote. Zygote 2016, 24, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Roh, S. Expression of polo-like kinase 1 in pre-implantation stage murine somatic cell nuclear transfer embryos. J. Vet. Sci. 2019, 20, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.J.; Griffin, E.E. PLK-1 Regulation of Asymmetric Cell Division in the Early C. elegans Embryo. Front. Cell Dev. Biol. 2020, 8, 632253. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.L.; Piehowski, P.D.; Orton, D.J.; Moore, R.J.; Qian, W.J.; Casey, C.P.; Sun, X.; Dey, S.K.; Burnum-Johnson, K.E.; Smith, R.D. SNaPP: Simplified Nanoproteomics Platform for Reproducible Global Proteomic Analysis of Nanogram Protein Quantities. Endocrinology 2016, 157, 1307–1314. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, D.; Vasconcelos, F.R.; Teles-Filho, A.; Viana, A.; Martins, A.; Sousa, M.V.; Castro, M.S.; Ricart, C.A.; Fontes, W.; Bertolini, M.; et al. Proteomic profile of pre-implantational ovine embryos produced in vivo. Reprod. Domest. Anim. 2021, 56, 586–603. [Google Scholar] [CrossRef]
- Chae, J.; Cho, Y.K.; Cho, S.; Kim, J.; Han, Y.; Koo, D.; Lee, K. Proteomic analysis of pancreas derived from adult cloned pig. Biochem. Bioph. Res. Commun. 2008, 366, 379–387. [Google Scholar] [CrossRef]
- He, W.; Li, C.; Dong, L.; Yang, G.; Liu, H. Tandem Mass Tag-Based Quantitative Proteomic Analysis of ISG15 Knockout PK15 Cells in Pseudorabies Virus Infection. Genes 2021, 12, 1557. [Google Scholar] [CrossRef]
- Shan, X.; Yu, T.; Yan, X.; Wu, J.; Fan, Y.; Guan, X.; Fang, F.; Lin, Y.; Zhang, Y.; Li, Y.; et al. Proteomic analysis of healthy and atretic porcine follicular granulosa cells. J. Proteom. 2021, 232, 104027. [Google Scholar] [CrossRef]
- Wang, H.; Jiao, P.; Zhang, X.; Xing, H. Quantitative proteomic analysis of trachea in fatting pig exposed to ammonia. J. Proteom. 2021, 247, 104330. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, S.; Fan, B.; Niu, B.; Guo, R.; Gu, J.; Gao, S.; Li, B. iTRAQ-based proteome analysis of porcine group A rotavirus-infected porcine IPEC-J2 intestinal epithelial cells. J. Proteom. 2021, 248, 104354. [Google Scholar] [CrossRef]
- Dawson, H.D.; Chen, C.; Gaynor, B.; Shao, J.; Urban, J.J. The porcine translational research database: A manually curated, genomics and proteomics-based research resource. BMC Genom. 2017, 18, 643. [Google Scholar] [CrossRef] [PubMed]
- la Cour, J.M.; Mollerup, J.; Winding, P.; Tarabykina, S.; Sehested, M.; Berchtold, M.W. Up-regulation of ALG-2 in hepatomas and lung cancer tissue. Am. J. Pathol. 2003, 163, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maki, M.; Takahara, T.; Shibata, H. Multifaceted Roles of ALG-2 in Ca(2+)-Regulated Membrane Trafficking. Int. J. Mol. Sci. 2016, 17, 1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huttemann, M.; Pecina, P.; Rainbolt, M.; Sanderson, T.H.; Kagan, V.E.; Samavati, L.; Doan, J.W.; Lee, I. The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: From respiration to apoptosis. Mitochondrion 2011, 11, 369–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manku, G.; Culty, M. Dynamic changes in the expression of apoptosis-related genes in differentiating gonocytes and in seminomas. Asian J. Androl. 2015, 17, 403–414. [Google Scholar] [CrossRef]
- Riedl, S.J.; Salvesen, G.S. The apoptosome: Signalling platform of cell death. Nat. Rev. Mol. Cell Biol. 2007, 8, 405–413. [Google Scholar] [CrossRef]
- Tong, C.; Fan, H.Y.; Lian, L.; Li, S.W.; Chen, D.Y.; Schatten, H.; Sun, Q.Y. Polo-like kinase-1 is a pivotal regulator of microtubule assembly during mouse oocyte meiotic maturation, fertilization, and early embryonic mitosis. Biol. Reprod. 2002, 67, 546–554. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.Y.; Tong, C.; Teng, C.B.; Lian, L.; Li, S.W.; Yang, Z.M.; Chen, D.Y.; Schatten, H.; Sun, Q.Y. Characterization of Polo-like kinase-1 in rat oocytes and early embryos implies its functional roles in the regulation of meiotic maturation, fertilization, and cleavage. Mol. Reprod. Dev. 2003, 65, 318–329. [Google Scholar] [CrossRef]

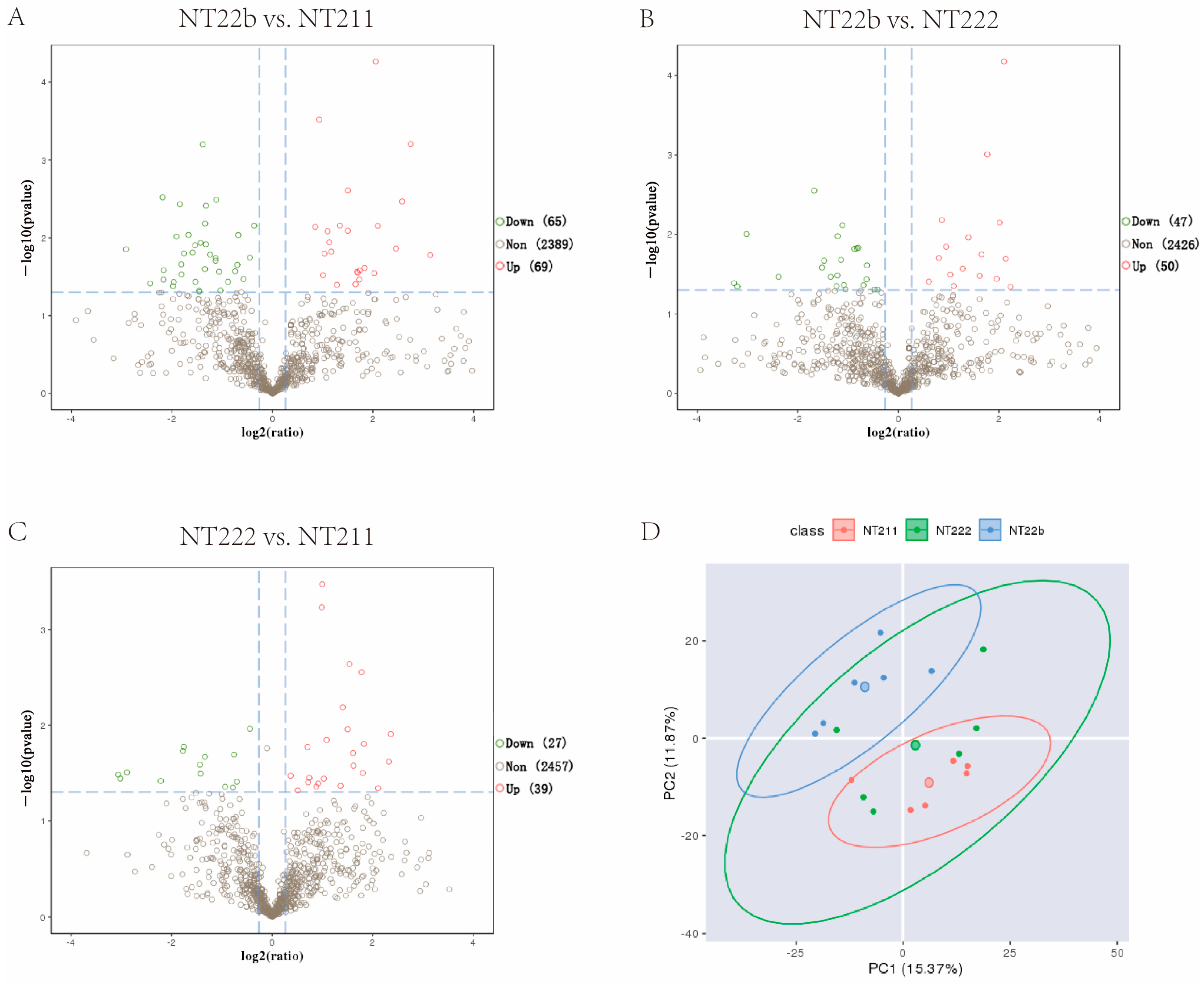



| Manipulated Two-Cell Stage Embryos | Arrested at the Two-Cell Stage | Arrested at the Four-Cell Stage | Developed to the Blastocyst Stage | Arrested at Other Stages | |
|---|---|---|---|---|---|
| Total number of embryos | 335 | 165 | 58 | 66 | 46 |
| Proportion(%) | 100% | 49.25% | 17.31% | 19.70% | 13.73% |
| Group name | - | NT-211 | NT-222 | NT-22b | - |
| Samples | Number of Peptides | Number of Proteins |
|---|---|---|
| NT211_1 | 2296 | 1135 |
| NT211_2 | 1867 | 823 |
| NT211_3 | 2039 | 936 |
| NT211_4 | 2120 | 963 |
| NT211_5 | 1897 | 840 |
| NT211_6 | 2366 | 1075 |
| NT222_1 | 2366 | 1075 |
| NT222_2 | 1632 | 740 |
| NT222_3 | 2248 | 1076 |
| NT222_4 | 1836 | 841 |
| NT222_5 | 2471 | 1156 |
| NT222_6 | 1294 | 648 |
| NT22b_1 | 2470 | 1176 |
| NT22b_2 | 2496 | 1186 |
| NT22b_3 | 1883 | 955 |
| NT22b_4 | 1971 | 935 |
| NT22b_5 | 2163 | 933 |
| NT22b_6 | 1554 | 825 |
| Total Number of Injected SCNT Embryos | Concentration of PDCD6 siRNA | Repetition Numbers | Cleavage Rate (%) | Blastocyst Rate (%) | Number of Cells per Blastocyst |
|---|---|---|---|---|---|
| 120 | 0 μM | 4 | 65.84 ± 9.18 | 11.67 ± 1.92 | 42.13 ± 7.40 |
| 126 | 5 μM | 4 | 71.40 ± 4.84 | 11.89 ± 1.49 | 46.50 ± 6.63 |
| 119 | 10 μM | 4 | 75.58 ± 4.68 | 14.43 ± 3.31 | 48.63 ± 7.48 |
| 114 | 20 μM | 4 | 79.13 ± 5.65 * | 17.55 ± 2.94 * | 47.75 ± 5.00 |
| Total Number of Injected SCNT Embryos | Concentration of PLK1 mRNA | Repetition Numbers | Cleavage Rate (%) | Blastocyst Rate (%) | Number of Cells per Blastocyst |
|---|---|---|---|---|---|
| 116 | 0 ng/μL | 3 | 63.22 ± 1.99 | 11.49 ± 5.27 | 44.83 ± 9.87 |
| 115 | 100 ng/μL | 3 | 54.56 ± 12.52 | 10.40 ± 3.45 | 47.83 ± 10.63 |
| 108 | 500 ng/μL | 3 | 66.66 ± 9.81 | 8.64 ± 4.28 | 43.83 ± 7.14 |
| 113 | 1000 ng/μL | 3 | 77.38 ± 5.45 * | 15.48 ± 2.06 | 48.67 ± 9.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yang, L.; Zhang, Y.; Liang, Y.; Zhao, H.; Li, Y.; Cai, G.; Wu, Z.; Li, Z. Identification of Important Factors Causing Developmental Arrest in Cloned Pig Embryos by Embryo Biopsy Combined with Microproteomics. Int. J. Mol. Sci. 2022, 23, 15975. https://doi.org/10.3390/ijms232415975
Zhang Y, Yang L, Zhang Y, Liang Y, Zhao H, Li Y, Cai G, Wu Z, Li Z. Identification of Important Factors Causing Developmental Arrest in Cloned Pig Embryos by Embryo Biopsy Combined with Microproteomics. International Journal of Molecular Sciences. 2022; 23(24):15975. https://doi.org/10.3390/ijms232415975
Chicago/Turabian StyleZhang, Yuxing, Liusong Yang, Yiqian Zhang, Yalin Liang, Huaxing Zhao, Yanan Li, Gengyuan Cai, Zhenfang Wu, and Zicong Li. 2022. "Identification of Important Factors Causing Developmental Arrest in Cloned Pig Embryos by Embryo Biopsy Combined with Microproteomics" International Journal of Molecular Sciences 23, no. 24: 15975. https://doi.org/10.3390/ijms232415975




