Macrophage Function Modulated by tPA Signaling in Mouse Experimental Kidney Disease Models
Abstract
:1. Introduction
2. tPA Structure and Dual Functions of Protease and Cytokine
3. Renal Origin and Distribution of tPA after Kidney Injury
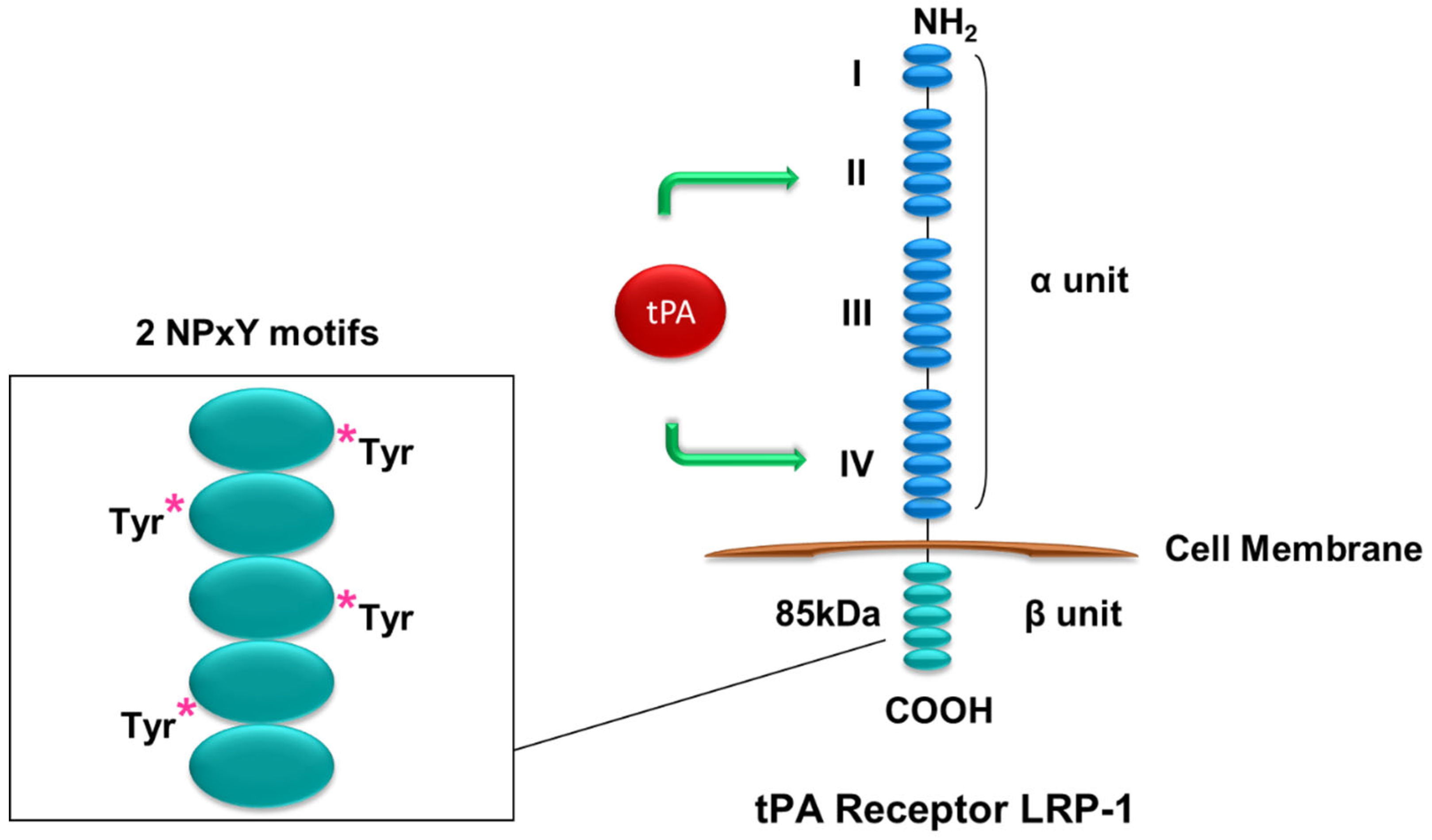
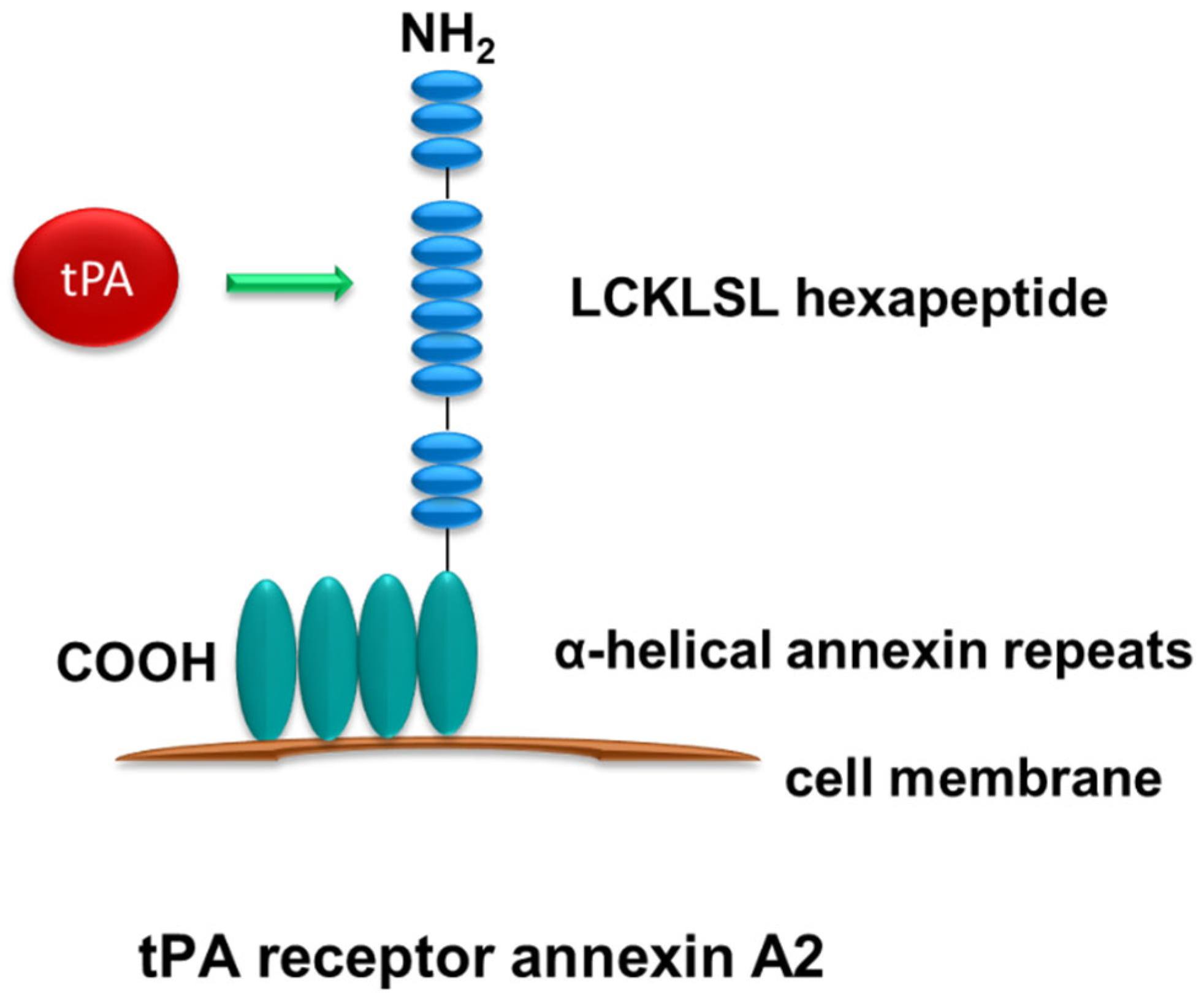
4. Renal Receptors of tPA Signaling
5. tPA and NF-kB Signaling in Renal Inflammation
6. tPA and Macrophage Motility in Kidney Disease
7. tPA and Macrophage Phenotypic Switch in Kidney Disease
8. tPA and Macrophage Survival in Renal Injury
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geissmann, F.; Manz, M.G.; Jung, S.; Sieweke, M.H.; Merad, M.; Ley, K. Development of monocytes, macrophages, and dendritic cells. Science 2010, 327, 656–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef] [Green Version]
- Ricardo, S.D.; van Goor, H.; Eddy, A.A. Macrophage diversity in renal injury and repair. J. Clin. Investig. 2008, 118, 3522–3530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Hu, K. Tissue-type plasminogen activator modulates macrophage M2 to M1 phenotypic change through annexin A2-mediated NF-kappaB pathway. Oncotarget 2017, 8, 88094–88103. [Google Scholar] [CrossRef] [Green Version]
- Ferrario, F.; Castiglione, A.; Colasanti, G.; Barbiano di Belgioioso, G.; Bertoli, S.; D’Amico, G. The detection of monocytes in human glomerulonephritis. Kidney Int. 1985, 28, 513–519. [Google Scholar] [CrossRef] [Green Version]
- Eardley, K.S.; Kubal, C.; Zehnder, D.; Quinkler, M.; Lepenies, J.; Savage, C.O.; Howie, A.J.; Kaur, K.; Cooper, M.S.; Adu, D.; et al. The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int. 2008, 74, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Hu, K.; Mars, W.M.; Liu, Y. Novel actions of tissue-type plasminogen activator in chronic kidney disease. Front. Biosci. 2008, 13, 5174–5186. [Google Scholar] [CrossRef] [Green Version]
- Vivien, D.; Gauberti, M.; Montagne, A.; Defer, G.; Touze, E. Impact of tissue plasminogen activator on the neurovascular unit: From clinical data to experimental evidence. J. Cereb. Blood Flow. Metab. 2011, 31, 2119–2134. [Google Scholar] [CrossRef] [Green Version]
- Olson, S.T.; Swanson, R.; Day, D.; Verhamme, I.; Kvassman, J.; Shore, J.D. Resolution of Michaelis complex, acylation, and conformational change steps in the reactions of the serpin, plasminogen activator inhibitor-1, with tissue plasminogen activator and trypsin. Biochemistry 2001, 40, 11742–11756. [Google Scholar] [CrossRef]
- White, S.; Lin, L.; Hu, K. NF-kappaB and tPA Signaling in Kidney and Other Diseases. Cells 2020, 9, 1348. [Google Scholar] [CrossRef]
- Hu, K.; Lin, L.; Tan, X.; Yang, J.; Bu, G.; Mars, W.M.; Liu, Y. tPA protects renal interstitial fibroblasts and myofibroblasts from apoptosis. J. Am. Soc. Nephrol. 2008, 19, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Hu, K.; Wu, C.; Mars, W.M.; Liu, Y. Tissue-type plasminogen activator promotes murine myofibroblast activation through LDL receptor-related protein 1-mediated integrin signaling. J. Clin. Investig. 2007, 117, 3821–3832. [Google Scholar] [CrossRef] [Green Version]
- Hu, K.; Yang, J.; Tanaka, S.; Gonias, S.L.; Mars, W.M.; Liu, Y. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J. Biol. Chem. 2006, 281, 2120–2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Bu, G.; Mars, W.M.; Reeves, W.B.; Tanaka, S.; Hu, K. tPA activates LDL receptor-related protein 1-mediated mitogenic signaling involving the p90RSK and GSK3beta pathway. Am. J. Pathol. 2010, 177, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wu, C.; Hu, K. Tissue Plasminogen Activator Activates NF-kappaB through a Pathway Involving Annexin A2/CD11b and Integrin-Linked Kinase. J. Am. Soc. Nephrol. 2012, 23, 1329–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higazi, A.A.; El-Haj, M.; Melhem, A.; Horani, A.; Pappo, O.; Alvarez, C.E.; Muhanna, N.; Friedman, S.L.; Safadi, R. Immunomodulatory effects of plasminogen activators on hepatic fibrogenesis. Clin. Exp. Immunol. 2008, 152, 163–173. [Google Scholar] [CrossRef]
- Zhang, X.; Polavarapu, R.; She, H.; Mao, Z.; Yepes, M. Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein mediate cerebral ischemia-induced nuclear factor-kappaB pathway activation. Am. J. Pathol. 2007, 171, 1281–1290. [Google Scholar] [CrossRef] [Green Version]
- Huber, D.; Cramer, E.M.; Kaufmann, J.E.; Meda, P.; Masse, J.M.; Kruithof, E.K.; Vischer, U.M. Tissue-type plasminogen activator (t-PA) is stored in Weibel-Palade bodies in human endothelial cells both in vitro and in vivo. Blood 2002, 99, 3637–3645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yepes, M.; Woo, Y.; Martin-Jimenez, C. Plasminogen Activators in Neurovascular and Neurodegenerative Disorders. Int. J. Mol. Sci. 2021, 22, 4380. [Google Scholar] [CrossRef]
- Roelofs, J.J.; Rouschop, K.M.; Leemans, J.C.; Claessen, N.; de Boer, A.M.; Frederiks, W.M.; Lijnen, H.R.; Weening, J.J.; Florquin, S. Tissue-type plasminogen activator modulates inflammatory responses and renal function in ischemia reperfusion injury. J. Am. Soc. Nephrol. 2006, 17, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Shultz, R.W.; Mars, W.M.; Wegner, R.E.; Li, Y.; Dai, C.; Nejak, K.; Liu, Y. Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J. Clin. Investig. 2002, 110, 1525–1538. [Google Scholar] [CrossRef]
- Cao, C.; Lawrence, D.A.; Li, Y.; Von Arnim, C.A.; Herz, J.; Su, E.J.; Makarova, A.; Hyman, B.T.; Strickland, D.K.; Zhang, L. Endocytic receptor LRP together with tPA and PAI-1 coordinates Mac-1-dependent macrophage migration. EMBO J. 2006, 25, 1860–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Jin, Y.; Mars, W.M.; Reeves, W.B.; Hu, K. Myeloid-derived tissue-type plasminogen activator promotes macrophage motility through FAK, Rac1, and NF-kappaB pathways. Am. J. Pathol. 2014, 184, 2757–2767. [Google Scholar] [CrossRef] [PubMed]
- Bu, G.; Williams, S.; Strickland, D.K.; Schwartz, A.L. Low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor is an hepatic receptor for tissue-type plasminogen activator. Proc. Natl. Acad. Sci. USA 1992, 89, 7427–7431. [Google Scholar] [CrossRef]
- Herz, J.; Strickland, D.K. LRP: A multifunctional scavenger and signaling receptor. J. Clin. Investig. 2001, 108, 779–784. [Google Scholar] [CrossRef]
- Hussain, M.M. Structural, biochemical and signaling properties of the low-density lipoprotein receptor gene family. Front. Biosci. 2001, 6, D417–D428. [Google Scholar] [PubMed] [Green Version]
- Strickland, D.K.; Ranganathan, S. Diverse role of LDL receptor-related protein in the clearance of proteases and in signaling. J. Thromb. Haemost. 2003, 1, 1663–1670. [Google Scholar] [CrossRef]
- Lin, L.; Hu, K. Tissue plasminogen activator and inflammation: From phenotype to signaling mechanisms. Am. J. Clin. Exp. Immunol. 2014, 3, 30–36. [Google Scholar]
- Obermoeller-McCormick, L.M.; Li, Y.; Osaka, H.; FitzGerald, D.J.; Schwartz, A.L.; Bu, G. Dissection of receptor folding and ligand-binding property with functional minireceptors of LDL receptor-related protein. J. Cell. Sci. 2001, 114, 899–908. [Google Scholar] [CrossRef]
- Barnes, H.; Ackermann, E.J.; van der Geer, P. v-Src induces Shc binding to tyrosine 63 in the cytoplasmic domain of the LDL receptor-related protein 1. Oncogene 2003, 22, 3589–3597. [Google Scholar] [CrossRef] [Green Version]
- Barnes, H.; Larsen, B.; Tyers, M.; van Der Geer, P. Tyrosine-phosphorylated low density lipoprotein receptor-related protein 1 (Lrp1) associates with the adaptor protein SHC in SRC-transformed cells. J. Biol. Chem. 2001, 276, 19119–19125. [Google Scholar] [CrossRef] [Green Version]
- Strickland, D.K.; Gonias, S.L.; Argraves, W.S. Diverse roles for the LDL receptor family. Trends Endocrinol. Metab. 2002, 13, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Loukinova, E.; Ranganathan, S.; Kuznetsov, S.; Gorlatova, N.; Migliorini, M.M.; Loukinov, D.; Ulery, P.G.; Mikhailenko, I.; Lawrence, D.A.; Strickland, D.K. Platelet-derived growth factor (PDGF)-induced tyrosine phosphorylation of the low density lipoprotein receptor-related protein (LRP). Evidence for integrated co-receptor function betwenn LRP and the PDGF. J. Biol. Chem. 2002, 277, 15499–15506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Hu, K. Annexin A2 and Kidney Diseases. Front. Cell. Dev. Biol. 2022, 10, 974381. [Google Scholar] [CrossRef] [PubMed]
- Siao, C.J.; Tsirka, S.E. Tissue plasminogen activator mediates microglial activation via its finger domain through annexin II. J. Neurosci. 2002, 22, 3352–3358. [Google Scholar] [CrossRef] [PubMed]
- Rescher, U.; Gerke, V. Annexins--unique membrane binding proteins with diverse functions. J. Cell. Sci. 2004, 117, 2631–2639. [Google Scholar] [CrossRef] [Green Version]
- Hajjar, K.A.; Mauri, L.; Jacovina, A.T.; Zhong, F.; Mirza, U.A.; Padovan, J.C.; Chait, B.T. Tissue plasminogen activator binding to the annexin II tail domain. Direct modulation by homocysteine. J. Biol. Chem. 1998, 273, 9987–9993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Hajjar, K.A. Annexin II: A plasminogen-plasminogen activator co-receptor. Front. Biosci. 2002, 7, d341–d348. [Google Scholar] [CrossRef]
- Cesarman, G.M.; Guevara, C.A.; Hajjar, K.A. An endothelial cell receptor for plasminogen/tissue plasminogen activator (t-PA). II. Annexin II-mediated enhancement of t-PA-dependent plasminogen activation. J. Biol. Chem. 1994, 269, 21198–21203. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Zapater, E.; Peiro, S.; Roda, O.; Corominas, J.M.; Aguilar, S.; Ampurdanes, C.; Real, F.X.; Navarro, P. Tissue plasminogen activator induces pancreatic cancer cell proliferation by a non-catalytic mechanism that requires extracellular signal-regulated kinase 1/2 activation through epidermal growth factor receptor and annexin A2. Am. J. Pathol. 2007, 170, 1573–1584. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Ownbey, R.T.; Sharma, M.C. Breast cancer cell surface annexin II induces cell migration and neoangiogenesis via tPA dependent plasmin generation. Exp. Mol. Pathol. 2010, 88, 278–286. [Google Scholar] [CrossRef]
- Paciucci, R.; Tora, M.; Diaz, V.M.; Real, F.X. The plasminogen activator system in pancreas cancer: Role of t-PA in the invasive potential in vitro. Oncogene 1998, 16, 625–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonizzi, G.; Karin, M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; Sanchez-Nino, M.D.; Ramos, A.M.; Moreno, J.A.; Santamaria, B.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A. NF-kappaB in renal inflammation. J. Am. Soc. Nephrol. 2010, 21, 1254–1262. [Google Scholar] [CrossRef] [Green Version]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Sterner, R.M.; Hartono, S.P.; Grande, J.P. The Pathogenesis of Lupus Nephritis. J. Clin. Cell. Immunol. 2014, 5, 1357–1366. [Google Scholar]
- Kiryluk, K.; Novak, J. The genetics and immunobiology of IgA nephropathy. J. Clin. Investig. 2014, 124, 2325–2332. [Google Scholar] [CrossRef] [Green Version]
- Guijarro, C.; Egido, J. Transcription factor-kappa B (NF-kappa B) and renal disease. Kidney Int. 2001, 59, 415–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Sun, S.C. NF-kappaB in inflammation and renal diseases. Cell. Biosci. 2015, 5, 63. [Google Scholar] [CrossRef] [Green Version]
- Morrissey, J.; Klahr, S. Transcription factor NF-kappaB regulation of renal fibrosis during ureteral obstruction. Semin. Nephrol. 1998, 18, 603–611. [Google Scholar]
- Volpini, R.A.; Costa, R.S.; da Silva, C.G.; Coimbra, T.M. Inhibition of nuclear factor-kappaB activation attenuates tubulointerstitial nephritis induced by gentamicin. Nephron Physiol. 2004, 98, p97–p106. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, C.K.; Antunes, G.R.; Mattar, A.L.; Malheiros, D.M.; Vieira, J.M., Jr.; Zatz, R. Chronic inhibition of nuclear factor-kappaB attenuates renal injury in the 5/6 renal ablation model. Am. J. Physiol. Ren. Physiol. 2007, 292, F92–F99. [Google Scholar] [CrossRef] [PubMed]
- Gonias, S.L. Plasminogen activator receptor assemblies in cell signaling, innate immunity, and inflammation. Am. J. Physiol. Cell. Physiol. 2021, 321, C721–C734. [Google Scholar] [CrossRef]
- Bliss, T.V.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef]
- Mantuano, E.; Azmoon, P.; Brifault, C.; Banki, M.A.; Gilder, A.S.; Campana, W.M.; Gonias, S.L. Tissue-type plasminogen activator regulates macrophage activation and innate immunity. Blood 2017, 130, 1364–1374. [Google Scholar] [CrossRef] [Green Version]
- Das, L.; Azmoon, P.; Banki, M.A.; Mantuano, E.; Gonias, S.L. Tissue-type plasminogen activator selectively inhibits multiple toll-like receptors in CSF-1-differentiated macrophages. PLoS ONE 2019, 14, e0224738. [Google Scholar] [CrossRef]
- Liot, G.; Roussel, B.D.; Lebeurrier, N.; Benchenane, K.; Lopez-Atalaya, J.P.; Vivien, D.; Ali, C. Tissue-type plasminogen activator rescues neurones from serum deprivation-induced apoptosis through a mechanism independent of its proteolytic activity. J. Neurochem. 2006, 98, 1458–1464. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.L.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Guan, J.L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug. Deliv. Rev. 2011, 63, 610–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobes, C.D.; Hall, A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell. Biol. 1999, 144, 1235–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaller, M.D. Cellular functions of FAK kinases: Insight into molecular mechanisms and novel functions. J. Cell. Sci. 2010, 123, 1007–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, C.E.; Brown, M.C.; Perrotta, J.A.; Riedy, M.C.; Nikolopoulos, S.N.; McDonald, A.R.; Bagrodia, S.; Thomas, S.; Leventhal, P.S. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: A role in cytoskeletal remodeling. J. Cell. Biol. 1999, 145, 851–863. [Google Scholar] [CrossRef] [Green Version]
- Frank, S.R.; Hansen, S.H. The PIX-GIT complex: A G protein signaling cassette in control of cell shape. Semin. Cell. Dev. Biol. 2008, 19, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Chang, F.; Lemmon, C.A.; Park, D.; Romer, L.H. FAK potentiates Rac1 activation and localization to matrix adhesion sites: A role for betaPIX. Mol. Biol. Cell. 2007, 18, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Huen, S.; Nishio, H.; Nishio, S.; Lee, H.K.; Choi, B.S.; Ruhrberg, C.; Cantley, L.G. Distinct macrophage phenotypes contribute to kidney injury and repair. J. Am. Soc. Nephrol. 2011, 22, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Braga, T.T.; Correa-Costa, M.; Azevedo, H.; Silva, R.C.; Cruz, M.C.; Almeida, M.E.; Hiyane, M.I.; Moreira-Filho, C.A.; Santos, M.F.; Perez, K.R.; et al. Early infiltration of p40IL12(+)CCR7(+)CD11b(+) cells is critical for fibrosis development. Immun. Inflamm. Dis. 2016, 4, 300–314. [Google Scholar] [CrossRef] [Green Version]
- Tang, P.M.; Nikolic-Paterson, D.J.; Lan, H.Y. Macrophages: Versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 2019, 15, 144–158. [Google Scholar] [CrossRef]
- Chen, T.; Cao, Q.; Wang, Y.; Harris, D.C.H. M2 macrophages in kidney disease: Biology, therapies, and perspectives. Kidney Int. 2019, 95, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Jin, Y.; Hu, K. Tissue-type plasminogen activator (tPA) promotes M1 macrophage survival through p90 ribosomal S6 kinase (RSK) and p38 mitogen-activated protein kinase (MAPK) pathway. J. Biol. Chem. 2015, 290, 7910–7917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.Z.; Yao, B.; Yang, S.; Jiang, L.; Wang, S.; Fan, X.; Yin, H.; Wong, K.; Miyazawa, T.; Chen, J.; et al. CSF-1 signaling mediates recovery from acute kidney injury. J. Clin. Investig. 2012, 122, 4519–4532. [Google Scholar] [CrossRef] [Green Version]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef]
- Steinberg, B.E.; Grinstein, S. Pathogen destruction versus intracellular survival: The role of lipids as phagosomal fate determinants. J. Clin. Investig. 2008, 118, 2002–2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marriott, H.M.; Bingle, C.D.; Read, R.C.; Braley, K.E.; Kroemer, G.; Hellewell, P.G.; Craig, R.W.; Whyte, M.K.; Dockrell, D.H. Dynamic changes in Mcl-1 expression regulate macrophage viability or commitment to apoptosis during bacterial clearance. J. Clin. Investig. 2005, 115, 359–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, R.; Blenis, J. The RSK family of kinases: Emerging roles in cellular signalling. Nat. Rev. 2008, 9, 747–758. [Google Scholar] [CrossRef]
- Romeo, Y.; Zhang, X.; Roux, P.P. Regulation and function of the RSK family of protein kinases. Biochem. J. 2012, 441, 553–569. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; White, S.A.; Hu, K. Role of p90RSK in Kidney and Other Diseases. Int. J. Mol. Sci. 2019, 20, 972. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Hu, K. Macrophage Function Modulated by tPA Signaling in Mouse Experimental Kidney Disease Models. Int. J. Mol. Sci. 2023, 24, 11067. https://doi.org/10.3390/ijms241311067
Lin L, Hu K. Macrophage Function Modulated by tPA Signaling in Mouse Experimental Kidney Disease Models. International Journal of Molecular Sciences. 2023; 24(13):11067. https://doi.org/10.3390/ijms241311067
Chicago/Turabian StyleLin, Ling, and Kebin Hu. 2023. "Macrophage Function Modulated by tPA Signaling in Mouse Experimental Kidney Disease Models" International Journal of Molecular Sciences 24, no. 13: 11067. https://doi.org/10.3390/ijms241311067





