A Novel Mechanically Robust and Biodegradable Egg White Hydrogel Membrane by Combined Unidirectional Nanopore Dehydration and Annealing
Abstract
:1. Introduction
2. Results
2.1. Preparation of EWG and the Effect of Temperature and Humidity
2.2. Effect of Annealing Mode on Mechanical Properties of EWHM
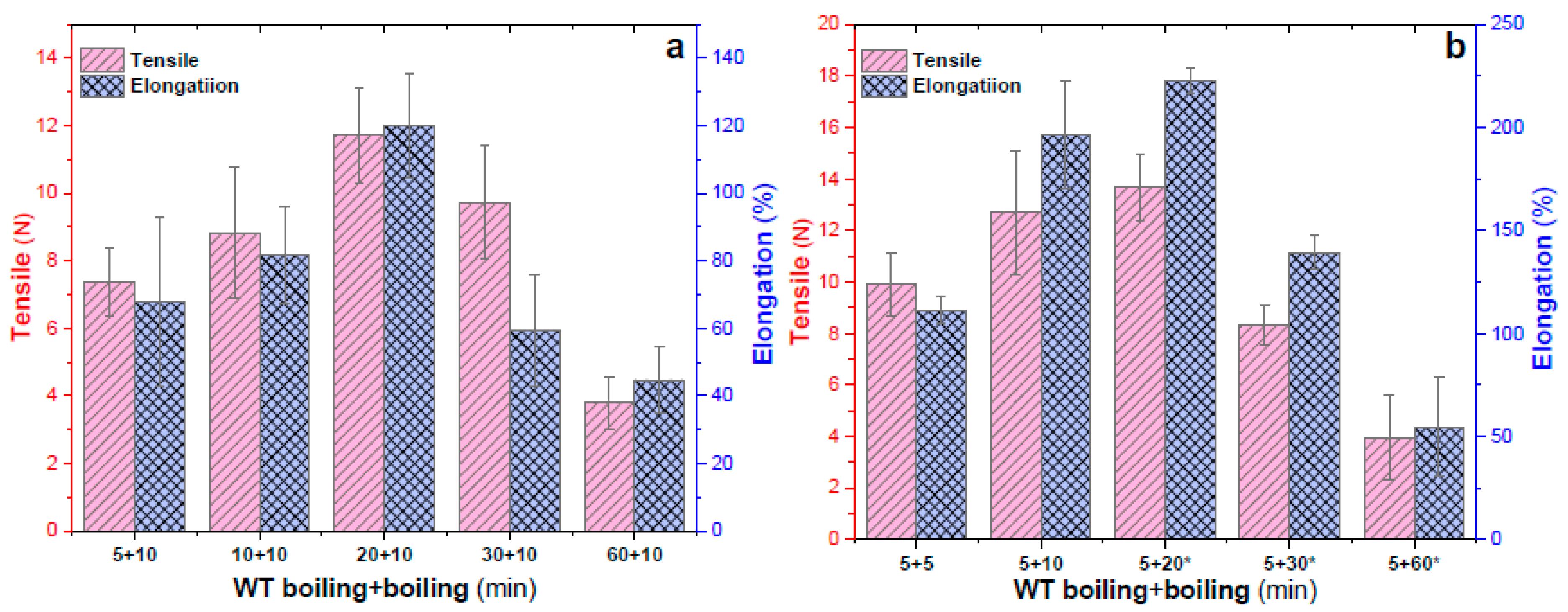
2.3. Effects of Alternating Water Boiling and Watertight Boiling on Mechanical Properties and Transparency of EWHM
2.4. Effect of High-Temperature Oven-Heating Treatment on Tensile and Swelling Properties
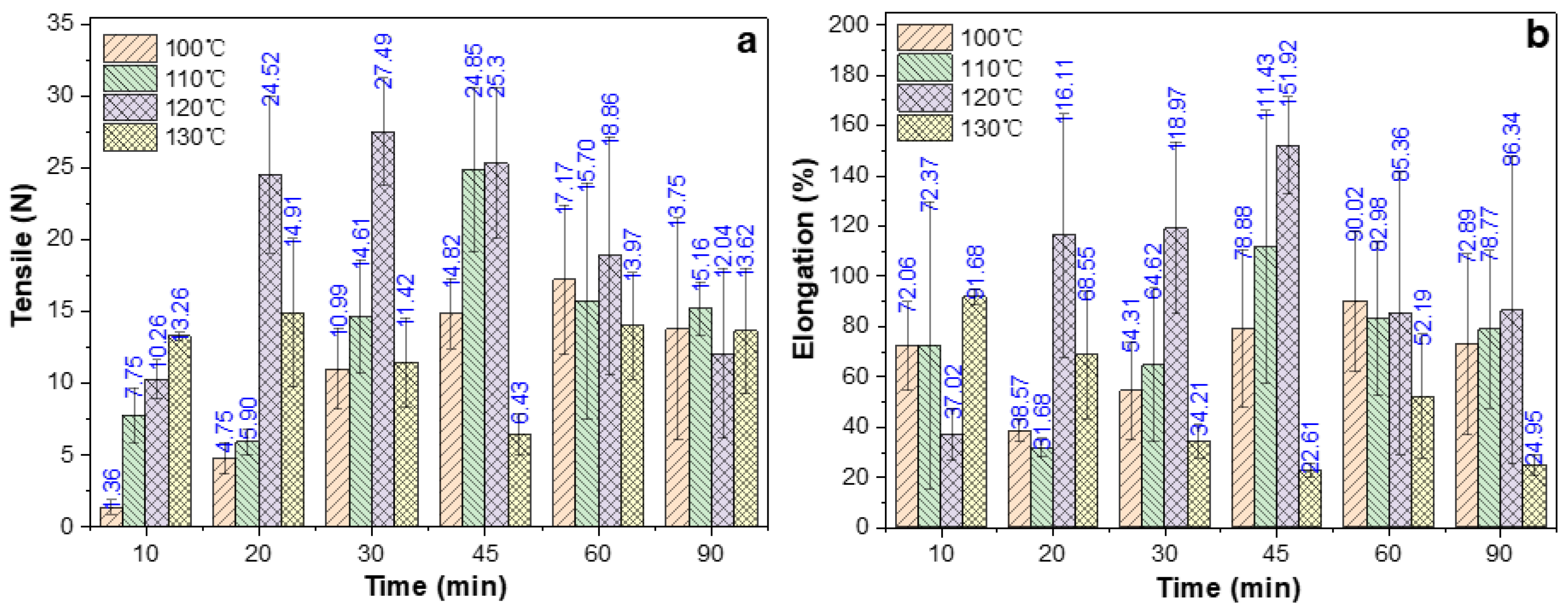
2.5. Effect of Oven-Heating Temperature and Time on Tensile Properties
2.6. Optical Properties of EWG and EWHM
2.7. Stress and Strain Curves of EWHMs
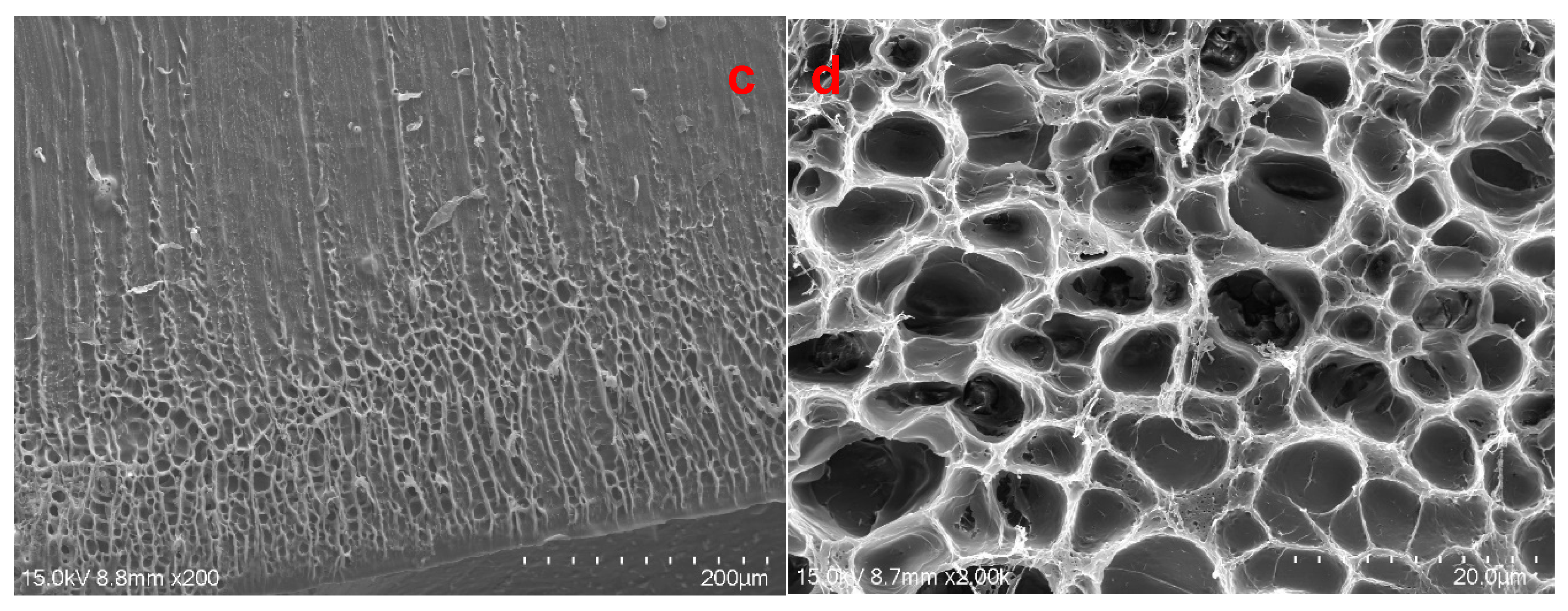
2.8. SEM of the Cross-Section of EWHMs
2.9. Thermal Analysis
2.10. FTIR Spectra
2.11. XRD Spectra
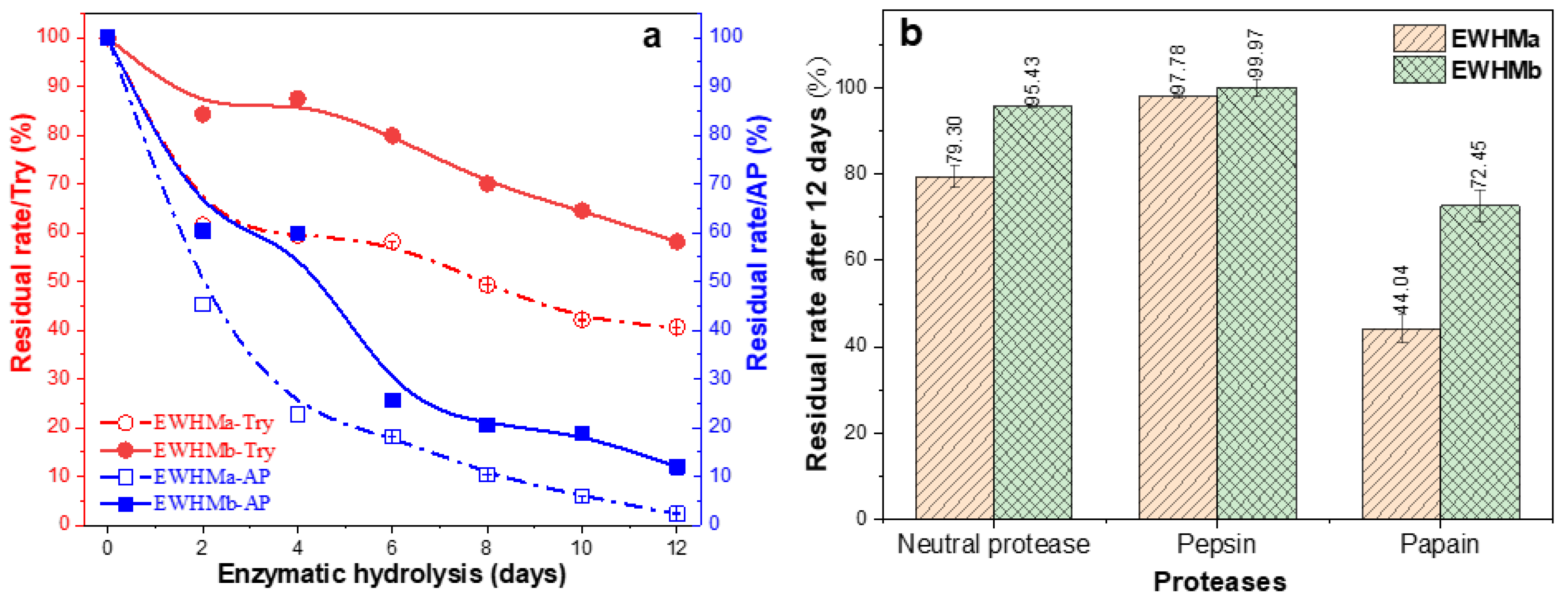
2.12. Biodegradation In Vitro
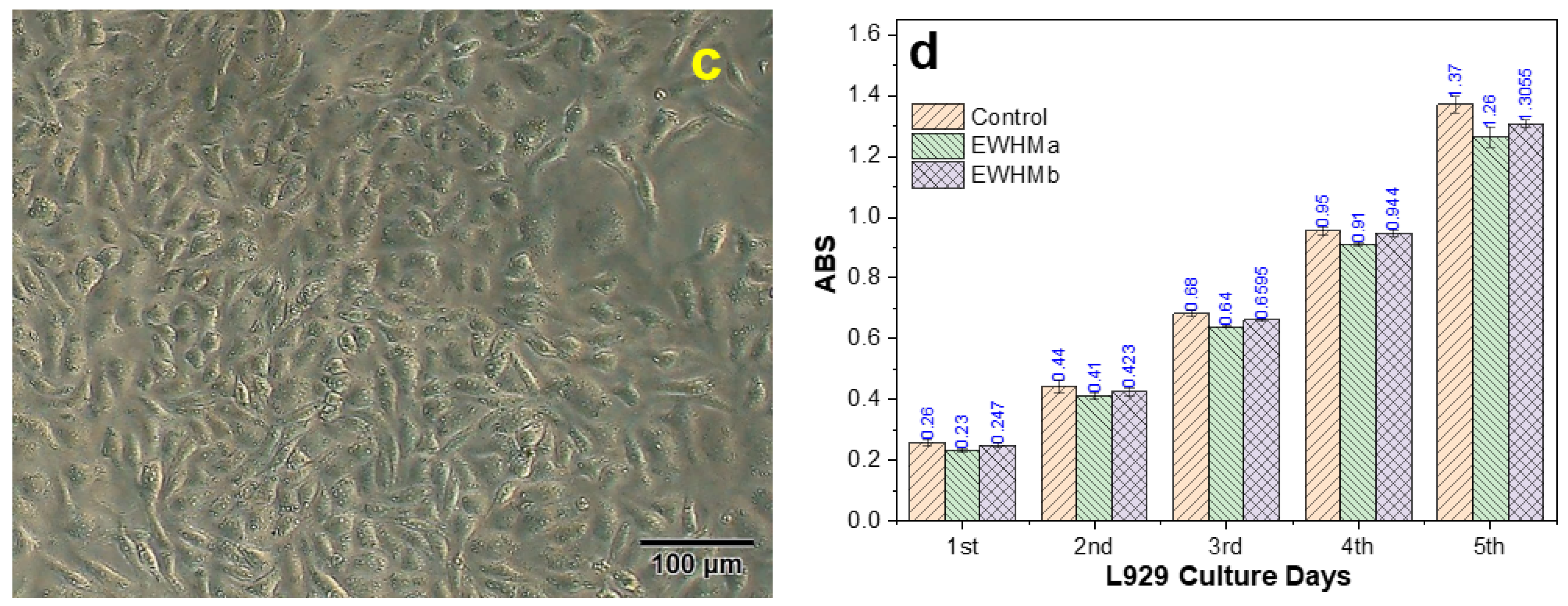
2.13. L929 Growth and Proliferation on EWHMs
3. Discussion
4. Materials and Methods
4.1. EW Homogenization
4.2. Preparation of Egg White Glass (EWG)
4.3. Preparation of EW Hydrogel Membrane (EWHM)
4.4. Determination of Mechanical Properties and Swelling Ratio
4.5. Determination of In Vitro Degradation Rate and Cell Culture
4.6. Thermostabilty, FTIR Spectrum, and XRD Pattern Analysis
4.7. SEM Observation
4.8. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jalili-Firoozinezhad, S.; Filippi, M.; Mohabatpour, F.; Letourneur, D.; Scherberich, A. Chicken egg white: Hatching of a new old biomaterial. Mater. Today 2020, 40, 193–214. [Google Scholar] [CrossRef]
- You, R.; Zhang, J.; Gu, S.; Zhou, Y.; Li, X.; Ye, D.; Xu, W. Regenerated egg white/silk fibroin composite films for biomedical applications. Mater. Sci. Eng. C 2017, 79, 430–435. [Google Scholar] [CrossRef]
- Okuno, K.; Xu, J.; Isogai, E.; Nakamura, S. Salmonella typhimurium is attracted to egg yolk and repelled by albumen. Curr. Microbiol. 2019, 76, 393–397. [Google Scholar] [CrossRef]
- Gennadios, A.; Weller, C.L.; Hanna, M.A.; Froning, G.W. Mechanical and barrier properties of egg albumen films. J. Food Sci. 1996, 61, 585–589. [Google Scholar] [CrossRef]
- Bagheri, S.; Shameli, K.; Bee, S.; Hamid, A. Synthesis and Characterization of Anatase Titanium Dioxide Nanoparticles Using Egg White Solution via Sol-Gel Method. J. Chem. 2013, 2013, 848205. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Zhang, T.; Chen, X.; Fang, K.; Hou, M. The effects of porosity and stiffness of genipin cross-linked egg white simulating aged extracellular matrix on proliferation and aggregation of ovarian cancer cells. Colloids Surf. A. 2017, 520, 649–660. [Google Scholar] [CrossRef]
- Chang, C.; Meikle, T.G.; Su, Y.; Wang, X.; Dekiwadia, C.; Drummond, C.J.; Conn, C.E.; Yang, Y. Encapsulation in egg white protein nanoparticles protects anti-oxidant activity of curcumin. Food Chem. 2019, 280, 65–72. [Google Scholar] [CrossRef]
- Meier, C.; Welland, M.E. Wet-spinning of amyloid protein nanofibers into multifunctional high-performance biofibers. Biomacromolecules 2011, 12, 3453–3459. [Google Scholar] [CrossRef]
- Albohani, S.; Minakshi Sundaram, M.; Laird, D.W. Egg shell membrane template stabilises formation of b-NiMoO4 nanowires and enhances hybrid supercapacitor behaviour. Mater. Lett. 2019, 236, 64–68. [Google Scholar]
- Zhuang, Y.; Xu, Y.; Wang, H.; Wang, L.; Liu, C.; Xu, W. Preparation of pure egg albumen fiber through coaxial wet-spinning. Mater. Lett. 2019, 253, 63–66. [Google Scholar] [CrossRef]
- Chang, J.; Wang, C.; Huang, C.; Tsai, T.; Guo, T. Chicken Albumen Dielectrics in Organic Field-Effect Transistors. Adv. Mater. 2011, 23, 4077–4081. [Google Scholar] [CrossRef]
- Lemos, A.F.; Ferreira, J.M.F. The Valences of Egg White for Designing Smart Porous Bioceramics: As Foaming and Consolidation Agent. Key Eng. Mater. 2004, 256, 1045–1049. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, Y.-Q. An insight on egg white: From most common functional food to biomaterial application. J. Biomed. Mater. Res. B 2021, 109, 1045–1058. [Google Scholar] [CrossRef]
- Tomimatsu, Y.; Donovan, J.W. Light Scattering Study of Ovomucin. J. Agric. Food Chem. 1972, 20, 1067–1073. [Google Scholar] [CrossRef]
- Huopalahti, R.; López-Fandio, R.; Anton, M.; Schade, R. Bioactive Egg Compounds; Springer: Heidelberg, Germany, 2007; p. 268. [Google Scholar]
- Handa, A.; Gennadios, A.; Froning, G.W.; Kuroda, N.; Hanna, M.A. Tensile, Solubility, and Electrophoretic Properties of Egg White Films as Affected by Surface Sulfhydryl Groups. J. Food Sci. 1999, 64, 82–85. [Google Scholar] [CrossRef]
- Jerez, A.; Partal, P.; Martinez Gallegos, C.; Guerrero, A. Egg white-based bioplastics developed by thermomechanical processing. J. Food Eng. 2007, 82, 608–617. [Google Scholar] [CrossRef]
- Lim, L.; Mine, Y.; Tung, M.A. Transglutaminase Cross-Linked Egg White Protein Films: Tensile Properties and Oxygen Permeability. J. Agric. Food Chem. 1998, 46, 4022–4029. [Google Scholar] [CrossRef]
- Handa, A.; Gennadios, A.; Hanna, M.A.; Weller, C.L.; Kuroda, N. Physical and Molecular Properties of Egg-white Lipid Films. J. Food Sci. 1999, 64, 860–864. [Google Scholar] [CrossRef]
- Venkateswarlu, U.; Boopalan, K.; Mohan, R.; Das, B.N.; Sastry, T.P. Studies on Chemically Modified Hen Egg White and Gelatin Composites. J. Appl. Polym. Sci. 2006, 100, 318–322. [Google Scholar] [CrossRef]
- Peng, N.; Gu, L.; Li, J.; Chang, C.; Li, X.; Su, Y. Films Based on Egg White Protein and Succinylated Casein Cross-Linked with Transglutaminase. Food Bioprocess Technol. 2017, 10, 1422–1430. [Google Scholar] [CrossRef]
- Cui, D.; Wang, Z.; Guo, L. Egg white-mediated green synthesis of silver nanoparticles with excellent biocompatibility and enhanced radiation effects on cancer cells. Int. J. Nanomed. 2012, 7, 2101–2107. [Google Scholar]
- Shaabani, Y.; Sirousazar, M.; Kheiri, F.; Shaabani, Y.; Sirousazar, M.; Kheiri, F. Synthetic-Natural Bionanocomposite Hydrogels on the Basis of Polyvinyl Alcohol and Egg White. J. Macromol. Sci. B 2016, 2348, 849–865. [Google Scholar] [CrossRef]
- Babaei, J.; Khodaiyan, F.; Mohammadian, M. Effects of enriching with gellan gum on the structural, functional, and degradation properties of egg white heat-induced hydrogels. Int. J. Biol. Macromol. 2019, 128, 94–100. [Google Scholar] [CrossRef]
- Priya, P.; Raja, A.; Raj, A. Interpenetrating polymeric networks of chitosan and egg white with dual crosslinking agents polyethylene glycol/polyvinylpyrrolidone as a novel drug carrier. Cellulose 2016, 23, 699–712. [Google Scholar] [CrossRef]
- Bashash, M.; Varidi, M.; Varshosaz, J. Ultrasound-triggered transglutaminase-catalyzed egg white-bovine gelatin composite hydrogel: Physicochemical and rheological studies. Innov. Food Sci. Emerg. Technol. 2022, 76, 102936. [Google Scholar] [CrossRef]
- Jahani-javanmardi, A.; Sirousazar, M.; Shaabani, Y. Egg white/poly (vinyl alcohol)/MMT nanocomposite hydrogels for wound dressing. J. Biomater. Sci. Polym. 2016, 27, 1262–1276. [Google Scholar] [CrossRef]
- Khallok, H.; Ojala, S.; Ezzahmouly, M.; Elouahli, A.; Gourri, E.H.; Jamil, M. Porous foams based hydroxyapatite prepared by direct foaming method using egg white as a pore promoter. J. Aust. Ceram Soc. 2019, 55, 611–619. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Zhou, X.; Yu, J.; Wang, H.; Zhao, S. Preparation of Si3N4 ceramic foams by simultaneously using egg white protein and fish collagen. Ceram Int. 2013, 39, 445–448. [Google Scholar] [CrossRef]
- Dhara, S.; Bhargava, P. Egg White as an Environmentally Friendly Low-Cost Binder for Gelcasting of Ceramics. J. Am. Ceram Soc. 2001, 50, 3048–3050. [Google Scholar] [CrossRef]
- Jalili-Firoozinezhad, S.; Rajabi-Zeleti, S.; Mohammadi, P.; Gaudiello, E.; Bonakdar, S.; Solati-Hashjin, M.; Marsano, A.; Aghdami, N.; Scherberich, A.; Baharvand, H. Facile Fabrication of Egg White Macroporous Sponges for Tissue Regeneration. Adv. Healthc. Mater. 2015, 4, 2281–2290. [Google Scholar] [CrossRef]
- della Vecchia, N.F.; Cerruti, P.; Gentile, G.; Errico, M.E.; Ambrogi, V.; D’Errico, G.; Longobardi, S.; Napolitano, A.; Paduano, L.; Carfagna, C.; et al. Artificial biomelanin: Highly light-absorbing nano-sized eumelanin by biomimetic synthesis in chicken egg white. Biomacromolecules 2014, 15, 3811–3816. [Google Scholar] [CrossRef]
- Xing, Y.; Liu, H.; Yu, W. Preparation and characterization of keratin and chicken egg white-templated luminescent Au cluster composite film. J. Mol. Struct. 2016, 1106, 53–58. [Google Scholar] [CrossRef]
- Jiang, B.; Yang, J.; Li, C.; Zhang, L.; Zhang, X.; Yang, P. Water-Based Photo- and Electron-Beam Lithography Using Egg White as a Resist. Adv. Mater. Interfaces 2017, 4, 1–9. [Google Scholar] [CrossRef]
- Gabal, M.A.; Al Angari, Y.M. A study on Cu substituted Ni–Cu–Zn ferrites synthesized using egg-white. J. Alloy Compd. 2010, 492, 411–415. [Google Scholar] [CrossRef]
- Zheng, L.; Hu, Y.; Ma, Y.; Zhou, Y.; Nie, F.; Liu, X.; Pei, C. Egg-white-mediated crystallization of calcium carbonate. J. Cryst. Growth 2012, 361, 217–224. [Google Scholar] [CrossRef]
- Wei, Z.-Z.; Dong, X.; Zhang, Y.-Q. A mechanically robust egg white hydrogel scaffold with excellent biocompatibility by three-step green processing. Sci. China Technol. Sci. 2022, 65, 1599–1612. [Google Scholar] [CrossRef]
- Honda, A.; Huroda, N. Functional improvement in dried egg white through the Maillard reaction. J. Agric. Food Chem. 1999, 47, 1845–1850. [Google Scholar] [CrossRef]
- Nakamura, A.; Hara, K.; Hiramatsu, N.; Matsumoto, A. Low frequency Raman peak and elastic anomaly of dehydrated heat-treated egg-white gel. Phys. B Condens. Matter 1999, 263–264, 327–329. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.Q. Unidirectional nanopore dehydration induces to form superstretchable, superthick, degradable and mechanically robust silk fibroin membranes dominated by type II beta-turns. ACS Biomater. Sci. Eng. 2023, 9, 2741–2754. [Google Scholar] [CrossRef]
- Jing, F.-Y.; Zhang, Y.-Q. Unidirectional Nanopore Dehydration Induces an Anisotropic Polyvinyl Alcohol Hydrogel Membrane with Enhanced Mechanical Properties. Gels 2022, 8, 1–19. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Wang, H.-D.; Zhang, Y.-Q. A mechanically robust PVA-xylosylated sericin composite hydrogel membrane with enhanced biocompatibility by unidirectional dehydration throught nanopore. Polym. Test. 2023, 124, 108062. [Google Scholar] [CrossRef]
- Tan, J.; Liu, T.; Yao, Y.; Wu, N.; Du, H.; Xu, M.; Liao, M.; Zhao, Y.; Tu, Y. Changes in physicochemical and antioxidant properties of egg white during the Maillard reaction induced by alkali. LWT-Food Sci. Technol. 2021, 143, 111151. [Google Scholar] [CrossRef]
- Mine, Y.; Noutomi, T.; Haga, N. Thermally induced changes in egg white proteins. J. Agric. Food Chem. 1990, 38, 2122–2125. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Zhang, Y.-Q. A Novel Mechanically Robust and Biodegradable Egg White Hydrogel Membrane by Combined Unidirectional Nanopore Dehydration and Annealing. Int. J. Mol. Sci. 2023, 24, 12661. https://doi.org/10.3390/ijms241612661
Dong X, Zhang Y-Q. A Novel Mechanically Robust and Biodegradable Egg White Hydrogel Membrane by Combined Unidirectional Nanopore Dehydration and Annealing. International Journal of Molecular Sciences. 2023; 24(16):12661. https://doi.org/10.3390/ijms241612661
Chicago/Turabian StyleDong, Xuan, and Yu-Qing Zhang. 2023. "A Novel Mechanically Robust and Biodegradable Egg White Hydrogel Membrane by Combined Unidirectional Nanopore Dehydration and Annealing" International Journal of Molecular Sciences 24, no. 16: 12661. https://doi.org/10.3390/ijms241612661




