Porcine Follicular Fluid-Derived Exosome: The Pivotal Material for Porcine Oocyte Maturation in Lipid Antioxidant Activity
Abstract
:1. Introduction
2. Results
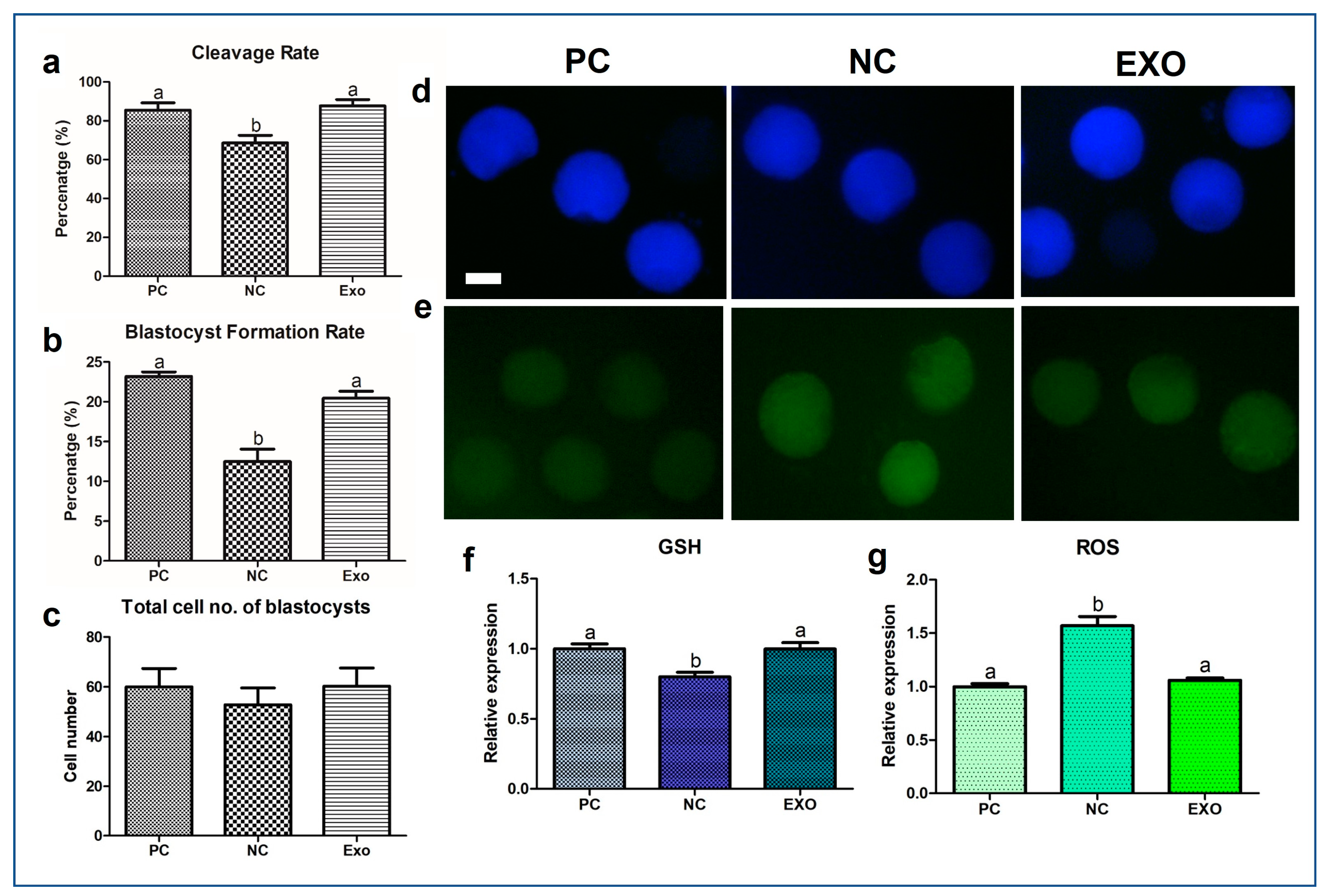
2.1. Characterization of PFF-Derived EVs and EV Treatments on Porcine Oocytes
2.2. GSH/ROS Measurement
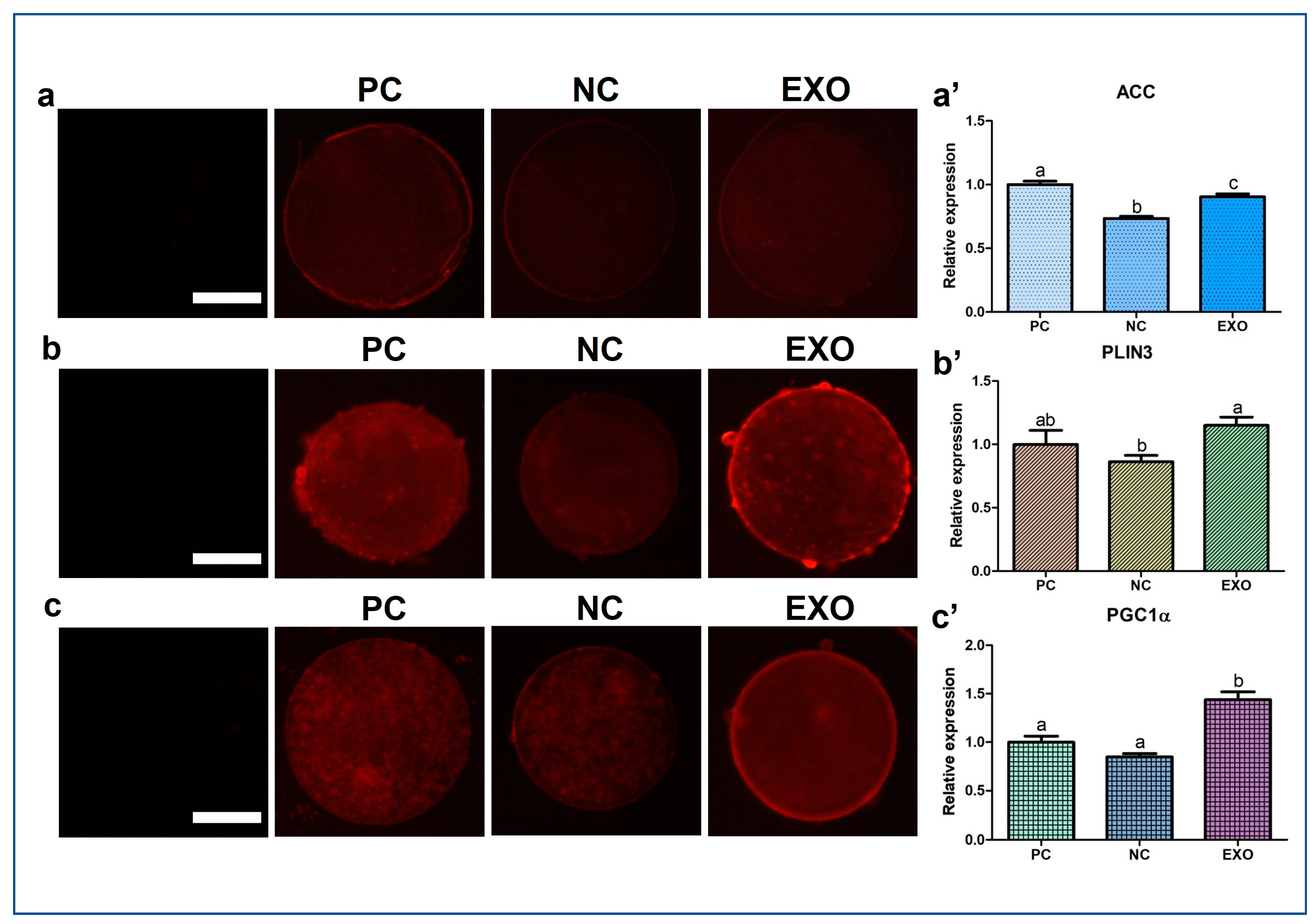
2.3. MRNA Gene Expression in Porcine Oocytes
2.4. Patterns of Protein Expression Evaluated by ICC, BODIPY, and JC-1 Matrix Metalloproteinase (MMP) Staining
3. Discussion
4. Materials and Methods
4.1. Chemicals and Research Ethics
4.2. Exosome Isolation and Characterization
4.3. Retrieval of COCs and IVM
4.4. Cumulus Cell Expansion Assessment
4.5. Parthenogenetic Activation (PA)
4.6. Embryo Evaluation and Total Cell Count after PA
4.7. Immunofluorescence Staining
4.8. ATP Content Assay
4.9. Measurement of Intracellular GSH and ROS Levels
4.10. Fluorescent FA Analog Assays
4.11. JC-1 MMP Assays
4.12. Analysis of Gene Expression by Quantitative Real-Time PCR (qRT-PCR)
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | Acetyl-CoA carboxylase |
| ANOVA | Univariate analysis variance |
| ATGL | Adipose triglyceride lipase |
| BAX | BCL2-associated X protein |
| BCL2 | B-cell lymphoma 2 |
| CAT | Catalase |
| CC | Cumulus cells |
| CD | Cluster of differentiation |
| CGI58 | Comparative gene identification-58 |
| CMF2HC | 4-chloromethyl-6.8-difluoro-7-hydroxycoumarin |
| COC | Cumulus-oocyte complexes |
| EV | Extracellular vesicles |
| EXO | Exosome |
| FA | Fatty acid |
| GSH | Glutathione |
| H2DCFDA | 2′, 7′-dichlorodihydrofluorescein diacetates |
| HSL | Hormone-sensitive lipase |
| IACUC | Institutional Animal Care and Use Committee |
| IVC | In vitro culture |
| IVM | In vitro maturation |
| MGL | Monoacylglycerol Lipase |
| MMP | Mitochondrial membrane potential |
| NC | Negative control |
| NTA | Nanoparticle Tracking Analysis |
| PA | Parthenogenetic Activation |
| PC | Positive control |
| pFF | porcine follicular fluid |
| PFA/PBS | Paraformaldehyde/Phosphate buffered saline |
| PGC1α | Peroxisome proliferator-activated receptor-gamma coactivator-1alpha |
| Nrf1 | Nuclear factor erythroid 2-related factor 1 |
| PLIN3 | Perilipin3 |
| PZM | Porcine zygote media |
| ROS | Reactive oxygen species |
| TALP | Tyrode’s albumin lactate pyruvate |
| TCM199 | Tissue culture media 199 |
| TEM | Transmission electron microscope |
| TFAM | Mitochondrial transcription factor A |
References
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, E.A.; Stephens, K.K.; Winuthayanon, W. Extracellular Vesicles and the Oviduct Function. Int. J. Mol. Sci. 2020, 21, 8280. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell. Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.A.; Tuna, K.M.; Alli, A.A.; Tribulo, P.; Hansen, P.J.; Koh, J.; Paula-Lopes, F.F. Follicular fluid exosomes act on the bovine oocyte to improve oocyte competence to support development and survival to heat shock. Reprod. Fertil. Dev. 2019, 31, 888–897. [Google Scholar] [CrossRef]
- Gabrys, J.; Kij-Mitka, B.; Sawicki, S.; Kochan, J.; Nowak, A.; Lojko, J.; Karnas, E.; Bugno-Poniewierska, M. Extracellular vesicles from follicular fluid may improve the nuclear maturation rate of in vitro matured mare oocytes. Theriogenology 2022, 188, 116–124. [Google Scholar] [CrossRef]
- Lee, S.H.; Saadeldin, I.M. Exosomes as a Potential Tool for Supporting Canine Oocyte Development. Animals 2020, 10, 1971. [Google Scholar] [CrossRef]
- Basuino, L.; Silveira, C.F., Jr. Human follicular fluid and effects on reproduction. JBRA Assist. Reprod. 2016, 20, 38–40. [Google Scholar] [CrossRef]
- Revelli, A.; Delle Piane, L.; Casano, S.; Molinari, E.; Massobrio, M.; Rinaudo, P. Follicular fluid content and oocyte quality: From single biochemical markers to metabolomics. Reprod. Biol. Endocrinol. 2009, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Brinca, A.T.; Ramalhinho, A.C.; Sousa, A.; Oliani, A.H.; Breitenfeld, L.; Passarinha, L.A.; Gallardo, E. Follicular Fluid: A Powerful Tool for the Understanding and Diagnosis of Polycystic Ovary Syndrome. Biomedicines 2022, 10, 1254. [Google Scholar] [CrossRef]
- Malyszka, N.; Pawlak, P.; Cieslak, A.; Szkudelska, K.; Lechniak, D. Distinct dynamics of lipid accumulation by porcine cumulus cells during in vitro maturation with follicular fluid of low and high fatty acid contents. Theriogenology 2023, 195, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, P.; Warzych, E.; Cieslak, A.; Malyszka, N.; Maciejewska, E.; Madeja, Z.E.; Lechniak, D. The consequences of porcine IVM medium supplementation with follicular fluid become reflected in embryo quality, yield and gene expression patterns. Sci. Rep. 2018, 8, 15306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reverchon, M.; Cornuau, M.; Rame, C.; Guerif, F.; Royere, D.; Dupont, J. Chemerin inhibits IGF-1-induced progesterone and estradiol secretion in human granulosa cells. Hum. Reprod. 2012, 27, 1790–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, M.; Li, M.; Zhou, J.; Ding, X.; Shao, Y.; Jing, J.; Liu, Y.; Yao, B. Brain-derived neurotrophic factor promotes human granulosa-like tumor cell steroidogenesis and proliferation by activating the FSH receptor-mediated signaling pathway. Sci. Rep. 2017, 7, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgarten, S.C.; Convissar, S.M.; Zamah, A.M.; Fierro, M.A.; Winston, N.J.; Scoccia, B.; Stocco, C. FSH Regulates IGF-2 Expression in Human Granulosa Cells in an AKT-Dependent Manner. J. Clin. Endocrinol. Metab. 2015, 100, E1046–E1055. [Google Scholar] [CrossRef] [Green Version]
- Yuan, C.; Li, Z.; Zhao, Y.; Wang, X.; Chen, L.; Zhao, Z.; Cao, M.; Chen, T.; Iqbal, T.; Zhang, B.; et al. Follicular fluid exosomes: Important modulator in proliferation and steroid synthesis of porcine granulosa cells. FASEB J. 2021, 35, e21610. [Google Scholar] [CrossRef]
- da Silveira, J.C.; Andrade, G.M.; Simas, R.C.; Martins-Junior, H.A.; Eberlin, M.N.; Smith, L.C.; Perecin, F.; Meirelles, F.V. Lipid profile of extracellular vesicles and their relationship with bovine oocyte developmental competence: New players in intra follicular cell communication. Theriogenology 2021, 174, 1–8. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, N.; Yan, T.; Shi, Y.N.; Chen, J.; Zhang, C.J.; Xie, X.J.; Liao, D.F.; Qin, L. The crosstalk: Exosomes and lipid metabolism. Cell Commun. Signal. 2020, 18, 119. [Google Scholar] [CrossRef]
- Mito, T.; Hoshi, H. In Vitro Culture of Late Stage Pig Embryos in a Chemically Defined Medium, Porcine Blastocyst Medium (PBM). Methods Mol. Biol. 2019, 2006, 105–113. [Google Scholar] [CrossRef]
- Jiao, X.; Ding, Z.; Meng, F.; Zhang, X.; Wang, Y.; Chen, F.; Duan, Z.; Wu, D.; Zhang, S.; Miao, Y.; et al. The toxic effects of Fluorene-9-bisphenol on porcine oocyte in vitro maturation. Environ. Toxicol. 2020, 35, 152–158. [Google Scholar] [CrossRef]
- Li, J.; Wang, R.; Chen, Q.; Tian, Y.; Gao, L.; Lei, A. Salidroside improves porcine oocyte maturation and subsequent embryonic development by promoting lipid metabolism. Theriogenology 2022, 192, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Guan, S.; Lv, D.; Zhao, M.; Yan, L.; Shi, L.; Ji, P.; Zhang, L.; Liu, G. Melatonin Modulates Lipid Metabolism in Porcine Cumulus-Oocyte Complex via Its Receptors. Front. Cell Dev. Biol. 2021, 9, 648209. [Google Scholar] [CrossRef]
- Lowe, J.L.; Bathgate, R.; Grupen, C.G. Effect of carbohydrates on lipid metabolism during porcine oocyte IVM. Reprod. Fertil. Dev. 2019, 31, 557–569. [Google Scholar] [CrossRef]
- Costermans, N.G.J.; Teerds, K.J.; Middelkoop, A.; Roelen, B.A.J.; Schoevers, E.J.; van Tol, H.T.A.; Laurenssen, B.; Koopmanschap, R.E.; Zhao, Y.; Blokland, M.; et al. Consequences of negative energy balance on follicular development and oocyte quality in primiparous sowsdagger. Biol. Reprod. 2020, 102, 388–398. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef] [Green Version]
- Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Chiaradia, E.; Urbanelli, L.; Emiliani, C. Lysosomal Exocytosis, Exosome Release and Secretory Autophagy: The Autophagic- and Endo-Lysosomal Systems Go Extracellular. Int. J. Mol. Sci. 2020, 21, 2576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuno, Y.; Kanke, T.; Maruyama, N.; Fujii, W.; Naito, K.; Sugiura, K. Characterization of mRNA profiles of the exosome-like vesicles in porcine follicular fluid. PLoS ONE 2019, 14, e0217760. [Google Scholar] [CrossRef] [Green Version]
- Yuan, C.; Chen, X.; Shen, C.; Chen, L.; Zhao, Y.; Wang, X.; Cao, M.; Zhao, Z.; Chen, T.; Zhang, B.; et al. Follicular fluid exosomes regulate oxidative stress resistance, proliferation, and steroid synthesis in porcine theca cells. Theriogenology 2022, 194, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, R.; Chen, Y.; Wang, M.; Du, J. Crosstalk between Oxidative Stress and Exosomes. Oxid. Med. Cell. Longev. 2022, 2022, 3553617. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Mizzoni, D.; Di Raimo, R.; Fais, S. Exosomes: A Source for New and Old Biomarkers in Cancer. Cancers 2020, 12, 2566. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Greening, D.W.; Zhu, H.J.; Takahashi, N.; Simpson, R.J. Extracellular vesicle isolation and characterization: Toward clinical application. J. Clin. Investig. 2016, 126, 1152–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.; Wiegmans, A.P.; Leimgruber, A.; Moller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Saeed-Zidane, M.; Linden, L.; Salilew-Wondim, D.; Held, E.; Neuhoff, C.; Tholen, E.; Hoelker, M.; Schellander, K.; Tesfaye, D. Cellular and exosome mediated molecular defense mechanism in bovine granulosa cells exposed to oxidative stress. PLoS ONE 2017, 12, e0187569. [Google Scholar] [CrossRef] [Green Version]
- MacPherson, R.E.; Ramos, S.V.; Vandenboom, R.; Roy, B.D.; Peters, S.J. Skeletal muscle PLIN proteins, ATGL and CGI-58, interactions at rest and following stimulated contraction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R644–R650. [Google Scholar] [CrossRef] [Green Version]
- Skryabin, G.O.; Komelkov, A.V.; Savelyeva, E.E.; Tchevkina, E.M. Lipid Rafts in Exosome Biogenesis. Biochemistry 2020, 85, 177–191. [Google Scholar] [CrossRef]
- Kim, E.H.; Ridlo, M.R.; Lee, B.C.; Kim, G.A. Crosstalk between Peroxisomal Activities and Nrf2 Signaling in Porcine Embryos. Antioxidants 2021, 10, 771. [Google Scholar] [CrossRef]
- Kim, E.H.; Ridlo, M.R.; Lee, B.C.; Kim, G.A. Melatonin-Nrf2 Signaling Activates Peroxisomal Activities in Porcine Cumulus Cell-Oocyte Complexes. Antioxidants 2020, 9, 1080. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, G.A.; Taweechaipaisankul, A.; Ridlo, M.R.; Lee, S.H.; Ra, K.; Ahn, C.; Lee, B.C. Phytanic acid-derived peroxisomal lipid metabolism in porcine oocytes. Theriogenology 2020, 157, 276–285. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, G.A.; Taweechaipaisankul, A.; Lee, S.H.; Qasim, M.; Ahn, C.; Lee, B.C. Melatonin enhances porcine embryo development via the Nrf2/ARE signaling pathway. J. Mol. Endocrinol. 2019, 63, 175–185. [Google Scholar] [CrossRef]
- Benador, I.Y.; Veliova, M.; Liesa, M.; Shirihai, O.S. Mitochondria Bound to Lipid Droplets: Where Mitochondrial Dynamics Regulate Lipid Storage and Utilization. Cell. Metab. 2019, 29, 827–835. [Google Scholar] [CrossRef] [Green Version]
- Park, J.E.; Lee, S.H.; Hwangbo, Y.; Park, C.K. Porcine follicular fluid derived from > 8 mm sized follicles improves oocyte maturation and embryo development during in vitro maturation of pigs. Zygote 2021, 29, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.X.; Lee, S.; Setyawan, E.M.N.; Taweechaipaisankul, A.; Kim, G.A.; Han, H.J.; Ahn, C.; Lee, B.C. A potential role of knockout serum replacement as a porcine follicular fluid substitute for in vitro maturation: Lipid metabolism approach. J. Cell. Physiol. 2018, 233, 6984–6995. [Google Scholar] [CrossRef]
- Srivastava, A.; Srivastava, P.; Mathur, S.; Abbas, S.; Rai, N.; Tiwari, S.; Tiwari, M.; Sharma, L. Lipid Metabolism and Mitochondria: Cross Talk in Cancer. Curr. Drug Targets 2022, 23, 606–627. [Google Scholar] [CrossRef] [PubMed]
- Brownsey, R.W.; Boone, A.N.; Elliott, J.E.; Kulpa, J.E.; Lee, W.M. Regulation of acetyl-CoA carboxylase. Biochem. Soc. Trans. 2006, 34, 223–227. [Google Scholar] [CrossRef]
- Chen, L.; Duan, Y.; Wei, H.; Ning, H.; Bi, C.; Zhao, Y.; Qin, Y.; Li, Y. Acetyl-CoA carboxylase (ACC) as a therapeutic target for metabolic syndrome and recent developments in ACC1/2 inhibitors. Expert. Opin. Investig. Drugs 2019, 28, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Grabner, G.F.; Xie, H.; Schweiger, M.; Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 2021, 3, 1445–1465. [Google Scholar] [CrossRef]
- Duncan, R.E.; Ahmadian, M.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 2007, 27, 79–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, L.; Gong, J.; Guo, Y.; Li, Y.; Huang, H.; Liu, X. Construction of a ceRNA network in polycystic ovary syndrome (PCOS) driven by exosomal lncRNA. Front. Genet. 2022, 13, 979924. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Kim, S.J.; Choi, Y.B.; Lee, B.C. Improvement of cloned embryos development by co-culturing with parthenotes: A possible role of exosomes/microvesicles for embryos paracrine communication. Cell. Reprogram 2014, 16, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.H.; Taweechaipaisankul, A.; Ridlo, M.R.; Kim, G.A.; Lee, B.C. Effect of Klotho protein during porcine oocyte maturation via Wnt signaling. Aging 2020, 12, 23808–23821. [Google Scholar] [CrossRef]
- Jin, J.X.; Lee, S.; Taweechaipaisankul, A.; Kim, G.A.; Lee, B.C. Melatonin regulates lipid metabolism in porcine oocytes. J. Pineal Res. 2017, 62, e12388. [Google Scholar] [CrossRef] [PubMed]
- Ducolomb, Y.; Gonzalez-Marquez, H.; Fierro, R.; Jimenez, I.; Casas, E.; Flores, D.; Bonilla, E.; Salazar, Z.; Betancourt, M. Effect of porcine follicular fluid proteins and peptides on oocyte maturation and their subsequent effect on in vitro fertilization. Theriogenology 2013, 79, 896–904. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Melo-Sterza, F.; Poehland, R. Lipid Metabolism in Bovine Oocytes and Early Embryos under In Vivo, In Vitro, and Stress Conditions. Int. J. Mol. Sci. 2021, 22, 3421. [Google Scholar] [CrossRef] [PubMed]
- Momozawa, K. Usefulness of modified Medium RD as a chemically defined medium for in vitro maturation of bovine oocytes. Reprod. Med. Biol. 2020, 19, 365–371. [Google Scholar] [CrossRef]




| Genes | * Primer Sequences (5′–3′) | Product Size (Bp) | Accession No. |
|---|---|---|---|
| GAPDH | F: GTCGGTTGTGGATCTGACCT R: TTGACGAAGTGGTCGTTGAG | 207 | NM_001206359 |
| CATALASE | F: AGGGAGAGGCGGTTTATTGC R: GGACTCGTTGGTGAAGCTCA | 117 | NM_001206359 |
| CD9 | F: CCGTGGTGATGATTTTCGGC R: ACAGGACCCCGAGAAGATGA | 147 | NM_214006.1 |
| CD63 | F: ACGTTCTTCTGCTGGCCTTT R: ACTGCGATGATGACCACAGG | 136 | XM_005663878.2 |
| CD81 | F: GGGTGCTGTGATGATGTTTG R: GCCTGGTCGTAGAACTGCTT | 108 | NM_001078679.1 |
| PGC1A | F: CACGGACAGAACTGAGGGAC R: ACCTGCGCAAAGTGTATCCA | 192 | XM_021100442.1 |
| NRF1 | F: CAGCAAGTACAGCAGGTCCA R: ATGAGGCCGTTTCCGTTTCT | 222 | XM_021078993.1 |
| TFAM | F: GCTCTCCGTTCAGTTTTGCG R: ACCTGCCAGTCTGCCCTATA | 238 | NM_001130211.1 |
| ATGL | F: GACGGTGGCATCTCAGACAA R: TGGATGTTGGTGGAGCTGTC | 113 | NM_001098605.1 |
| HSL | F: GCCTTTCCTGCAGACCATCT R: CACTGGTGAAGAGGGAGCTG | 104 | NM_214315.3 |
| MGL | F: ACCCCACAGAGTGTCCCATA R: GGGTGTAGCTGAGGGTTTCC | 96 | XM_013982013.2 |
| CGI58 | F: TCTTGCTGGGACACAACCTG R: CCAAAGGGTCCTGCAATCCT | 220 | NM_001012407.1 |
| PLIN3 | F: ATGTTTGCCAGCGAGACAGA R: GTAGGCAGCAGACACCATGT | 141 | NM_001031778.1 |
| BAX | F: CATGAAGACAGGGGCCCTTT R: CATCCTCTGCAGCTCCATGT | 181 | XM_003127290 |
| BCL2 | F: AATGTCTCAGAGCAACCGGG R: GGGGCCTCAGTTCTGTTCTC | 193 | NM_214285 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.; Ra, K.; Lee, M.-S.; Kim, G.A. Porcine Follicular Fluid-Derived Exosome: The Pivotal Material for Porcine Oocyte Maturation in Lipid Antioxidant Activity. Int. J. Mol. Sci. 2023, 24, 9807. https://doi.org/10.3390/ijms24129807
Kim E, Ra K, Lee M-S, Kim GA. Porcine Follicular Fluid-Derived Exosome: The Pivotal Material for Porcine Oocyte Maturation in Lipid Antioxidant Activity. International Journal of Molecular Sciences. 2023; 24(12):9807. https://doi.org/10.3390/ijms24129807
Chicago/Turabian StyleKim, Euihyun, Kihae Ra, Myung-Shin Lee, and Geon A. Kim. 2023. "Porcine Follicular Fluid-Derived Exosome: The Pivotal Material for Porcine Oocyte Maturation in Lipid Antioxidant Activity" International Journal of Molecular Sciences 24, no. 12: 9807. https://doi.org/10.3390/ijms24129807





