Integrated Proteomics and Metabolomics Analysis of Nitrogen System Regulation on Soybean Plant Nodulation and Nitrogen Fixation
Abstract
:1. Introduction
2. Results
2.1. Effects of Nitrogen Supply on Nodule Growth and Nitrogenase Activity
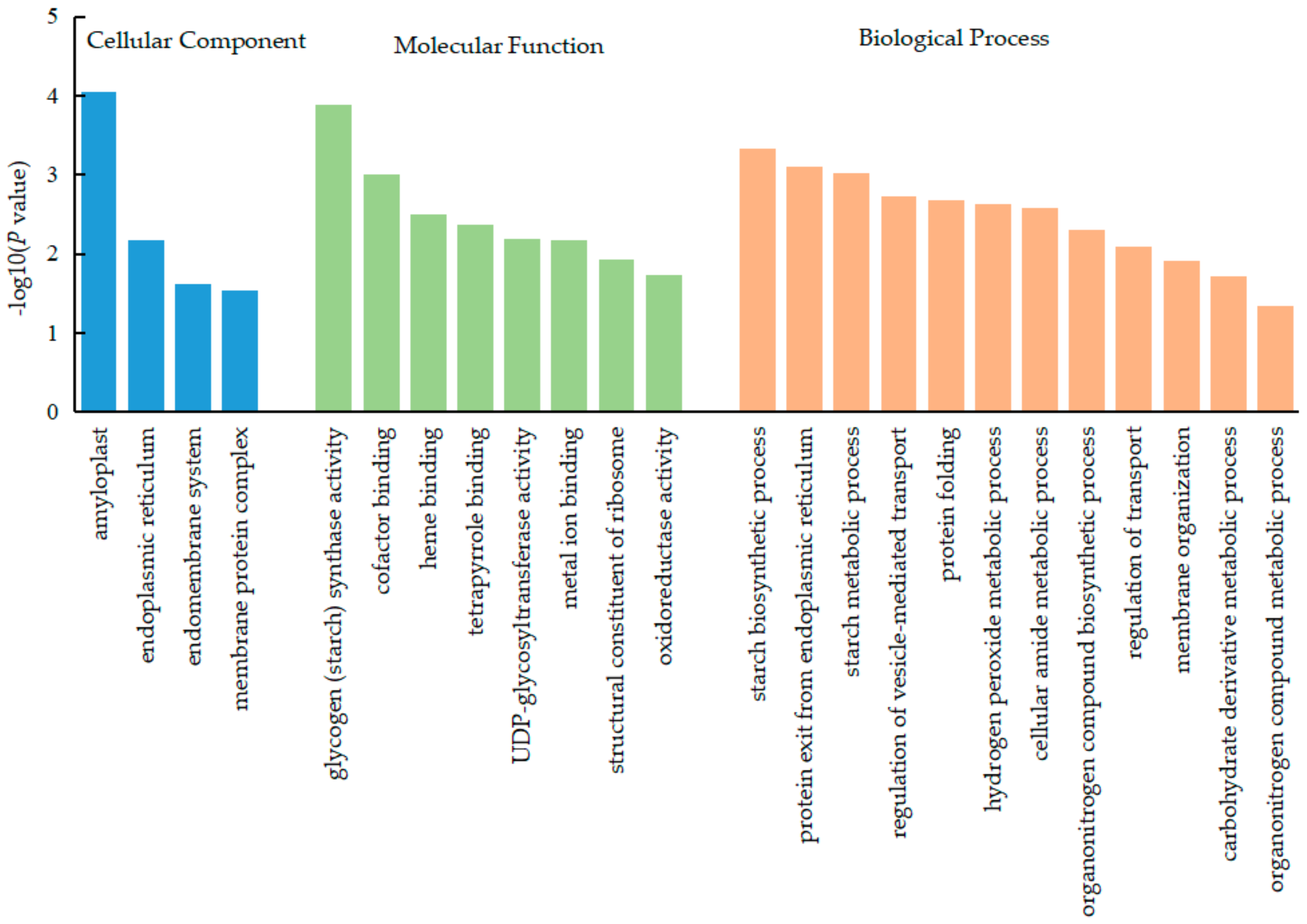
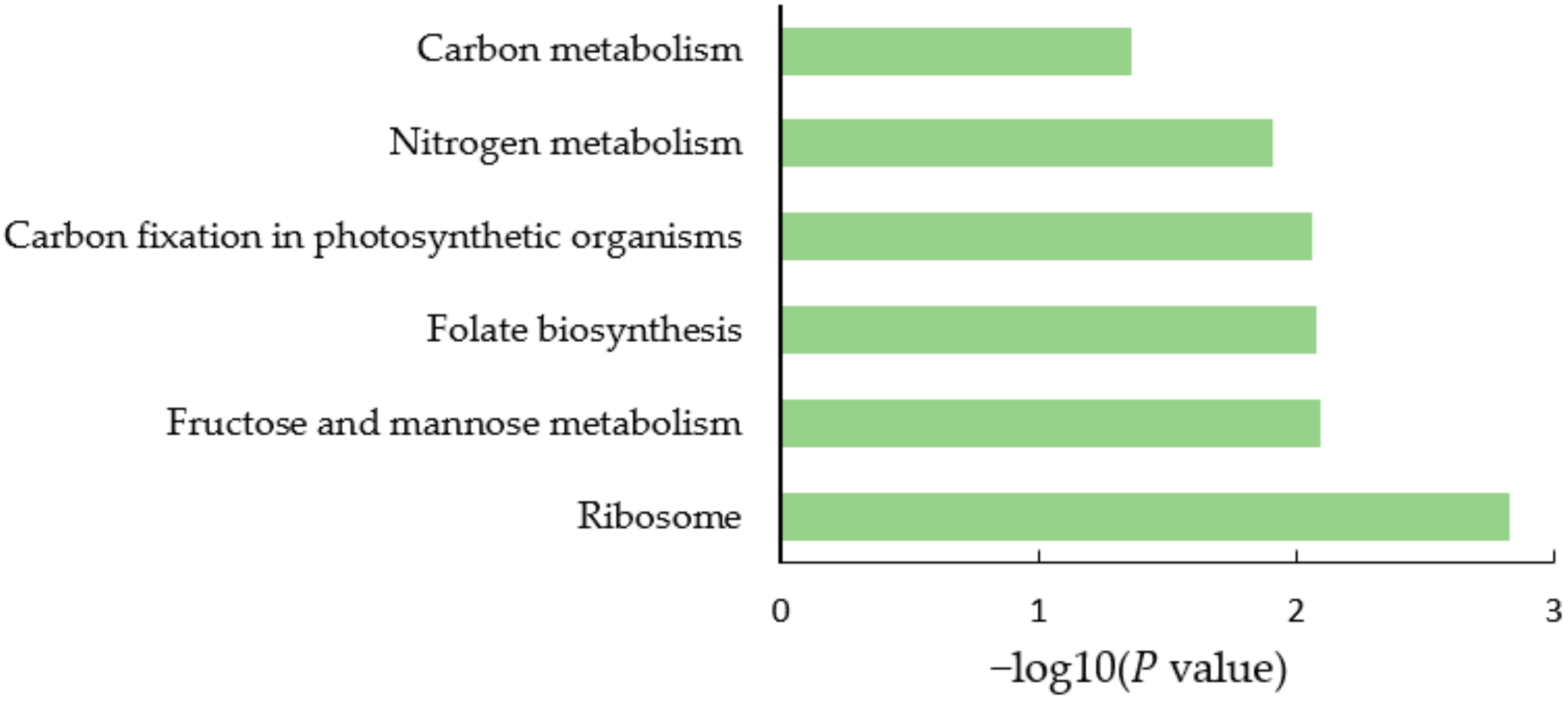
2.2. Proteomics Analysis of Soybean Nodules
2.3. Metabolomics Analysis of Soybean Nodules
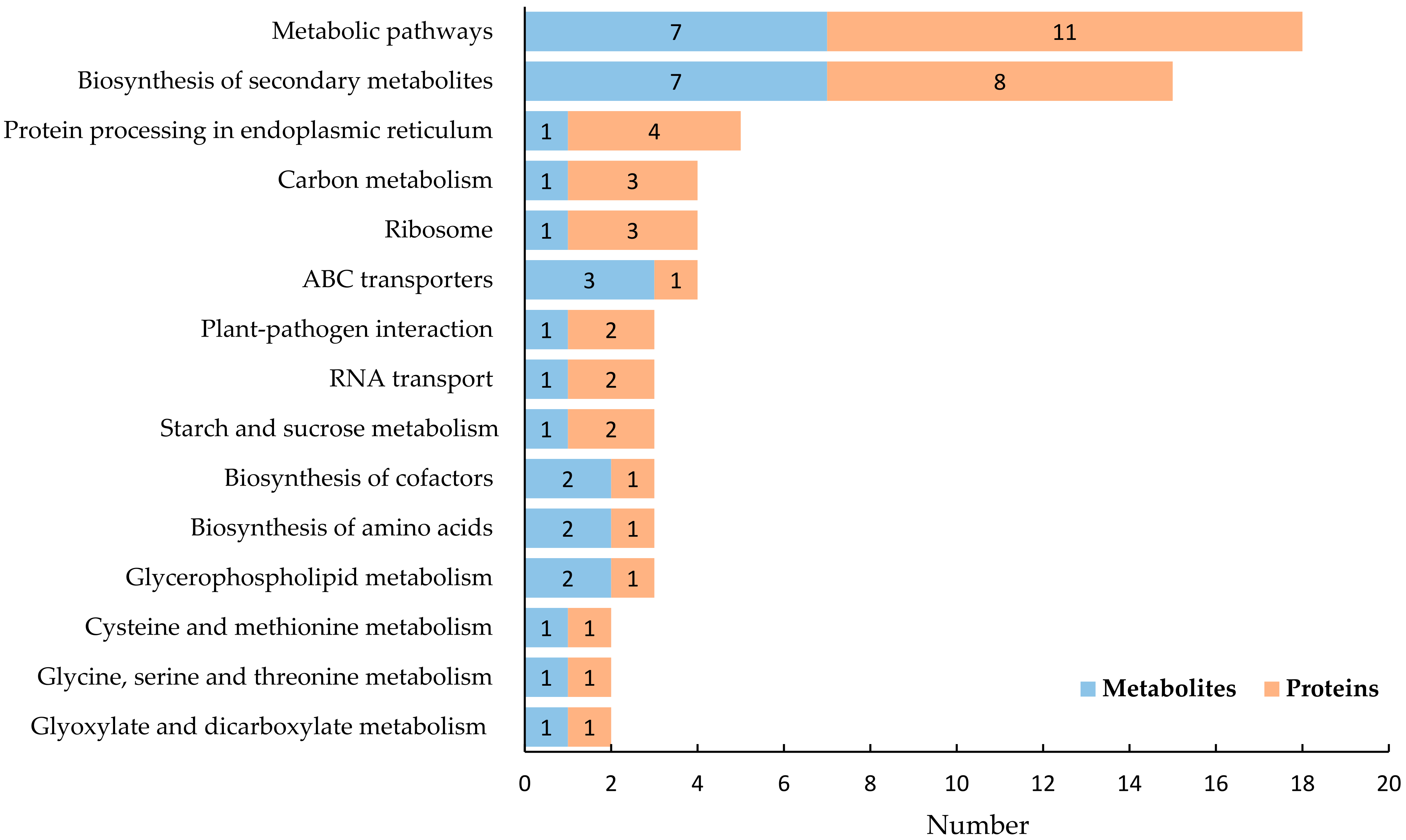
2.4. Integrated Proteomic and Metabonomic Analysis
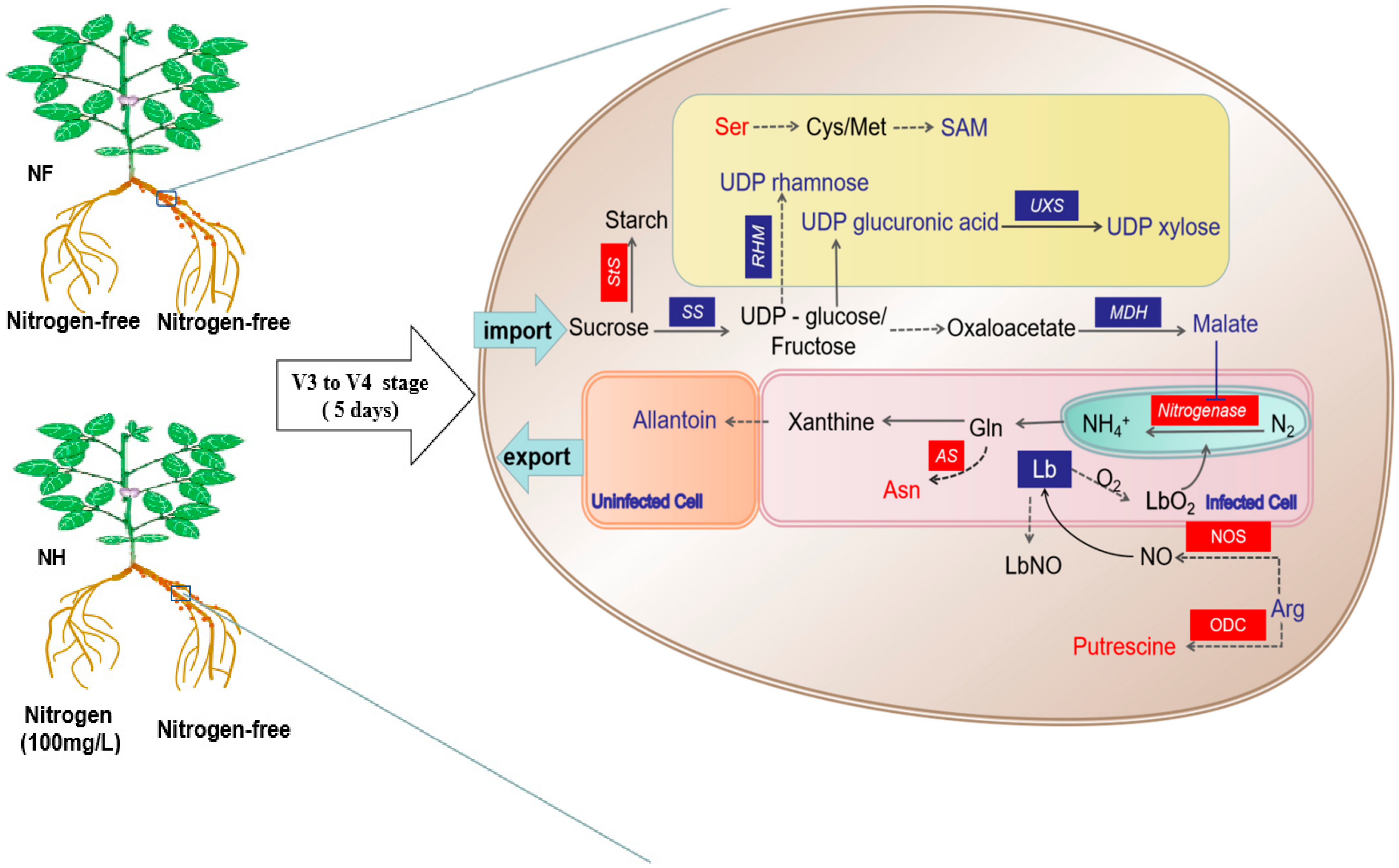
3. Discussion
3.1. Regulation of Nitrogen Supply on Nodules Energy Metabolism
3.2. Regulation of Nitrogen Supply on the Nodules Cell Wall
3.3. Regulation of Nitrogen Supply on Nitrogen Fixation Signaling Substances in Nodules
4. Materials and Methods
4.1. Plant Materials, Growth Conditions, and Treatments
4.2. Sampling Methods
4.3. Determination of Acetylene Reduction Assay
4.4. Production and Content of NO in Nodules
4.5. Proteomics Analysis
4.6. Metabolomics Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harper, J.E.; Gibson, A.H. Differential Nodulation Tolerance to Nitrate Among Legume Species. Crop Sci. 1984, 24, 797–801. [Google Scholar] [CrossRef]
- Streeter, J.; Wong, P.P. Inhibition of legume nodule formation and N2 fixation by nitrate. Crit. Rev. Plant Sci. 1988, 7, 1–23. [Google Scholar] [CrossRef]
- Carroll, B.J.; McNeil, D.L.; Gresshoff, P.M. Isolation and properties of soybean [Glycine max (L.) Merr.] mutants that nodulate in the presence of high nitrate concentrations. Proc. Natl. Acad. Sci. USA 1985, 82, 4162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, A.H.; Harper, J.E. Nitrate Effect on Nodulation of Soybean by Bradyrhizobium japonicum1. Crop Sci. 1985, 25, 497–501. [Google Scholar] [CrossRef]
- Mizukoshi, K.; Nishiwaki, T.; Ohtake, N.; Minagawa, R.; Ikarashi, T.; Ohyama, T. Nitrate transport pathway into soybean nodules traced by tungstate and 15NO3. Soil Sci. Plant Nutr. 1995, 41, 75–88. [Google Scholar] [CrossRef]
- Fujikake, H.; Tamura, Y.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Photoassimilate partitioning in hypernodulation mutant of soybean (Glycine max (L.) Merr.) NOD1-3 and its parent williams in relation to nitrate inhibition of nodule growth. Soil Sci. Plant Nutr. 2003, 49, 583–590. [Google Scholar] [CrossRef]
- Fujikake, H.; Yamazaki, A.; Ohtake, N.; Sueyoshi, K.; Matsuhashi, S.; Ito, T.; Mizuniwa, C.; Kume, T.; Hashimoto, S.; Ishioka, N.S.; et al. Quick and reversible inhibition of soybean root nodule growth by nitrate involves a decrease in sucrose supply to nodules. J. Exp. Bot. 2003, 54, 1379–1388. [Google Scholar] [CrossRef] [Green Version]
- Ono, Y.; Fukasawa, M.; Sueyoshi, K.; Ohtake, N.; Sato, T.; Tanabata, S.; Toyota, R.; Higuchi, K.; Saito, A.; Ohyama, T. Application of Nitrate, Ammonium, or Urea Changes the Concentrations of Ureides, Urea, Amino Acids and Other Metabolites in Xylem Sap and in the Organs of Soybean Plants (Glycine max (L.) Merr.). Int. J. Mol. Sci. 2021, 22, 4573. [Google Scholar] [CrossRef]
- Yashima, H.; Fujikake, H.; Sato, T.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Systemic and local effects of long-term application of nitrate on nodule growth and N2 fixation in soybean (Glycine max [L.] Merr.). Soil Sci. Plant Nutr. 2003, 49, 825–834. [Google Scholar] [CrossRef]
- Yashima, H.; Fujikake, H.; Yamazaki, A.; Ito, S.; Sato, T.; Tewari, K.; Ohtake, N.; Sueyoshi, K.; Takahashi, Y.; Ohyama, T. Long-Term Effect of Nitrate Application from Lower Part of Roots on Nodulation and N2 Fixation in Upper Part of Roots of Soybean (Glycine max (L.) Merr.) in Two-Layered Pot Experiment. Soil Sci. Plant Nutr. 2005, 51, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Ma, C.; Dong, S.; Xu, Y.; Gong, Z. Effects of nitrogen concentrations on nodulation and nitrogenase activity in dual root systems of soybean plants. Soil Sci. Plant Nutr. 2017, 63, 470–482. [Google Scholar] [CrossRef]
- Lyu, X.; Xia, X.; Wang, C.; Ma, C.; Dong, S.; Gong, Z. Effects of changes in applied nitrogen concentrations on nodulation, nitrogen fixation and nitrogen accumulation during the soybean growth period. Soil Sci. Plant Nutr. 2019, 65, 479–489. [Google Scholar] [CrossRef]
- IMSANDE, J. Inhibition of Nodule Development in Soybean by Nitrate or Reduced Nitrogen. J. Exp. Bot. 1986, 37, 348–355. [Google Scholar] [CrossRef]
- Fujikake, H.; Yashima, H.; Sato, T.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Rapid and reversible nitrate inhibition of nodule growth and N2 fixation activity in soybean (Glycine max (L.) Merr.). Soil Sci. Plant Nutr. 2002, 48, 211–217. [Google Scholar] [CrossRef]
- Tanaka, A.; Fujlta, K.; Terasawa, H. Growth and Dinitrogen Fixation, of Soybean Root System Affected by Partial Exposure to Nitrate. Soil Sci. Plant Nutr. 1985, 31, 637–645. [Google Scholar] [CrossRef] [Green Version]
- Lyu, X.; Li, M.; Li, X.; Li, S.; Yan, C.; Ma, C.; Gong, Z. Assessing the Systematic Effects of the Concentration of Nitrogen Supplied to Dual-Root Systems of Soybean Plants on Nodulation and Nitrogen Fixation. Agronomy 2020, 10, 763. [Google Scholar] [CrossRef]
- Yamashita, N.; Tanabata, S.; Ohtake, N.; Sueyoshi, K.; Sato, T.; Higuchi, K.; Saito, A.; Ohyama, T. Effects of Different Chemical Forms of Nitrogen on the Quick and Reversible Inhibition of Soybean Nodule Growth and Nitrogen Fixation Activity. Front. Plant Sci. 2019, 10, 131. [Google Scholar] [CrossRef]
- Dean, J.V.; Harper, J.E. The Conversion of Nitrite to Nitrogen Oxide(s) by the Constitutive NAD(P)H-Nitrate Reductase Enzyme from Soybean. Plant Physiol. 1988, 88, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, H.; Sakihama, Y. Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: In vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Lett. 2000, 468, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Kato, K.; Kanahama, K.; Kanayama, Y. Involvement of nitric oxide in the inhibition of nitrogenase activity by nitrate in Lotus root nodules. J. Plant Physiol. 2010, 167, 238–241. [Google Scholar] [CrossRef]
- Serraj, R.; Vadez, V.; Denison, R.; Sinclair, T. Involvement of Ureides in Nitrogen Fixation Inhibition in Soybean. Plant Physiol. 1999, 119, 289–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vadez, V.; Sinclair, T.R.; Serraj, R. Asparagine and ureide accumulation in nodules and shoots as feedback inhibitors of N2 fixation in soybean. Physiol. Plant. 2000, 110, 215–223. [Google Scholar] [CrossRef]
- Udvardi, M.; Poole, P.S. Transport and metabolism in legume-rhizobia symbioses. Annu. Rev. Plant Biol. 2013, 64, 781–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrest, S.; Verma, D.; Dhindsa, R. Starch content and activities of starch-metabolizing enzymes in effective and ineffective root nodules of soybean. Can. J. Bot. 2011, 69, 697–701. [Google Scholar] [CrossRef]
- Gordon, A.; James, C. Enzymes of carbohydrate and amino acid metabolism in developing and mature nodules of white clover. J. Exp. Bot. 1997, 48, 895–903. [Google Scholar] [CrossRef]
- Gordon, A.J.; Minchin, F.R.; James, C.L.; Komina, O. Sucrose synthase in legume nodules is essential for nitrogen fixation. Plant Physiol. 1999, 120, 867–878. [Google Scholar] [CrossRef] [Green Version]
- Udvardi, M.K.; Price, G.D.; Gresshoff, P.M.; Day, D.A. A dicarboxylate transporter on the peribacteroid membrane of soybean nodules. FEBS Lett. 1988, 231, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Miller, S.S.; Driscoll, B.T.; Gregerson, R.G.; Gantt, J.S.; Vance, C.P. Alfalfa malate dehydrogenase (MDH): Molecular cloning and characterization of five different forms reveals a unique nodule-enhanced MDH. Plant J. 1998, 15, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Fedorova, M.; Tikhonovich, I.A.; Vance, C.P. Expression of C-assimilating enzymes in pea (Pisum sativum L.) root nodules. In situ localization in effective nodules. Plant Cell Environ. 1999, 22, 1249–1262. [Google Scholar] [CrossRef]
- Takanashi, K.; Sasaki, T.; Kan, T.; Saida, Y.; Sugiyama, A.; Yamamoto, Y.; Yazaki, K. A Dicarboxylate Transporter, LjALMT4, Mainly Expressed in Nodules of Lotus japonicus. Mol. Plant-Microbe Interact. 2016, 29, 584–592. [Google Scholar] [CrossRef] [Green Version]
- Tesfaye, M.; Temple, S.J.; Allan, D.L.; Vance, C.P.; Samac, D.A. Overexpression of Malate Dehydrogenase in Transgenic Alfalfa Enhances Organic Acid Synthesis and Confers Tolerance to Aluminum. Plant Physiol. 2001, 127, 1836–1844. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, L.; El-Hamdaoui, A.; Bonilla, I. Recovery of development and functionality of nodules and plant growth in salt-stressed Pisum sativum—Rhizobium leguminosarum symbiosis by boron and calcium. J. Plant Physiol. 2003, 160, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Muneer, S.; Kim, T.H.; Qureshi, M.I. Fe modulates Cd-induced oxidative stress and the expression of stress responsive proteins in the nodules of Vigna radiata. Plant Growth Regul. 2012, 68, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Fan, R.; Huang, Y.C.; Grusak, M.A.; Huang, C.P.; Sherrier, D.J. Effects of nano-TiO2 on the agronomically-relevant Rhizobium–legume symbiosis. Sci. Total Environ. 2014, 466, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Li, C.; Tarczynski, M.C. High free-methionine and decreased lignin content result from a mutation in the Arabidopsis S-adenosyl-L-methionine synthetase 3 gene. Plant J. 2002, 29, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Atici, Ö.; Ogutcu, H.; Algur, Ö.F. Effect of putrescine on inducing symbiosis in chickpea and vetch inoculated with commercial or indigenous strains of Rhizobium. Symbiosis 2005, 38, 163–174. [Google Scholar]
- Shimoda, Y.; Nagata, M.; Suzuki, A.; Abe, M.; Sato, S.; Kato, T.; Tabata, S.; Higashi, S.; Uchiumi, T. Symbiotic Rhizobium and Nitric Oxide Induce Gene Expression of Non-symbiotic Hemoglobin in Lotus japonicus. Plant Cell Physiol. 2005, 46, 99–107. [Google Scholar] [CrossRef]
- Horchani, F.; Prévot, M.; Boscari, A.; Evangelisti, E.; Meilhoc, E.; Bruand, C.; Raymond, P.; Boncompagni, E.; Aschi-Smiti, S.; Puppo, A.; et al. Both Plant and Bacterial Nitrate Reductases Contribute to Nitric Oxide Production in Medicago truncatula Nitrogen-Fixing Nodules. Plant Physiol. 2011, 155, 1023–1036. [Google Scholar] [CrossRef] [Green Version]
- Cam, Y.; Pierre, O.; Boncompagni, E.; Hérouart, D.; Meilhoc, E.; Bruand, C. Nitric oxide (NO): A key player in the senescence of Medicago truncatula root nodules. New Phytol. 2012, 196, 548–560. [Google Scholar] [CrossRef]
- Blanquet, P.; Silva, L.; Catrice, O.; Bruand, C.; Carvalho, H.; Meilhoc, E. Sinorhizobium meliloti Controls Nitric Oxide-Mediated Post-Translational Modification of a Medicago truncatula Nodule Protein. Mol. Plant-Microbe Interact. 2015, 28, 1353–1363. [Google Scholar] [CrossRef] [Green Version]
- Hichri, I.; Boscari, A.; Castella, C.; Rovere, M.; Puppo, A.; Brouquisse, R. Nitric oxide: A multifaceted regulator of the nitrogen-fixing symbiosis. J. Exp. Bot. 2015, 66, 2877–2887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo-Begueria, L.; Rubio, M.C.; Martínez, J.I.; Pérez-Rontomé, C.; Delgado, M.J.; Bedmar, E.J.; Becana, M. Redefining nitric oxide production in legume nodules through complementary insights from electron paramagnetic resonance spectroscopy and specific fluorescent probes. J. Exp. Bot. 2018, 69, 3703–3714. [Google Scholar] [CrossRef]
- Sulieman, S.; Fischinger, S.A.; Gresshoff, P.M.; Schulze, J. Asparagine as a major factor in the N-feedback regulation of N2 fixation in Medicago truncatula. Physiol. Plant. 2010, 140, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Sulieman, S.; Tran, L.-S.P. Asparagine: An amide of particular distinction in the regulation of symbiotic nitrogen fixation of legumes. Crit. Rev. Biotechnol. 2013, 33, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Ono, Y.; Ohtake, N.; Sueyoshi, K.; Tanabata, S.; Ohyama, T. Transcriptome and Metabolome Analyses Reveal That Nitrate Strongly Promotes Nitrogen and Carbon Metabolism in Soybean Roots, but Tends to Repress It in Nodules. Plants 2018, 7, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahiri, K.; Chattopadhyay, S.; Chattopadhyay, S.; Ghosh, B. Polyamine metabolism in nodules of Vigna mungo during senescence. Phytochemistry 1992, 31, 4087–4090. [Google Scholar] [CrossRef]
- Lahiri, K.; Chattopadhyay, S.; Ghosh, B. Correlation of endogenous free polyamine levels with root nodule senescence in different genotypes in Vigna mungo L. J. Plant Physiol. 2004, 161, 563–571. [Google Scholar] [CrossRef]
- Terakado, J.; Yoneyama, T.; Fujihara, S. Shoot-applied polyamines suppress nodule formation in soybean (Glycine max). J. Plant Physiol. 2006, 163, 497–505. [Google Scholar] [CrossRef]
- Maskall, C.S.; Gibson, J.F.; Dart, P.J. Electron-paramagnetic-resonance studies of leghaemoglobins from soya-bean and cowpea root nodules. Identification of nitrosyl-leghaemoglobin in crude leghaemoglobin preparations. Biochem. J. 1977, 167, 435–445. [Google Scholar] [CrossRef] [Green Version]
- Nishiwaki, T.; Sato, T.; Yashima, H.; Ikarashi, T.; Ohyama, T.; Harper, J.E.; Akao, S.; Kouchi, H. Changes in concentration of leghemoglobin components in hypernodulation mutants of soybean. Soil Sci. Plant Nutr. 1997, 43, 1091–1096. [Google Scholar] [CrossRef]
- Li, S.; Xiao, F.; Yang, D.; Lyu, X.; Ma, C.; Dong, S.; Yan, C.; Gong, Z. Nitrate Transport and Distribution in Soybean Plants with Dual-Root Systems. Front. Plant Sci. 2021, 12, 892. [Google Scholar] [CrossRef] [PubMed]
- Bacanamwo, M.; Harper, J.E. The feedback mechanism of nitrate inhibition of nitrogenase activity in soybean may involve asparagine and/or products of its metabolism. Physiol. Plant. 1997, 100, 371–377. [Google Scholar] [CrossRef]
- Yang, J.K.; Zhang, W.T.; Yuan, T.Y.; Zhou, J.C. Genotypic characteristics of the rrn operon and genome of indigenous soybean Bradyrhizobia in cropping zones of China. Can. J. Microbiol 2006, 52, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, W.; Han, X.; Wang, E.; Zou, W.; Zhang, Z. Genetic diversity of indigenous soybean-nodulating rhizobia in response to locally-based long term fertilization in a Mollisol of Northeast China. World J. Microbiol. Biotechnol. 2016, 33, 6. [Google Scholar] [CrossRef] [PubMed]





| Treatments | Nodule Number (Per Plant) | Nodule Weight (g/Plant) |
|---|---|---|
| NF | 103.0 ± 3.91 a | 0.41 ± 0.044 a |
| NH | 98.2 ± 7.71 a | 0.37 ± 0.027 a |
| UniProt Database | Upregulated Protein | Downregulated Protein | Total |
|---|---|---|---|
| Glycine max (Soybean) (Glycine hispida) | 42 | 10 | 52 |
| Bradyrhizobium japonicum | 55 | 57 | 112 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, X.; Sun, C.; Zhang, J.; Wang, C.; Zhao, S.; Ma, C.; Li, S.; Li, H.; Gong, Z.; Yan, C. Integrated Proteomics and Metabolomics Analysis of Nitrogen System Regulation on Soybean Plant Nodulation and Nitrogen Fixation. Int. J. Mol. Sci. 2022, 23, 2545. https://doi.org/10.3390/ijms23052545
Lyu X, Sun C, Zhang J, Wang C, Zhao S, Ma C, Li S, Li H, Gong Z, Yan C. Integrated Proteomics and Metabolomics Analysis of Nitrogen System Regulation on Soybean Plant Nodulation and Nitrogen Fixation. International Journal of Molecular Sciences. 2022; 23(5):2545. https://doi.org/10.3390/ijms23052545
Chicago/Turabian StyleLyu, Xiaochen, Chunyan Sun, Jin Zhang, Chang Wang, Shuhong Zhao, Chunmei Ma, Sha Li, Hongyu Li, Zhenping Gong, and Chao Yan. 2022. "Integrated Proteomics and Metabolomics Analysis of Nitrogen System Regulation on Soybean Plant Nodulation and Nitrogen Fixation" International Journal of Molecular Sciences 23, no. 5: 2545. https://doi.org/10.3390/ijms23052545






