Biomarkers of Glucose Metabolism Alterations and the Onset of Metabolic Syndrome in Survivors of Childhood Acute Lymphoblastic Leukemia
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Biochemical Analysis
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tai, E.W.; Ward, K.C.; Bonaventure, A.; Siegel, D.A.; Coleman, M.P. Survival Among Children Diagnosed With Acute Lymphoblastic Leukemia in the United States, by Race and Age, 2001 to 2009: Findings From the CONCORD-2 Study. Cancer 2017, 123 (Suppl. 24), 5178–5189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Childhood Cancer Survivor Study. Chronic Health Conditions in Adult Survivors of Childhood Cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Krawczuk-Rybak, M.; Panasiuk, A.; Stachowicz-Stencel, T.; Zubowska, M.; Skalska-Sadowska, J.; Sęga-Pondel, D.; Czajńska-Deptuła, A.; Sławińska, D.; Badowska, W.; Kamieńska, E.; et al. Health status of polish children and adolescents after cancer treatment. Eur. J. Pediatr. 2018, 177, 437–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lima Junior, E.A.; Yamashita, A.S.; Pimentel, G.D.; De Sousa, L.G.O.; Santos, R.V.T.; Gonçalves, C.L.; Streck, E.L.; de Lira, F.S.; Rosa Neto, J.C. Doxorubicin Caused Severe Hyperglycaemia and Insulin Resistance, Mediated by Inhibition in AMPk Signalling in Skeletal Muscle. J. Cachexia Sarcopenia Muscle 2016, 7, 615–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowas, S.R.; Marks, D.; Malempati, S. Prevalence of Transient Hyperglycemia during Induction Chemotherapy for Pediatric Acute Lymphoblastic Leukemia. Pediatr. Blood Cancer 2009, 52, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Kelly, M.J.; Saltzman, E.; Must, A.; Roberts, S.B.; Parsons, S.K. Obesity in Pediatric ALL Survivors: A Meta-Analysis. Pediatrics 2014, 133, e704–e715. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.F.; Saltzman, E.; Kelly, M.J.; Liu, S.; Must, A.; Parsons, S.K.; Roberts, S.B. Comparison of Childhood Cancer Survivors’ Nutritional Intake with US Dietary Guidelines. Pediatr. Blood Cancer 2015, 62, 1461–1467. [Google Scholar] [CrossRef] [Green Version]
- Pluimakers, V.G.; van Santen, S.S.; Fiocco, M.; Bakker, M.E.; van der Lelij, A.J.; van den Heuvel-Eibrink, M.M.; Neggers, S.J.C.M.M. Can Biomarkers Be Used to Improve Diagnosis and Prediction of Metabolic Syndrome in Childhood Cancer Survivors? A Systematic Review. Obes. Rev. 2021, 22, e13312. [Google Scholar] [CrossRef]
- Vejrazkova, D.; Vankova, M.; Lukasova, P.; Vcelak, J.; Bendlova, B. Insights Into the Physiology of C-Peptide. Physiol. Res. 2020, 69 (Suppl. 2), S237–S243. [Google Scholar] [CrossRef]
- Alamri, B.N.; Shin, K.; Chappe, V.; Anini, Y. The Role of Ghrelin in the Regulation of Glucose Homeostasis. Horm. Mol. Biol. Clin. Investig. 2016, 26, 3–11. [Google Scholar] [CrossRef]
- Kolb, H.; Kempf, K.; Röhling, M.; Martin, S. Insulin: Too Much of a Good Thing Is Bad. BMC Med. 2020, 18, 224. [Google Scholar] [CrossRef] [PubMed]
- Kulina, G.R.; Rayfield, E.J. The role of glucagon in the pathophysiology and management of diabetes. Endocr. Pract. 2016, 22, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Pfeiffer, A.F.H. The Evolving Story of Incretins (GIP and GLP-1) in Metabolic and Cardiovascular Disease: A Pathophysiological Update. Diabetes Obes. Metab. 2021, 23 (Suppl. 3), 5–29. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Orlando, G.; Ferrante, C.; Chiavaroli, A.; Brunetti, L.; Leone, S. Adipokines: New Potential Therapeutic Target for Obesity and Metabolic, Rheumatic, and Cardiovascular Diseases. Front. Physiol. 2020, 11, 578966. [Google Scholar] [CrossRef] [PubMed]
- Atawia, R.T.; Bunch, K.L.; Toque, H.A.; Caldwell, R.B.; Caldwell, R.W. Mechanisms of Obesity-Induced Metabolic and Vascular Dysfunctions. Front. Biosci. (Landmark Ed.) 2019, 24, 890–934. [Google Scholar]
- Park, H.K.; Kwak, M.K.; Kim, H.J.; Ahima, R.S. Linking Resistin, Inflammation, and Cardiometabolic Diseases. Korean J. Intern. Med. 2017, 32, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.E.; Howell, C.R.; Chemaitilly, W.; Wilson, C.L.; Karol, S.E.; Nolan, V.; Smeltzer, M.P.; Green, D.M.; Ehrhardt, M.J.; Mulrooney, D.A.; et al. Diabetes Mellitus among Adult Survivors of Childhood Acute Lymphoblastic Leukemia: A Report from the St. Jude Lifetime Cohort Study. Cancer 2020, 126, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Cabrera de León, A.; Oliva García, J.G.; Marcelino Rodríguez, I.; Almeida González, D.; Alemán Sánchez, J.J.; Brito Díaz, B.; Domínguez Coello, S.; Bertomeu Martínez, V.; Aguirre Jaime, A.; Rodríguez Pérez, M. C-Peptide as a Risk Factor of Coronary Artery Disease in the General Population. Diabetes Vasc. Dis. Res. 2015, 12, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Morel, S.; Léveillé, P.; Samoilenko, M.; Franco, A.; England, J.; Malaquin, N.; Tu, V.; Cardin, G.B.; Drouin, S.; Rodier, F.; et al. Biomarkers of Cardiometabolic Complications in Survivors of Childhood Acute Lymphoblastic Leukemia. Sci. Rep. 2020, 10, 21507. [Google Scholar] [CrossRef]
- Manell, H.; Staaf, J.; Manukyan, L.; Kristinsson, H.; Cen, J.; Stenlid, R.; Ciba, I.; Forslund, A.; Bergsten, P. Altered Plasma Levels of Glucagon, GLP-1 and Glicentin During OGTT in Adolescents With Obesity and Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 1181–1189. [Google Scholar] [CrossRef]
- Calanna, S.; Christensen, M.; Holst, J.J.; Laferrère, B.; Gluud, L.L.; Vilsbøll, T.; Knop, F.K. Secretion of Glucose-Dependent Insulinotropic Polypeptide in Patients with Type 2 Diabetes: Systematic Review and Meta-Analysis of Clinical Studies. Diabetes Care 2013, 36, 3346–3352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timper, K.; Grisouard, J.; Sauter, N.S.; Herzog-Radimerski, T.; Dembinski, K.; Peterli, R.; Frey, D.M.; Zulewski, H.; Keller, U.; Müller, B.; et al. Glucose-Dependent Insulinotropic Polypeptide Induces Cytokine Expression, Lipolysis, and Insulin Resistance in Human Adipocytes. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1–E13. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Okahara, F.; Osaki, N.; Shimotoyodome, A. Increased GIP Signaling Induces Adipose Inflammation via a HIF-1α-Dependent Pathway and Impairs Insulin Sensitivity in Mice. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E414–E425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinehr, T.; Sousa, G.D.; Roth, C.L. Obestatin and Ghrelin Levels in Obese Children and Adolescents before and after Reduction of Overweight. Clin. Endocrinol. 2008, 68, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Fittipaldi, A.S.; Hernández, J.; Castrogiovanni, D.; Lufrano, D.; Francesco, P.N.D.; Garrido, V.; Vitaux, P.; Fasano, M.V.; Fehrentz, J.-A.; Fernández, A.; et al. Plasma Levels of Ghrelin, Des-Acyl Ghrelin and LEAP2 in Children with Obesity: Correlation with Age and Insulin Resistance. Eur. J. Endocrinol. 2020, 182, 165–175. [Google Scholar] [CrossRef]
- Sawicka-Żukowska, M.; Krawczuk-Rybak, M.; Muszynska-Roslan, K.; Panasiuk, A.; Latoch, E.; Konstantynowicz, J. Does Q223R Polymorphism of Leptin Receptor Influence on Anthropometric Parameters and Bone Density in Childhood Cancer Survivors? Int. J. Endocrinol. 2013, 2013, 805312. [Google Scholar] [CrossRef]
- Latoch, E.; Muszynska-Roslan, K.; Panas, A.; Panasiuk, A.; Sawicka-Zukowska, M.; Zelazowska-Rutkowska, B.; Zabrocka, E.; Krawczuk-Rybak, M. Adipokines and Insulin Resistance in Young Adult Survivors of Childhood Cancer. Int. J. Endocrinol. 2016, 2016, 6349134. [Google Scholar] [CrossRef] [Green Version]
- Brouwer, C.A.J.; Postma, A.; Hooimeijer, H.L.H.; Smit, A.J.; Vonk, J.M.; van Roon, A.M.; van den Berg, M.P.; Dolsma, W.V.; Lefrandt, J.D.; Bink-Boelkens, M.T.E.; et al. Endothelial Damage in Long-Term Survivors of Childhood Cancer. JCO 2013, 31, 3906–3913. [Google Scholar] [CrossRef] [Green Version]
- Altalhi, R.; Pechlivani, N.; Ajjan, R.A. PAI-1 in Diabetes: Pathophysiology and Role as a Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 3170. [Google Scholar] [CrossRef]
- Vecchiola, A.; García, K.; González-Gómez, L.M.; Tapia-Castillo, A.; Artigas, R.; Baudrand, R.; Kalergis, A.M.; Carvajal, C.A.; Fardella, C.E. Plasminogen Activator Inhibitor-1 and Adiponectin Are Associated With Metabolic Syndrome Components. Am. J. Hypertens. 2021, No. hpab138. [Google Scholar] [CrossRef]
- Siviero-Miachon, A.A.; Spinola-Castro, A.M.; Andreoni, S.; de Martino Lee, M.L.; Calixto, A.R.; Geloneze, B.; Guerra-Junior, G. Adipokines in Young Survivors of Childhood Acute Lymphocytic Leukemia Revisited: Beyond Fat Mass. Ann. Pediatr. Endocrinol. Metab. 2020, 25, 174–181. [Google Scholar] [CrossRef]
- Sandeep, S.; Velmurugan, K.; Deepa, R.; Mohan, V. Serum Visfatin in Relation to Visceral Fat, Obesity, and Type 2 Diabetes Mellitus in Asian Indians. Metabolism 2007, 56, 565–570. [Google Scholar] [CrossRef]
- Yin, C.; Hu, W.; Wang, M.; Xiao, Y. The Role of the Adipocytokines Vaspin and Visfatin in Vascular Endothelial Function and Insulin Resistance in Obese Children. BMC Endocr. Disord. 2019, 19, 127. [Google Scholar] [CrossRef]
- Moschen, A.R.; Kaser, A.; Enrich, B.; Mosheimer, B.; Theurl, M.; Niederegger, H.; Tilg, H. Visfatin, an Adipocytokine with Proinflammatory and Immunomodulating Properties. J. Immunol. 2007, 178, 1748–1758. [Google Scholar] [CrossRef] [Green Version]
- Friedman, D.N.; Tonorezos, E.S.; Cohen, P. Diabetes and Metabolic Syndrome in Survivors of Childhood Cancer. Horm. Res. Paediatr. 2019, 91, 118–127. [Google Scholar] [CrossRef]
- Kułaga, Z.; Litwin, M.; Tkaczyk, M.; Palczewska, I.; Zajączkowska, M.; Zwolińska, D.; Krynicki, T.; Wasilewska, A.; Moczulska, A.; Morawiec-Knysak, A.; et al. Polish 2010 growth references for school-aged children and adolescents. Eur. J. Pediatr. 2011, 170, 599–609. [Google Scholar] [CrossRef] [Green Version]
- Kułaga, Z.; Grajda, A.; Gurzkowska, B.; Góźdź, M.; Wojtyło, M.; Świąder, A.; Różdżyńska-Świątkowska, A.; Litwin, M. Polish 2012 growth references for preschool children. Eur. J. Pediatr. 2013, 172, 753–761. [Google Scholar] [CrossRef] [Green Version]
- Kułaga, Z.; Litwin, M.; Grajda, A.; Gurzkowska, B.; Napieralska, E.; Kułaga, K.; Grupa Badaczy, O.L.A.F. Distribution of blood pressure in school-aged children and adolescents reference population. Stand. Med. 2010, 7, 853–864. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Zimmet, P.; Alberti, K.G.M.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S. The metabolic syndrome in children and adolescents—an IDF consensus report. Pediatric Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef]
| Biomarker | Function |
|---|---|
| C-peptide |
|
| Ghrelin |
|
| Gastric inhibitory peptide (GIP) |
|
| Glucagon |
|
| Insulin |
|
| Plasminogen activator inhibitor-1 (PAI-1) |
|
| Resistin |
|
| Leptin |
|
| Visfatin |
|
| Study Group | ||
|---|---|---|
| Number (%) a n = 56 | Mean ± SD b | |
| Male | 26 (46.4) | |
| Female | 30 (53.6) | |
| Age at diagnosis (years) | 5.01 ± 3.46 | |
| Age at study (years) | 12.36 ± 5.15 | |
| Follow-up after treatment (years) | 6.58 ± 4.64 | |
| Chemotherapy | ||
| Methotrexate (cumulative dose (mg/m2)) | 10,321.43 ± 6644.50 | |
| Cumulative corticosteroid dose (mg/m2) c | 3538.05 ± 901.85 | |
| Prednisone (cumulative dose (mg/m2)) | 1680.00 ± 0.00 | |
| Dexamethasone (cumulative dose (mg/m2)) | 277.32 ± 134.60 | |
| Cyclophosphamide (cumulative dose (mg/m2)) | 3957.14 ± 2632.93 | |
| Anthracycline (cumulative dose (mg/m2)) | 225.00 ± 45.41 | |
| Radiotherapy | 9 (16.1) | |
| Cranial radiotherapy (CRT) (cumulative dose (Gy)) | 8 (14.3) | 12.75 ± 2.12 |
| Total body irradiation (TBI) | 2 (3.6) | 12 ± 0.00 |
| No | 47 (83.9) | |
| HSCT | 6 (10.7) | |
| Metabolic derangements | ||
| 1 Metabolic risk factor | 21 (37.5) | |
| 2 Metabolic risk factors | 7 (12.5) | |
| 3 Metabolic risk factors | 4 (7.1) | |
| 4 Metabolic risk factors | 1 (1.8) | |
| ALL Survivors n = 56 | Control Group n = 22 | p Value | |
|---|---|---|---|
| C-peptide (pg/mL) | 611.08 (332.59; 962.57) | 479.47 (268.64; 799.61) | 0.386 |
| Ghrelin (pg/mL) | 224.07 (161.76; 356.32) | 634.33 (377.65; 1070.13) | <0.001 |
| GIP (pg/mL) | 1050.12 (592.44; 1479.55) | 417.67 (280.34; 741.02) | 0.026 |
| Glucagon (pg/mL) | 394.94 (234.92; 612.39) | 237.62 (140.22; 324.11) | 0.001 |
| Insulin (pg/mL) | 530.18 (296.77; 964.83) | 377.87 (140.28; 631.33) | 0.158 |
| Leptin (pg/dL) | 5219.36 (1329.38; 12551.94) | 1846.23 (765.72; 3361.22) | 0.022 |
| PAI-1 (pg/mL) | 4914.04 (3638.52; 6040.11) | 3936.78 (3091.16; 4900.93) | 0.047 |
| Resistin (pg/mL) | 8448.39 (4983.02; 14698.13) | 7420.48 (4239.87; 12889.74) | 0.429 |
| Visfatin (pg/mL) | 1032.53 (689.50; 2632.74) | 133.00 (55.58; 1296.86) | 0.066 |
| ≥1 MetS Risk Factor n = 33 | Control Group n = 22 | p Value | |
|---|---|---|---|
| C-peptide (pg/mL) | 792.42 (444.15; 1046.09) | 475.47 (268.64; 799.61) | 0.094 |
| Ghrelin (pg/mL) | 220.49 (183.58; 351.21) | 634.33 (377.65; 1070.13) | <0.001 |
| GIP (pg/mL) | 1151.39 (592.44; 1731.89) | 417.67 (280.34; 741.02) | 0.030 |
| Glucagon (pg/mL) | 400.89 (269.81; 608.71) | 237.62 (140.22; 324.11) | <0.001 |
| Insulin (pg/dL) | 588.46 (365.81; 974.98) | 377.87 (140.28; 631.33) | 0.071 |
| Leptin (pg/mL) | 6999.47 (3347.83; 16,562.53) | 1846.23 (765.72; 3361.22) | 0.001 |
| PAI-1 (pg/mL) | 5305.50 (3814.72; 6898.52) | 3936.78 (3091.16; 4900.93) | 0.009 |
| Resistin (pg/mL) | 10,585.10 (5861.14; 15,774.47) | 7420.48 (4239.87; 12,889.74) | 0.129 |
| Visfatin (pg/mL) | 1285.36 (827.91; 3103.90) | 133.00 (55.58; 1296.86) | 0.068 |
| ≥2 MetS risk factor n = 12 | Control Group n = 22 | pValue | |
| C-peptide (pg/mL) | 936.48 (591.03; 1546.72) | 475.47 (268.64; 799.61) | 0.008 |
| Ghrelin (pg/mL) | 216.77 (193.13; 286.35) | 634.33 (377.65; 1070.13) | 0.001 |
| GIP (pg/mL) | 704.05 (438.26; 1403.35) | 417.67 (280.34; 741.02) | 0.138 |
| Glucagon (pg/mL) | 445.20 (355.39; 765.60) | 237.62 (140.22; 324.11) | <0.001 |
| Insulin (pg/dL) | 967.72 (454.60; 1729.53) | 377.87 (140.28; 631.33) | 0.020 |
| Leptin (pg/mL) | 10,981.25 (5654.11; 16,772.59) | 1846.23 (765.72; 3361.22) | <0.001 |
| PAI-1 (pg/mL) | 5757.94 (4203.10; 7416.83) | 3936.78 (3091.16; 4900.93) | 0.001 |
| Resistin (pg/mL) | 12,026.93 (5382.83; 16,882.36) | 7420.48 (4239.87; 12,889.74) | 0.299 |
| Visfatin (pg/mL) | 1016.43 (971.42; 1760.47) | 133.00 (55.58; 1296.86) | 0.240 |
| ≥1 MetS Risk Factor n = 33 | No MetS Risk Factors n = 23 | p Value | |
|---|---|---|---|
| C-peptide (pg/mL) | 792.42 (444.15; 1046.09) | 419.15 (258.64; 727.40) | 0.028 |
| Ghrelin (pg/mL) | 220.49 (183.58; 351.21) | 225.45 (118.91; 360.40) | 0.817 |
| GIP (pg/mL) | 1151.39 (592.44; 1731.89) | 1031.18 (605.88; 1093.86) | 0.409 |
| Glucagon (pg/mL) | 400.89 (269.81; 608.71) | 319.44 (174.91; 616.06) | 0.220 |
| Insulin (pg/dL) | 588.46 (365.81; 974.98) | 386.98 (201.06; 814.23) | 0.136 |
| Leptin (pg/mL) | 6999.47 (3347.83; 16562.53) | 3613.77 (664.69; 6269.79) | 0.003 |
| PAI-1 (pg/mL) | 5305.50 (3814.72; 6898.52) | 4478.74 (3409.32; 5383.39) | 0.034 |
| Resistin (pg/mL) | 10,585.10 (5861.14; 15774.47) | 6608.98 (3902.95; 12059.42) | 0.059 |
| Visfatin (pg/mL) | 1285.36 (827.91; 3103.90) | 729.03 (326.13; 2250.56) | 0.253 |
| ≥2 MetS risk factor n = 12 | No MetS risk factors n = 23 | pvalue | |
| C-peptide (pg/mL) | 936.48 (591.03; 1546.72) | 419.15 (258.64; 727.40) | 0.002 |
| Ghrelin (pg/mL) | 216.77 (193.13; 286.35) | 225.45 (118.91; 360.40) | 0.959 |
| GIP (pg/mL) | 704.05 (438.26; 1403.35) | 1031.18 (605.88; 1093.86) | 0.788 |
| Glucagon (pg/mL) | 445.20 (355.39; 765.60) | 319.44 (174.91; 616.06) | 0.263 |
| Insulin (pg/dL) | 967.72 (454.60; 1729.53) | 386.98 (201.06; 814.23) | 0.021 |
| Leptin (pg/mL) | 10,981.25 (5654.11; 16772.59) | 3613.77 (664.69; 6269.79) | <0.001 |
| PAI-1 (pg/mL) | 5757.94 (4203.10; 7416.83) | 4478.74 (3409.32; 5383.39) | 0.031 |
| Resistin (pg/mL) | 12,026.93 (5382.83; 16882.36) | 6608.98 (3902.95; 12059.42) | 0.176 |
| Visfatin (pg/mL) | 1016.43 (971.42; 1760.47) | 729.03 (326.13; 2250.56) | 0.408 |
| AUC | 95% Cl | |
|---|---|---|
| C-peptide (pg/mL) | 0.733 | (0.596–0.871) |
| Ghrelin (pg/mL) | 0.473 | (0.317–0.630) |
| GIP (pg/mL) | 0.638 | (0.377–0.899) |
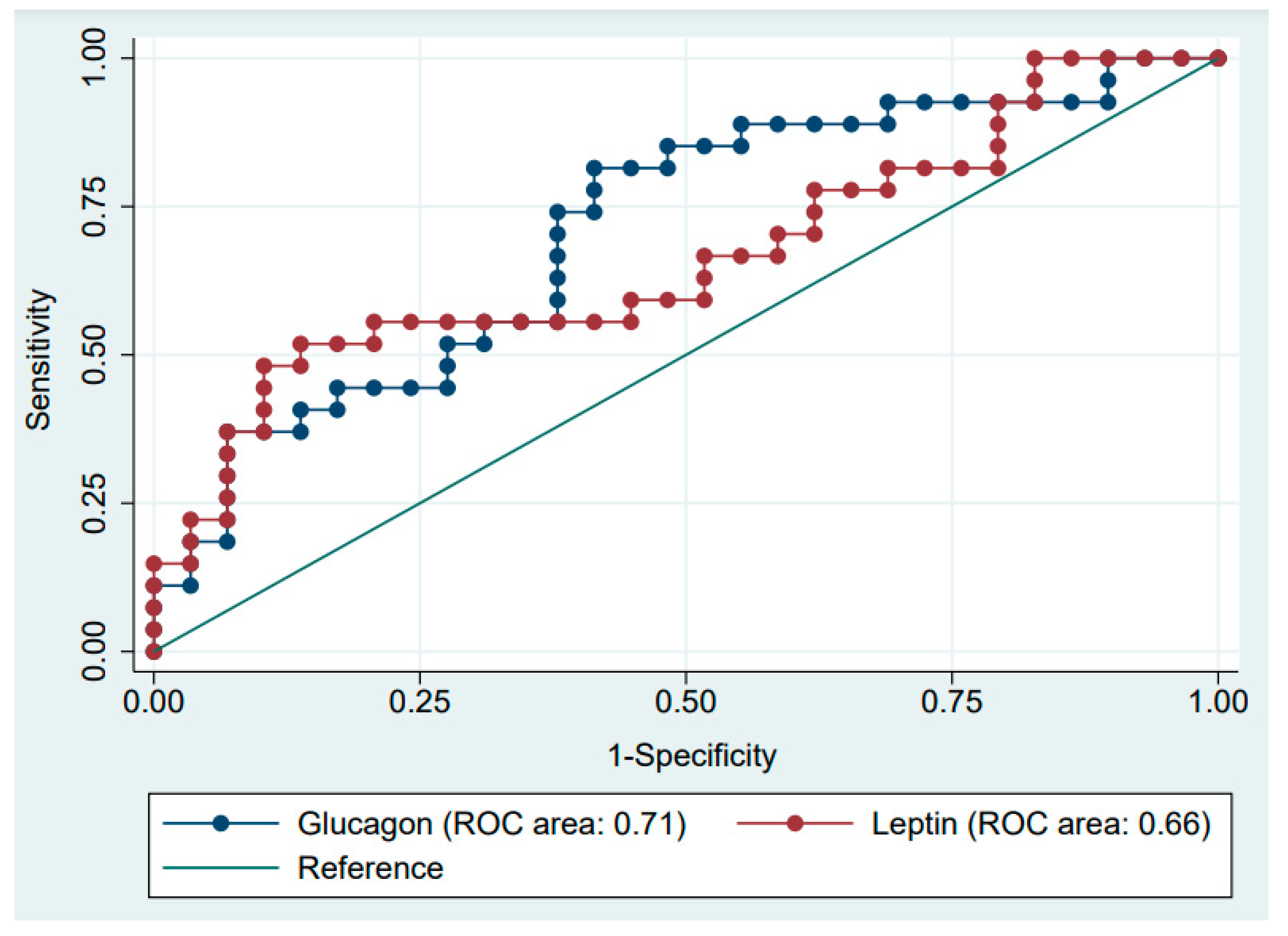
| Glucagon (pg/mL) | 0.642 | (0.490–0.795) |
| Insulin (pg/dL) | 0.675 | (0.527–0.823) |
| Leptin (pg/mL) | 0.797 | (0.684–0.911) |
| PAI-1 (pg/mL) | 0.685 | (0.542–0.828) |
| Resistin (pg/mL) | 0.676 | (0.531–0.822) |
| Visfatin (pg/mL) | 0.649 | (0.429–0.868) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konończuk, K.; Muszyńska-Rosłan, K.; Konstantynowicz-Nowicka, K.; Krawczuk-Rybak, M.; Chabowski, A.; Latoch, E. Biomarkers of Glucose Metabolism Alterations and the Onset of Metabolic Syndrome in Survivors of Childhood Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2022, 23, 3712. https://doi.org/10.3390/ijms23073712
Konończuk K, Muszyńska-Rosłan K, Konstantynowicz-Nowicka K, Krawczuk-Rybak M, Chabowski A, Latoch E. Biomarkers of Glucose Metabolism Alterations and the Onset of Metabolic Syndrome in Survivors of Childhood Acute Lymphoblastic Leukemia. International Journal of Molecular Sciences. 2022; 23(7):3712. https://doi.org/10.3390/ijms23073712
Chicago/Turabian StyleKonończuk, Katarzyna, Katarzyna Muszyńska-Rosłan, Karolina Konstantynowicz-Nowicka, Maryna Krawczuk-Rybak, Adrian Chabowski, and Eryk Latoch. 2022. "Biomarkers of Glucose Metabolism Alterations and the Onset of Metabolic Syndrome in Survivors of Childhood Acute Lymphoblastic Leukemia" International Journal of Molecular Sciences 23, no. 7: 3712. https://doi.org/10.3390/ijms23073712






