Neural Crest-Derived Stem Cells (NCSCs) Obtained from Dental-Related Stem Cells (DRSCs): A Literature Review on Current Knowledge and Directions toward Translational Applications
Abstract
:1. Introduction
2. The Neural Crest and Its Adult Stem Cell Derivatives
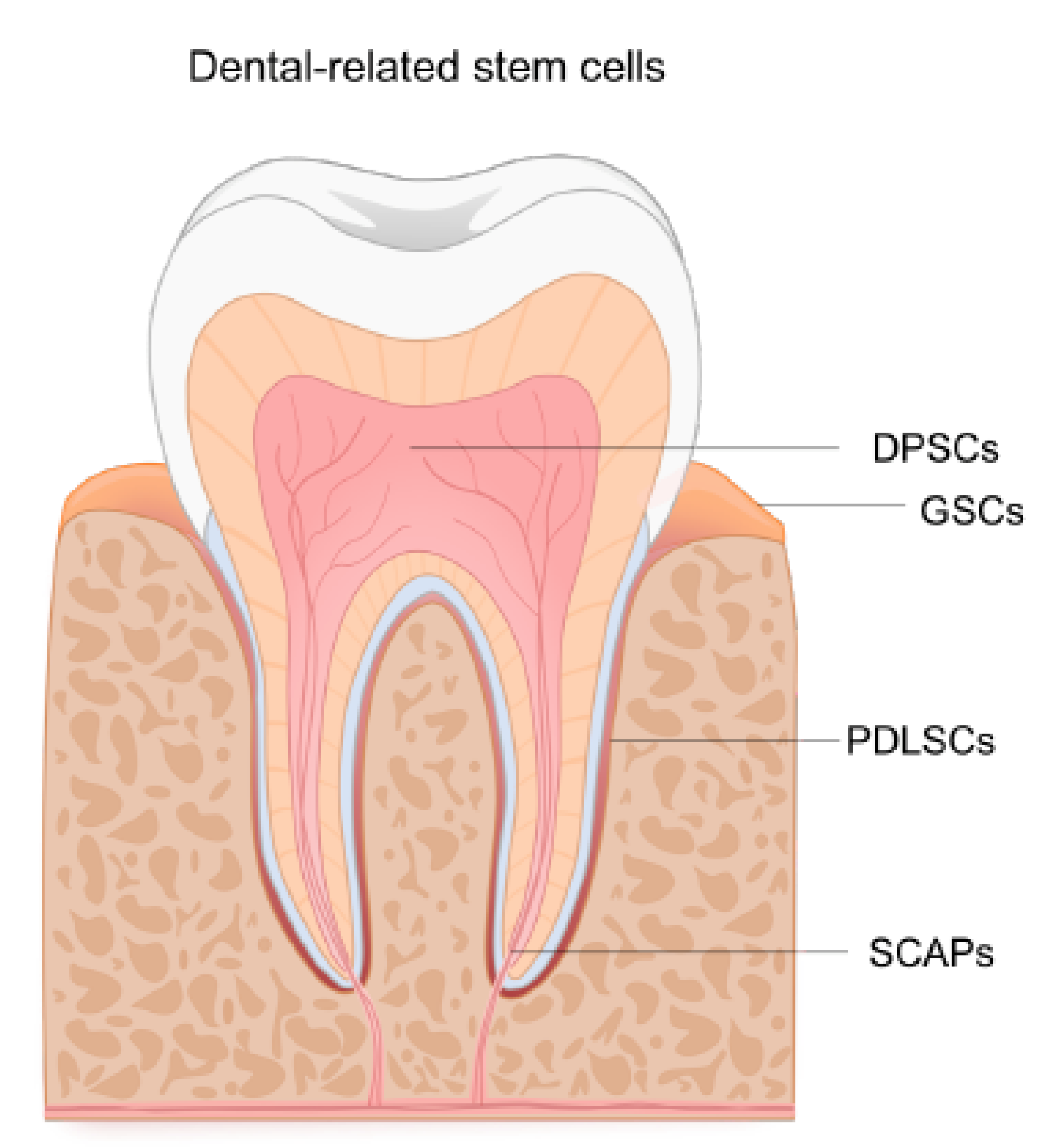
3. Dental-Related Sources of Human Neural Crest-Derived Stem Cells
3.1. NCSCs Derived from Gingiva
| Origin | Method | Medium Components | Characterization | Functional Assay | Ref |
|---|---|---|---|---|---|
| Gingiva | Self-induced spheres and sphere-derived monolayer cultures From monolayer cultures grown in standard FBS-containing medium | NCSC medium (PLO/LAM coating): DMEM/F12, neurobasal medium, human bFGF (20 ng/mL), human EGF (20 ng/mL), βMe (55 µM), N2 (1%), B27 (1%) | ICC: P75, NES, SOX9, SNAIL1 RT-qPCR: SOX9, FOXD3, SLUG, SNAIL1 | Nerve conduits in rats: functional recovery | [26] |
| Small molecules (monolayer) NCSCs with WNT activation and TGFβ inhibition | Supplemented medium: NCSCs medium + CHIR99021 (2.5 µM), SB43152 (5 µM) | Flow cytometry: Loss: CD29, CD44, CD73, CD90 Gain: P75 | None | ||
| Neurospheres From monolayer cultures grown in standard FBS-containing medium | Neurosphere medium: DMEM/F12, L-Glut (2 mM), EGF (20 ng/mL), FGF2 (40 ng/mL), B27 (2%) | Monolayer and neurospheres: RT-qPCR: NES, PAX3, TWIST1, SNAIL1, FOXD3 AND SOX9 Neurospheres: ICC: VIM, NES, COL1, FN | In vitro neural differentiation | [25] | |
| Periodontal ligament | EGF/FGF2 (monolayer) From monolayer cultures grown in standard FBS-containing medium | a-MEM, FBS (10%), chicken embryo extract (2%), transferrin (10 mg/mL), hydrocortisone (0.1 mg/mL), glucagon (0.01 ng/mL), insulin (1 ng/mL), triiodothyronine (0.4 ng/mL), EGF (0.1 ng/mL), FGF2 (1 ng/mL), pen/strep (100 U/100 µg/mL) | NCSC conditions: ICC: P75, HNK-1, NES RT-PCR: NES, βTUBIII, NFM, MAP2, PER, P0, GFAP, aSMA. | None | [27] |
| Direct isolation Connexin 43+ve magnetic cell sorting | DMEM 10% FBS | CX43+ve population: ICC: NANOG, SOX2, SOX10, OCT4, NES, P75 | Teratoma formation as pluripotency test | [28] | |
| Small molecules (monolayer) From monolayer cultures grown in a defined MSC medium and changed to a NCSCm | MESENDEM (FN-coating): Optimem, ITS (1×), βME (50 µM), Glutamax (1×), FGF2 (5 ng/mL), EGF (10 ng/mL), Anti Anti (1×), 0.5, 1, 2, 10% FBS NCSCm (FN-coating): DMEM/F12, N2 (1×) B27 (1×), FGF2 (10 ng/mL), EGF (10 ng/mL), BIO (2 µM), REPSOX (1 µM) | NCSCs medium: Flow cytometry: increase in P75 and HNK1, E-CAD RT-PCR: OCT4, SOX2, MYC, CDH1, ZEB, SOX10 | None | [29] | |
| Human exfoliated deciduous teeth | Small molecules (monolayer) From monolayer cultures grown in a defined MSC medium and changed to a NCSCm | DentEpiMesMed (FN-coating): DMEM/F12, N2 (1×) ascorbic acid (100 µg/mL), βME (50 µM), Glutamax (1×), FGF2 (2.5 ng/mL), EGF (10 ng/mL) IGF (10 ng/mL), MEM AA (1×), Anti Anti (1×), 1 or 2% FBS NCSCm (FN-coating): DentEpiMesMed + BIO (1 µM), REPSOX (5 µM) | NCSC medium: Flow cytometry: Upregulation of P75 and E-cad, downregulation of CD73 ICC: Upregulation of SOX10 RT-qPCR: upregulation of SOX10, ZO-1, E-cadherin | In vitro neural differentiation (from defined MSC culture, but not from NCSCm cultures) | [30] |
| Apical pulp | Monolayer: Cultures grown in standard FBS-containing medium | DMEM, 10% FBS, L-Glut (2 mM) | RT-PCR: SNAIL1, SNAIL2, SOX9, TWIST1, MSX2 and DLX6 Flow cytometry: STRO-1 negative | In vitro neural differentiation | [31] |
| Neurospheres: Initial growth in standard FBS-containing medium and induced neurosphere formation | Neurosphere medium: DMEM/F12, FGF2 (20 ng/mL), EGF (20 ng/mL), N2 | Neurospheres: ICC: NES, MSI1, P75 RT-PCR: NES, MSI1, Slug, Snail, P75 | In vitro neural differentiation | [32] | |
| Neurosphere Initial growth in standard FBS-containing medium and induced neurosphere formation | Neurosphere medium (Matrigel coating after first passage): DMEM/F12, FGF2 (20 ng/mL), EGF (20 ng/mL), N2, B27 | ICC: HNK-1 and P75 | In vitro odontogenic differentiation | [33] | |
| Dental follicle | Monolayer: Cultures grown in standard FBS-containing medium | DMEM/F12, 20% FBS, ascorbic acid (100 µg/mL), L-Glut (2 mM) | ICC: HNK-1, NES, P75, SOX2 | None | [34] |
| Dental pulp | EGF/FGF2 (monolayer): Dental pulp cells outgrown from explants | Neurobasal medium, B27 without vitamin A, FGF2 (20 ng/mL), EGF (20 ng/mL), insulin (2.5 µM), L-Glut (2 mM), neuregulin-β1 (10 nM) | NCSC explants: ICC: P75, NES, SOX10 | Schwann cell, osteogenic and melanocytic differentiation | [35] |
| Neurospheres: From monolayer cultures grown in standard FBS-containing medium | DMEM, 10% FBS, L-ascorbic acid (100 µM) | Flow cytometry: CD34 negative ICC: P75, NES, c-KIT, MART-1 | In vitro mesenchymal lineage differentiation | [36] | |
| Direct isolation + neurosphere: STRO-1+ve, c-KIT+ve, CD34+ve magnetic sorting and maintained in neurosphere conditions | Neurosphere medium: DMEM/F12, FGF2 (20 ng/mL), EGF (20 ng/mL), B27 (2%), L-Glut (2 mM) | Neurosphere-derived cultures: ICC: SOX10, P75 | In vitro neural differentiation | [37] | |
| Direct isolation STRO-1+ve, c-KIT+ve, CD34+ve magnetic sorting | a-MEM, 10% FBS, L-Glut (2 mM) | ICC: P75, SOX10, NES | In vitro neural differentiation (from standard FBS-containing cultures) and in vivo grafting for peripheral nerve repair (magnetic sorted cells) | [38] | |
| Monolayer: Cultures established in serum-containing medium and transferred to serum-free, MSC commercial medium (STP) | STP+NTP Medium: StemPro MSC SFM, BDNF (500 ng/mL), NT3 (20 ng/mL) | RT-PCR: Upregulation of HNK-1 and P75 ICC: P75 | In vitro glial and neural differentiation | [39] | |
| Neurospheres: From monolayer cultures grown in serum-containing medium or serum-free medium | Monolayer (LAM coating)/Neurosphere medium: DMEM/F12, L-Glut (2 mM), EGF (20 ng/mL), FGF2 (20 ng/mL), IGF (50 ng/mL), B27 (2%), N2 (1%),with or without BMP4 (10 ng/mL) | Monolayer: ICC: NES, SLUG, SNAIL1, SOX9, P75. Neurospheres: ICC: NES, SOX2, SLUG, SNAIL1, SOX9. SOX10 and P75 (when supplemented with BMP4). RT-PCR: AP2a, P75, SNAIL1, SOX10 (when supplemented with BMP4) | In vitro neural differentiation | [40] | |
| Neurosphere Cultures grown in standard FBS-containing medium | Neurosphere: DMEM/F12, L-Glut (2 mM), EGF (20 ng/mL), FGF2 (20 ng/mL), heparin (5 ng/mL), B27 (1%), N2 (1%) | RT-PCR: AP2a, P75, NES, Sox2 | In vitro differentiation into corneal endothelial-like cells | [41] |
3.2. NCSCs Derived from Periodontal Ligament
3.3. NCSCs from Developing Teeth and the Adult Dental Pulp
3.3.1. NCSCs Derived from the Dental Pulp of Exfoliated Deciduous Teeth (SHEDs)
3.3.2. NCSCs Derived from the Apical Pulp or Apical Papilla (SCAPs)
3.3.3. NCSCs Derived from Dental Follicle
3.3.4. NCSCs Derived from the Dental Pulp
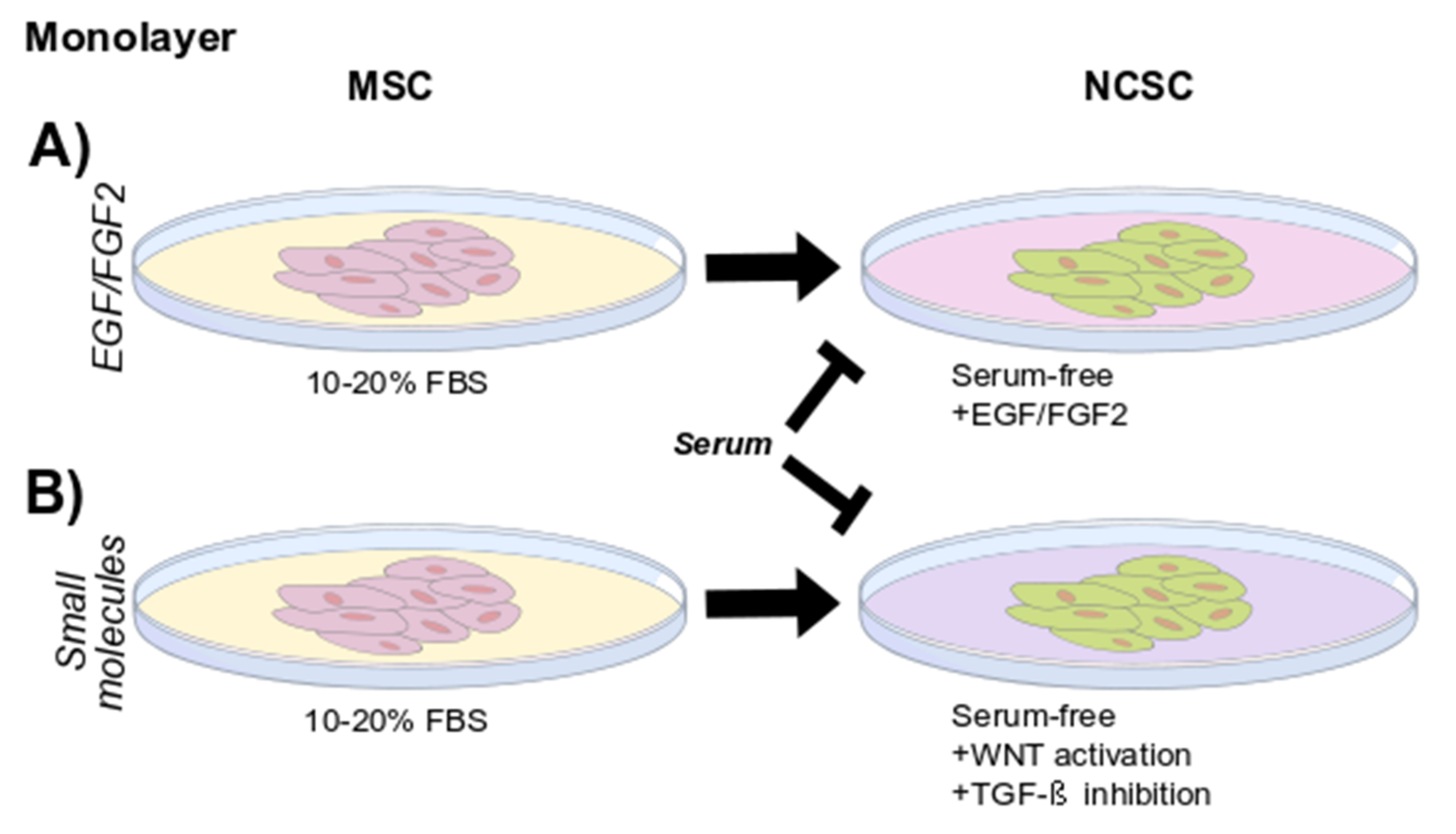
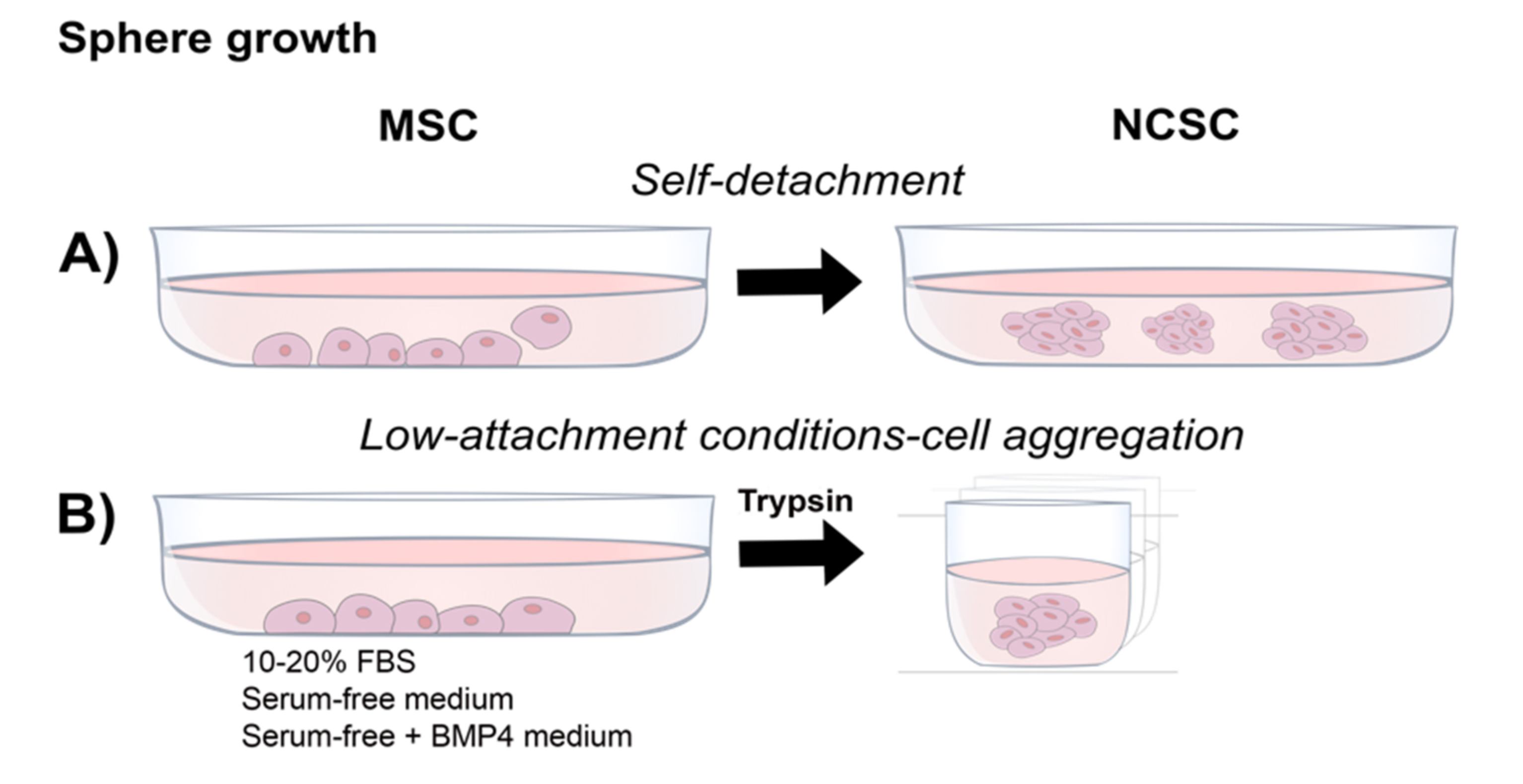
4. Considerations of Growth Conditions and Strategies for Isolation
4.1. EGF/FGF2 Containing Media
4.2. Small Molecules
4.3. Neurosphere Formation
4.4. Direct Isolation (Cell Sorting)
5. Important Considerations from Previous Reports of DRSCs Characterized as MSCs
6. Conclusions and Considerations for Future Applications
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AP2A | Activator Protein 2a |
| BDNF | Brain-Derived Neurotrophic Factor |
| ΒME | 2-Mercaptoethanol |
| BMP4 | Bone Morphogenetic Protein 4 |
| COL1 | Collagen Type I |
| CX43 | Connexin 43 |
| DLX6 | Distal-Less Homeobox 6 |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DPSC | Dental Pulp Stem Cell |
| DRSCS | Dental-Related Stem Cells |
| E-CAD/CDH1 | E-Cadherin |
| EGF | Epidermal Growth Factor |
| EMT | Epithelial to Mesenchymal Transition |
| FBS | Fetal Bovine Serum |
| FGF | Fibroblast Growth Factor |
| FN | Fibronectin |
| FOXD3 | Forkhead Box D3 |
| GAP-43 | Growth Associated Protein 43 |
| GCP | Good Clinical Practice |
| GFP | Green Fluorescent Protein |
| GMP | Good Manufacturing Practice |
| HDPSC | Human Dental Pulp Stem Cell |
| HNK-1 | Human Natural Killer-1 |
| ICC | Immunocytochemistry |
| IFG | Insulin Growth Factor |
| LAM | Laminin |
| MART-1 | Melanoma Antigen Recognized by T-Cells |
| MEM | Minimum Essential Medium |
| MET | Mesenchymal to Mesenchymal Transition |
| MSCS | Mesenchymal Stromal Cells |
| MSI1 | Mushashi-1 |
| MSX2 | Msh Homeobox 2 |
| NANOG | Nanog Homeobox |
| NCAD | N-Cadherin |
| NCC | Neural Crest Cells |
| NCSC | Neural Crest-Derived Stem Cell |
| NCSCM | Neural Crest-Derived Stem Cell Medium |
| NES | Nestin |
| NF | Neurofilament |
| NFH | Neurofilament-High |
| NFM | Neurofilament-Medium |
| NT3 | Neurotrophin-3 |
| OCT4 | Octamer-Binding Transcription Factor 4 |
| P75 | Low-Affinity Nerve Growth Factor Receptor |
| PAX3 | Paired Box 3 |
| PDL | Periodontal Ligament |
| PLO | Poly-Ornithine |
| RT-QPCR | Retro-Transcriptase Quantitative Polymerase Chain Reaction |
| SCAP | Stem Cells from Apical Pulp |
| SHED | Stem Cells from Human Exfoliated Deciduous Teeth |
| SLUG | Snail Family Transcriptional Repressor 2 |
| SNAIL | Snail Family Transcriptional Repressor |
| SOX2 | Sry-Box Transcription Factor 2 |
| SOX9 | Sry-Box Transcription Factor 9 |
| SOX10 | Sry-Box Transcription Factor 10 |
| SYN | Synapsin |
| TGF-Β | Transforming Growth Factor Beta |
| TUBIII | Class III Β-Tubulin |
| TWIST | Twist Family Bhlh Transcription Factor |
| VIM | Vimentin |
| WNT1 | Wingless-Related Integration Site-1 |
| ZEB | Zinc Finger E-Box Binding Homeobox |
References
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [Green Version]
- Kaewkhaw, R.; Scutt, A.M.; Haycock, J.W. Anatomical site influences the differentiation of adipose-derived stem cells for Schwann-cell phenotype and function. Glia 2011, 59, 734–749. [Google Scholar] [CrossRef] [PubMed]
- Koide, Y.; Morikawa, S.; Mabuchi, Y.; Muguruma, Y.; Hiratsu, E.; Hasegawa, K.; Kobayashi, M.; Ando, K.; Kinjo, K.; Okano, H.; et al. Two distinct stem cell lineages in murine bone marrow. Stem Cells 2007, 25, 1213–1221. [Google Scholar] [CrossRef]
- Lindner, U.; Kramer, J.; Rohwedel, J.; Schlenke, P. Mesenchymal stem or stromal cells: Toward a better understanding of their biology? Transfus. Med. Hemother. 2010, 37, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Heng, B.C.; Lim, L.W.; Wu, W.; Zhang, C. An overview of protocols for the neural induction of dental and oral stem cells in vitro. Tissue Eng. Part B Rev. 2016, 22, 220–250. [Google Scholar] [CrossRef]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Concise review: Dental pulp stem cells: A novel cell therapy for retinal and central nervous system repair. Stem Cells 2017, 35, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Földes, A.; Kádár, K.; Kerémi, B.; Zsembery, Á.; Gyires, K.; Zádori, Z.S.; Varga, G. Mesenchymal stem cells of dental origin—their potential for antiinflammatory and regenerative actions in brain and gut damage. Curr. Neuropharmacol. 2016, 14, 914–934. [Google Scholar] [CrossRef] [Green Version]
- Crane, J.F.; Trainor, P.A. Neural crest stem and progenitor cells. Annu. Rev. Cell Dev. Biol. 2006, 22, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Janebodin, K.; Horst, O.V.; Ieronimakis, N.; Balasundaram, G.; Reesukumal, K.; Pratumvinit, B.; Reyes, M. Isolation and characterization of neural crest-derived stem cells from dental pulp of neonatal mice. PLoS ONE 2011, 6, e27526. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M.; Hayashi, R.; Kanakubo, S.; Ogasawara, A.; Yamato, M.; Osumi, N.; Nishida, K. Neural crest-derived multipotent cells in the adult mouse iris stroma. Genes Cells 2011, 16, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Nagoshi, N.; Shibata, S.; Kubota, Y.; Nakamura, M.; Nagai, Y.; Satoh, E.; Morikawa, S.; Okada, Y.; Mabuchi, Y.; Katoh, H.; et al. Ontogeny and multipotency of neural crest-derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell 2008, 2, 392–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.Y.C.; Pelaez, D.; Dominguez-Bendala, J.; Garcia-Godoy, F.; Cheung, H.S. Plasticity of stem cells derived from adult periodontal ligament. Regen. Med. 2009, 4, 809–821. [Google Scholar] [CrossRef]
- Bressan, R.B.; Melo, F.R.; Almeida, P.A.; Bittencourt, D.A.; Visoni, S.; Jeremias, T.S.; Costa, A.P.; Leal, R.B.; Trentin, A.G. EGF-FGF2 stimulates the proliferation and improves the neuronal commitment of mouse epidermal neural crest stem cells (EPI-NCSCs). Exp. Cell Res. 2014, 327, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Locher, H.; Saadah, N.; de Groot, S.; de Groot, J.C.M.J.; Frijns, J.H.M.; Huisman, M.A. Hair follicle bulge cultures yield class III β-tubulin-positive melanoglial cells. Histochem. Cell Biol. 2015, 144, 87–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widera, D.; Heimann, P.; Zander, C.; Imielski, Y.; Heidbreder, M.; Heilemann, M.; Kaltschmidt, C.; Kaltschmidt, B. Schwann cells can be reprogrammed to multipotency by culture. Stem Cells Dev. 2011, 20, 2053–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schomann, T.; Mezzanotte, L.; De Groot, J.C.M.J.; Rivolta, M.N.; Hendriks, S.H.; Frijns, J.H.M.; Huisman, M.A. Neuronal differentiation of hair-follicle-bulge-derived stem cells co-cultured with mouse cochlear modiolus explants. PLoS ONE 2017, 12, e0187183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widera, D.; Zander, C.; Heidbreder, M.; Kasperek, Y.; Noll, T.; Seitz, O.; Saldamli, B.; Sudhoff, H.; Sader, R.; Kaltschmidt, C.; et al. Adult palatum as a novel source of neural crest-related stem cells. Stem Cells 2009, 27, 1899–1910. [Google Scholar] [CrossRef] [Green Version]
- Clewes, O.; Narytnyk, A.; Gillinder, K.R.; Loughney, A.D.; Murdoch, A.P.; Sieber-Blum, M. Human epidermal neural crest stem cells (hEPI-NCSC)—characterization and directed differentiation into osteocytes and melanocytes. Stem Cell Rev. Rep. 2011, 7, 799–814. [Google Scholar] [CrossRef] [Green Version]
- Hauser, S.; Widera, D.; Qunneis, F.; Müller, J.; Zander, C.; Greiner, J.; Strauss, C.; Lüningschrör, P.; Heimann, P.; Schwarze, H.; et al. Isolation of novel multipotent neural crest-derived stem cells from adult human inferior turbinate. Stem Cells Dev. 2012, 21, 742–756. [Google Scholar] [CrossRef]
- Kaku, M.; Komatsu, Y.; Mochida, Y.; Yamauchi, M.; Mishina, Y.; Ko, C.-C. Identification and characterization of neural crest-derived cells in adult periodontal ligament of mice. Arch. Oral Biol. 2012, 57, 1668–1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fawzy El-Sayed, K.M.; Dörfer, C.E. Gingival mesenchymal stem/progenitor cells: A unique tissue engineering gem. Stem Cells Int. 2016, 2016, 7154327. [Google Scholar] [CrossRef] [Green Version]
- Tomar, G.B.; Srivastava, R.K.; Gupta, N.; Barhanpurkar, A.P.; Pote, S.T.; Jhaveri, H.M.; Mishra, G.C.; Wani, M.R. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem. Biophys. Res. Commun. 2010, 393, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, S.; Liu, Y.; Uyanne, J.; Shi, Y.; Shi, S.; Le, A.D. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J. Immunol. 2009, 183, 7787–7798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fournier, B.P.; Loison-Robert, L.S.; Ferré, F.C.; Owen, G.R.; Larjava, H.; Häkkinen, L. Characterisation of human gingival neural crest-derived stem cells in monolayer and neurosphere cultures. Eur. Cell. Mater. 2016, 31, 40–58. [Google Scholar] [CrossRef]
- Zhang, Q.; Nguyen, P.D.; Shi, S.; Burrell, J.C.; Xu, Q.; Cullen, K.D.; Le, A.D. Neural crest stem-like cells non-genetically induced from human gingiva-derived mesenchymal stem cells promote facial nerve regeneration in rats. Mol. Neurobiol. 2018, 55, 6965–6983. [Google Scholar] [CrossRef]
- Coura, G.S.; Garcez, R.C.; Mendes De Aguiar, C.B.N.; Alvarez-Silva, M.; Magini, R.S.; Trentin, A.G. Human periodontal ligament: A niche of neural crest stem cells. J. Periodontal Res. 2008, 43, 531–536. [Google Scholar] [CrossRef]
- Pelaez, D.; Huang, C.-Y.C.; Cheung, H.S. Isolation of pluripotent neural crest-derived stem cells from adult human tissues by connexin-43 enrichment. Stem Cells Dev. 2013, 22, 2906–2914. [Google Scholar] [CrossRef]
- Ramírez-García, L.; Cevallos, R.; Gazarian, K. Unveiling and initial characterization of neural crest-like cells in mesenchymal populations from the human periodontal ligament. J. Periodontal Res. 2017, 52, 609–616. [Google Scholar] [CrossRef]
- Gazarian, K.G.; Ramírez-García, L.R. Human deciduous teeth stem cells (SHED) display neural crest signature characters. PLoS ONE 2017, 12, e0170321. [Google Scholar] [CrossRef]
- Degistirici, Ö.; Jaquiery, C.; Schönebeck, B.; Siemonsmeier, J.; Götz, W.; Martin, I.; Thie, M. Defining properties of neural crest-derived progenitor cells from the apex of human developing tooth. Tissue Eng. Part A 2008, 14, 317–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, S.; Hamada, K.; Miura, M.; Yamaguchi, S. Neural crest stem cell property of apical pulp cells derived from human developing tooth. Cell Biol. Int. 2012, 36, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiang, L.; Guan, C.; Yang, X.; Hu, X.; Zhang, X.; Zhang, W. Effects of platelet-rich plasma on proliferation, viability, and odontogenic differentiation of neural crest stem-like cells derived from human dental apical papilla. Biomed. Res. Int. 2020, 2020, 4671989. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.L.; Holanda-Afonso, R.C.; Moura-Neto, V.; Bolognese, A.M.; DosSantos, M.F.; Souza, M.M. Human dental follicle cells express embryonic, mesenchymal and neural stem cells markers. Arch. Oral Biol. 2016, 73, 121–128. [Google Scholar] [CrossRef]
- Al-Zer, H.; Apel, C.; Heiland, M.; Friedrich, R.E.; Jung, O.; Kroeger, N.; Eichhorn, W.; Smeets, R. Enrichment and Schwann cell differentiation of neural crest-derived dental pulp stem cells. In Vivo 2015, 29, 319–326. [Google Scholar]
- Stevens, A.; Zuliani, T.; Olejnik, C.; LeRoy, H.; Obriot, H.; Kerr-Conte, J.; Formstecher, P.; Bailliez, Y.; Polakowska, R.R. Human dental pulp stem cells differentiate into neural crest-derived melanocytes and have label-retaining and sphere-forming abilities. Stem Cells Dev. 2008, 17, 1175–1184. [Google Scholar] [CrossRef]
- Pisciotta, A.; Bertoni, L.; Riccio, M.; Mapelli, J.; Bigiani, A.; La Noce, M.; Orciani, M.; de Pol, A.; Carnevale, G. Use of a 3D floating sphere culture system to maintain the neural crest-related properties of human dental pulp stem cells. Front. Physiol. 2018, 9, 547. [Google Scholar] [CrossRef] [Green Version]
- Carnevale, G.; Pisciotta, A.; Riccio, M.; Bertoni, L.; De Biasi, S.; Gibellini, L.; Zordani, A.; Cavallini, G.M.; La Sala, G.B.; Bruzzesi, G.; et al. Human dental pulp stem cells expressing STRO-1, c-kit and CD34 markers in peripheral nerve regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e774–e785. [Google Scholar] [CrossRef]
- Luzuriaga, J.; Pineda, J.R.; Irastorza, I.; Uribe-Etxebarria, V.; García-Gallastegui, P.; Encinas, J.M.; Chamero, P.; Unda, F.; Ibarretxe, G. BDNF and NT3 reprogram human ectomesenchymal dental pulp stem cells to neurogenic and gliogenic neural crest progenitors cultured in serum-free medium. Cell. Physiol. Biochem. 2019, 52, 1361–1380. [Google Scholar] [CrossRef]
- Solis-Castro, O.O.; Boissonade, F.M.; Rivolta, M.N. Establishment and neural differentiation of neural crest-derived stem cells from human dental pulp in serum-free conditions. Stem Cells Transl. Med. 2020, 9, 1462–1476. [Google Scholar] [CrossRef]
- Bosch, B.M.; Salero, E.; Núñez-Toldrà, R.; Sabater, A.L.; Gil, F.J.; Perez, R.A. Discovering the potential of dental pulp stem cells for corneal endothelial cell production: A proof of concept. Front. Bioeng. Biotechnol. 2021, 9, 617724. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.-M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.-Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Shi, S.; Gronthos, S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J. Bone Miner. Res. 2003, 18, 696–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenebeck, B.; Hartschen, H.J.; Schindel, M.; Degistirici, O.; Siemonsmeier, J.; Goetz, W.; Thie, M. Molecular characterization of human impacted third molars: Diversification of compartments. Cells Tissues Organs 2009, 189, 356–370. [Google Scholar] [CrossRef]
- Zhou, T.; Pan, J.; Wu, P.; Huang, R.; Du, W.; Zhou, Y.; Wan, M.; Fan, Y.; Xu, X.; Zhou, X.; et al. Dental follicle cells: Roles in development and beyond. Stem Cells Int. 2019, 2019, 9159605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisciotta, A.; Carnevale, G.; Meloni, S.; Riccio, M.; De Biasi, S.; Gibellini, L.; Ferrari, A.; Bruzzesi, G.; De Pol, A. Human dental pulp stem cells (hDPSCs): Isolation, enrichment and comparative differentiation of two sub-populations. BMC Dev. Biol. 2015, 15, 14. [Google Scholar] [CrossRef] [Green Version]
- Bakopoulou, A.; Apatzidou, D.; Aggelidou, E.; Gousopoulou, E.; Leyhausen, G.; Volk, J.; Kritis, A.; Koidis, P.; Geurtsen, W. Isolation and prolonged expansion of oral mesenchymal stem cells under clinical-grade, GMP-compliant conditions differentially affects “stemness” properties. Stem Cell Res. Ther. 2017, 8, 247. [Google Scholar] [CrossRef]
- Jung, J.; Kim, J.-W.; Moon, H.-J.; Hong, J.Y.; Hyun, J.K. Characterization of neurogenic potential of dental pulp stem cells cultured in xeno/serum-free condition: In vitro and in vivo assessment. Stem Cells Int. 2016, 2016, 6921097. [Google Scholar] [CrossRef] [Green Version]
- Arthur, A.; Rychkov, G.; Shi, S.; Koblar, S.A.; Gronthos, S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells 2008, 26, 1787–1795. [Google Scholar] [CrossRef] [Green Version]
- Menendez, L.; Yatskievych, T.A.; Antin, P.B.; Dalton, S. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc. Natl. Acad. Sci. USA 2011, 108, 19240–19245. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, R.; Lee, H.L.; Hong, C.; Wang, C.Y. Single CD271 marker isolates mesenchymal stem cells from human dental pulp. Int. J. Oral Sci. 2015, 7, 205–212. [Google Scholar] [CrossRef]
- Mikami, Y.; Ishii, Y.; Watanabe, N.; Shirakawa, T.; Suzuki, S.; Irie, S.; Isokawa, K.; Honda, M.J. CD271/ p75NTR inhibits the differentiation of mesenchymal stem cells into osteogenic, adipogenic, chondrogenic, and myogenic lineages. Stem Cells Dev. 2011, 20, 901–913. [Google Scholar] [CrossRef]
- Pan, W.; Kremer, K.L.; Kaidonis, X.; Ludlow, V.E.; Rogers, M.-L.; Xie, J.; Proud, C.G.; Koblar, S.A. Characterization of p75 neurotrophin receptor expression in human dental pulp stem cells. Int. J. Dev. Neurosci. 2016, 53, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M.R.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S.T. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gervois, P.; Struys, T.; Hilkens, P.; Bronckaers, A.; Ratajczak, J.; Politis, C.; Brône, B.; Lambrichts, I.; Martens, W. Neurogenic maturation of human dental pulp stem cells following neurosphere generation induces morphological and electrophysiological characteristics of functional neurons. Stem Cells Dev. 2015, 24, 296–311. [Google Scholar] [CrossRef] [Green Version]
- Király, M.; Porcsalmy, B.; Pataki, Á.; Kádár, K.; Jelitai, M.; Molnár, B.; Hermann, P.; Gera, I.; Grimm, W.-D.; Ganss, B.; et al. Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem. Int. 2009, 55, 323–332. [Google Scholar] [CrossRef]
- Li, D.; Zou, X.-Y.; El-Ayachi, I.; Romero, L.O.; Yu, Z.; Iglesias-Linares, A.; Cordero-Morales, J.F.; Huang, G.T.-J. Human dental pulp stem cells and gingival mesenchymal stem cells display action potential capacity in vitro after neuronogenic differentiation. Stem Cell Rev. Rep. 2019, 15, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Yamamoto, A.; Matsubara, K.; Nakamura, S.; Naruse, M.; Yamagata, M.; Sakamoto, K.; Tauchi, R.; Wakao, N.; Imagama, S.; et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J. Clin. Invest. 2012, 122, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Matsubara, K.; Sakai, K.; Ito, M.; Ohno, K.; Ueda, M.; Yamamoto, A. Dopaminergic differentiation of stem cells from human deciduous teeth and their therapeutic benefits for Parkinsonian rats. Brain Res. 2015, 1613, 59–72. [Google Scholar] [CrossRef]
- Martens, W.; Sanen, K.; Georgiou, M.; Struys, T.; Bronckaers, A.; Ameloot, M.; Phillips, J.; Lambrichts, I. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J. 2014, 28, 1634–1643. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Ide, R.; Saiki, C.; Kumazawa, Y.; Okamura, H. Human dental pulp cells differentiate toward neuronal cells and promote neuroregeneration in adult organotypic hippocampal slices in vitro. Int. J. Mol. Sci. 2017, 18, 1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonmanee, T.; Sritanaudomchai, H.; Vongsavan, K.; Faisaikarm, T.; Songsaad, A.; White, K.L.; Thonabulsombat, C. Neuronal differentiation of dental pulp stem cells from human permanent and deciduous teeth following coculture with rat auditory brainstem slices. Anat. Rec. 2020, 303, 2931–2946. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solis-Castro, O.O.; Rivolta, M.N.; Boissonade, F.M. Neural Crest-Derived Stem Cells (NCSCs) Obtained from Dental-Related Stem Cells (DRSCs): A Literature Review on Current Knowledge and Directions toward Translational Applications. Int. J. Mol. Sci. 2022, 23, 2714. https://doi.org/10.3390/ijms23052714
Solis-Castro OO, Rivolta MN, Boissonade FM. Neural Crest-Derived Stem Cells (NCSCs) Obtained from Dental-Related Stem Cells (DRSCs): A Literature Review on Current Knowledge and Directions toward Translational Applications. International Journal of Molecular Sciences. 2022; 23(5):2714. https://doi.org/10.3390/ijms23052714
Chicago/Turabian StyleSolis-Castro, Oscar O., Marcelo N. Rivolta, and Fiona M. Boissonade. 2022. "Neural Crest-Derived Stem Cells (NCSCs) Obtained from Dental-Related Stem Cells (DRSCs): A Literature Review on Current Knowledge and Directions toward Translational Applications" International Journal of Molecular Sciences 23, no. 5: 2714. https://doi.org/10.3390/ijms23052714






