Association Mapping and Expression Analysis of the Genes Involved in the Wood Formation of Poplar
Abstract
:1. Introduction
2. Results
2.1. Wood Property Variation of Large-Diameter Poplars
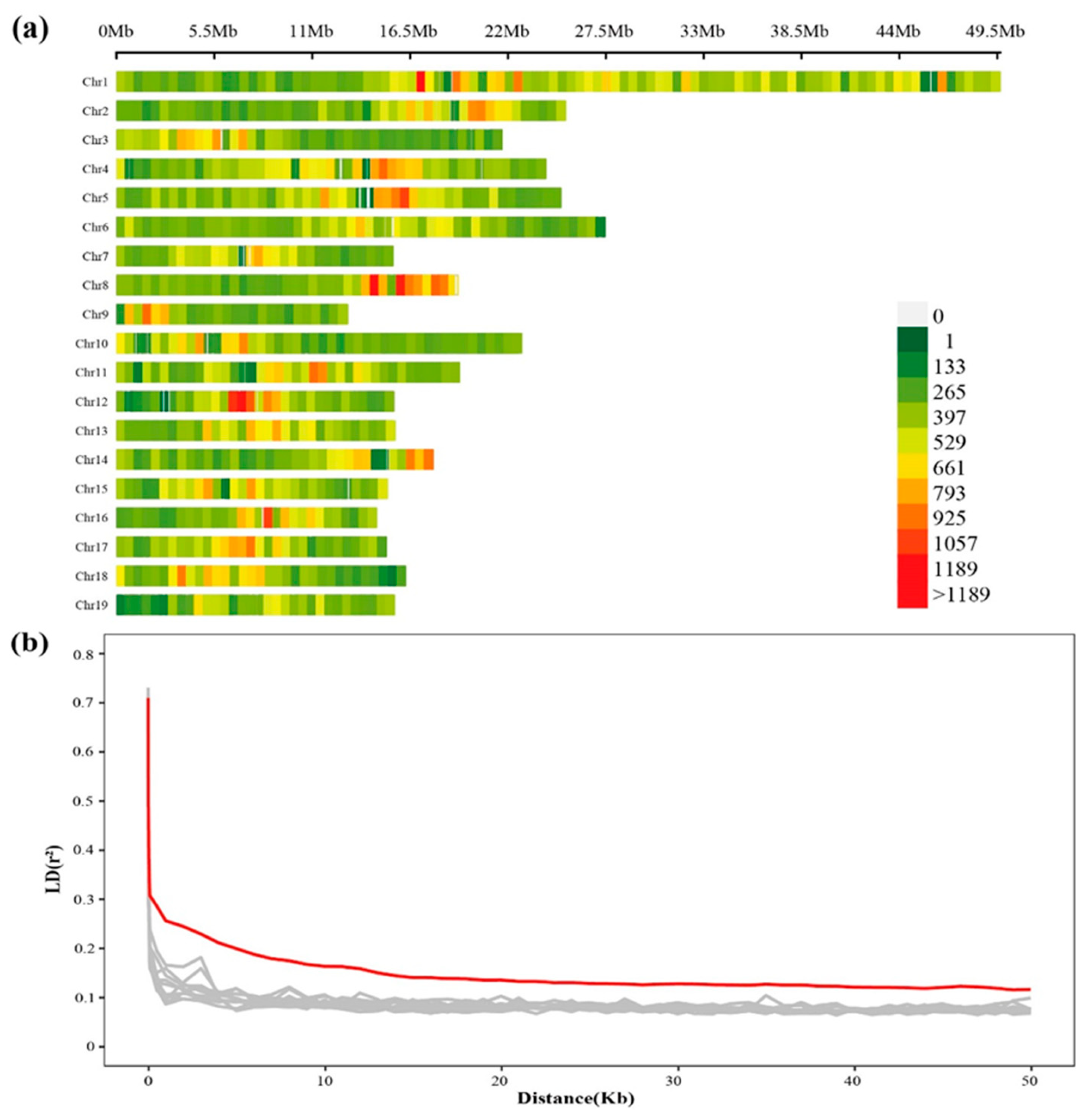
2.2. Genome-Wide Variations in a Full-Sib Population
2.3. Genetic Recombination and LD Decay
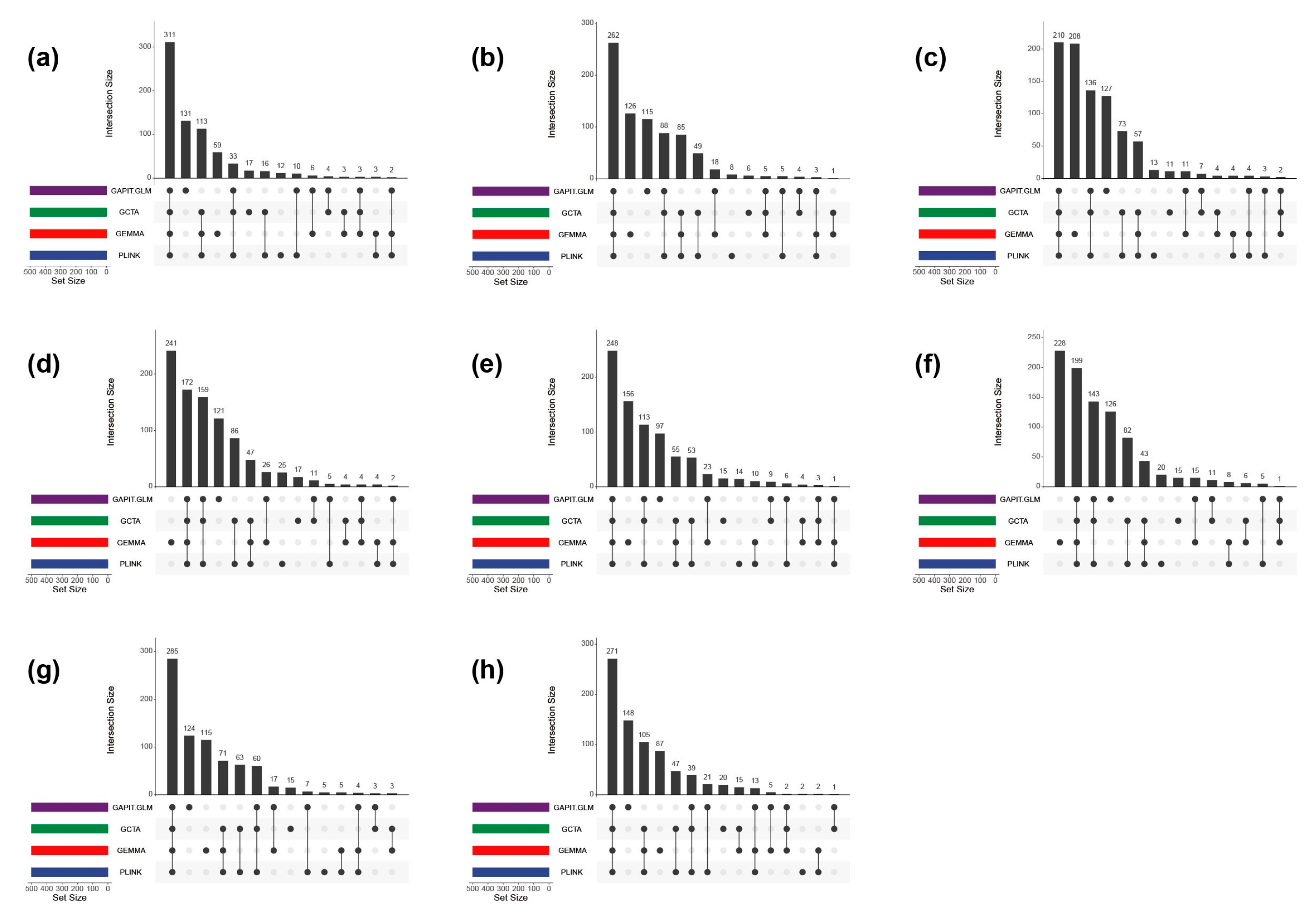
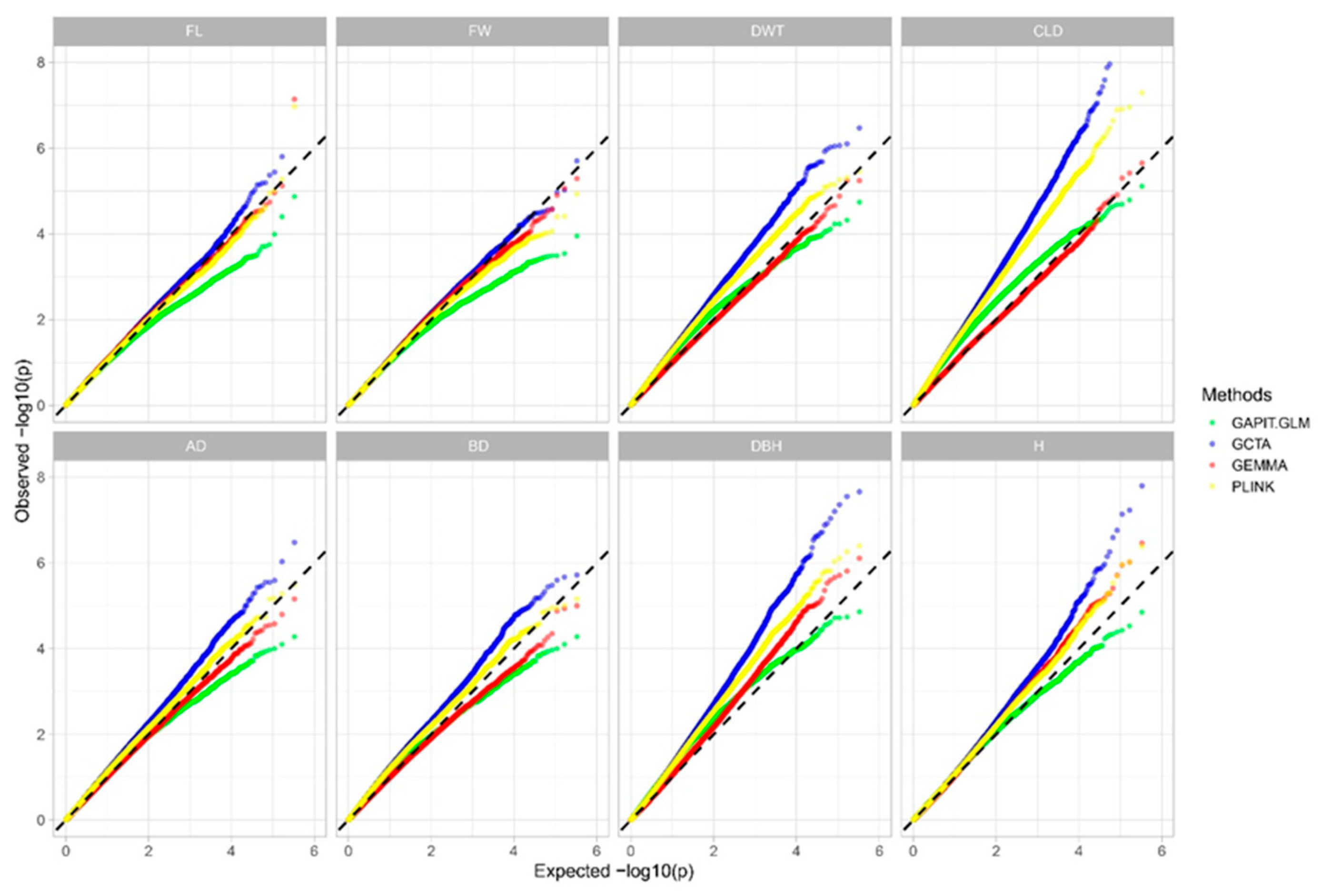
2.4. Association Model Selection
2.5. Full-Sib Population-Based Association Mapping
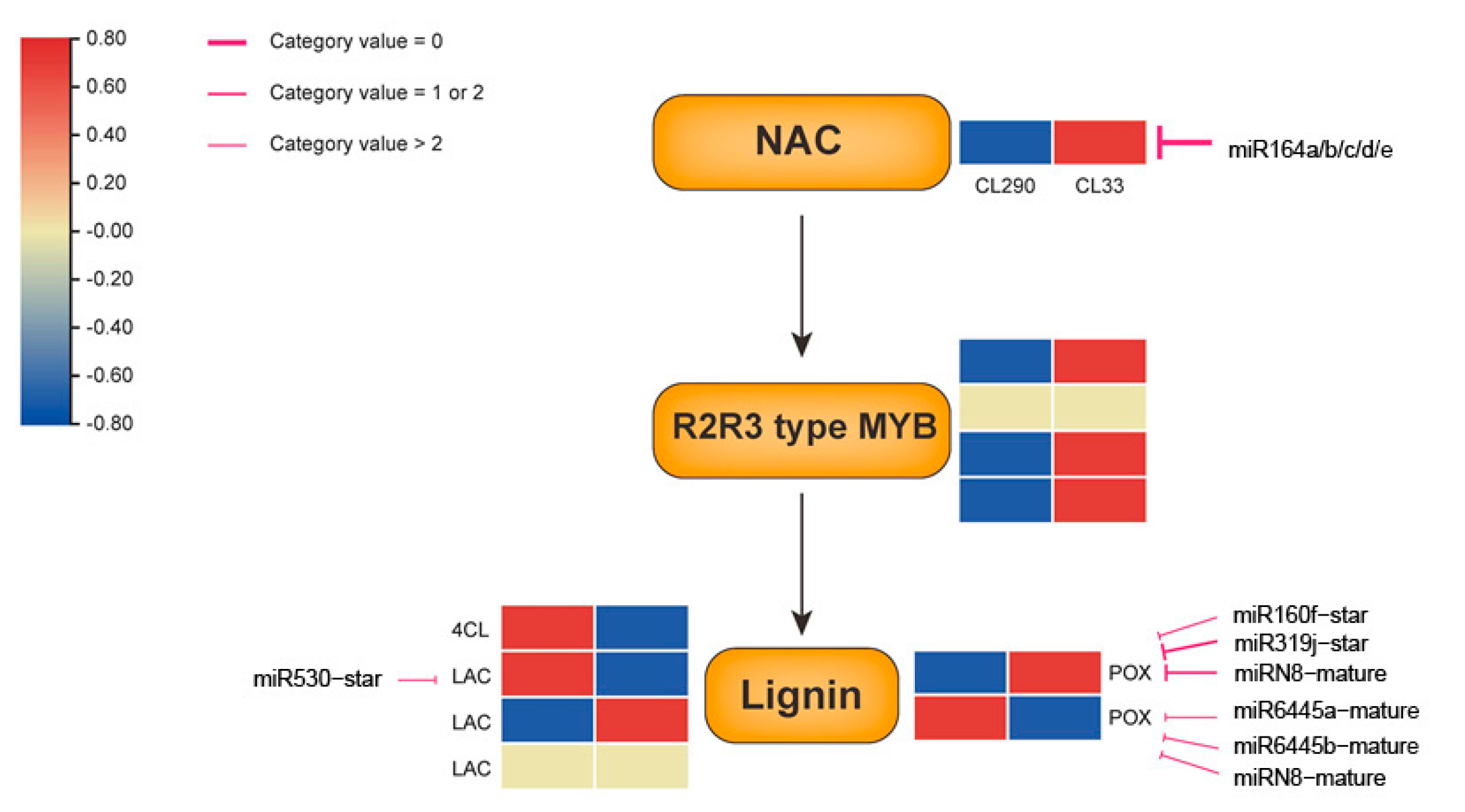
2.6. Transcriptional Regulatory Network for SCW Biosynthesis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Wood Property Determination
4.3. Genome Resequencing and SNP Genotyping
4.4. LD Decay Analysis
4.5. Statistical Algorithms
4.6. Enrichment and Transcriptional Expression Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, H.; Xing, X.; Guan, Y.; Zhou, L.; Liu, S.; Gao, H. Radial variation studies on wood properties of Populus deltoides parents and their hybrids. BioResources 2021, 16, 4905. [Google Scholar] [CrossRef]
- Ding, X.; Diao, S.; Luan, Q.; Wu, H.X.; Zhang, Y.; Jiang, J. A transcriptome-based association study of growth, wood quality, and oleoresin traits in a slash pine breeding population. PLoS Genet. 2022, 18, e1010017. [Google Scholar] [CrossRef] [PubMed]
- Plomion, C.; Leprovost, G.; Stokes, A. Wood Formation in Trees. Plant Physiol. 2001, 127, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nieminen, K.; Serra, J.A.A.; Helariutta, Y. The formation of wood and its control. Curr. Opin. Plant Biol. 2014, 17, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Yamaguchi, M.; Endo, H.; Rejab, N.A.; Ohtani, M. NAC-MYB-based transcriptional regulation of secondary cell wall biosynthesis in land plants. Front. Plant Sci. 2015, 6, 288. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xie, M.; Tuskan, G.A.; Muchero, W.; Chen, J.-G. Recent Advances in the Transcriptional Regulation of Secondary Cell Wall Biosynthesis in the Woody Plants. Front. Plant Sci. 2018, 9, 1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortés, A.J.; Restrepo-Montoya, M.; Bedoya-Canas, L.E. Modern Strategies to Assess and Breed Forest Tree Adaptation to Changing Climate. Front. Plant Sci. 2020, 11, 583323. [Google Scholar] [CrossRef]
- Du, Q.; Lu, W.; Quan, M.; Xiao, L.; Song, F.; Li, P.; Zhou, D.; Xie, J.; Wang, L.; Zhang, D. Genome-Wide Association Studies to Improve Wood Properties: Challenges and Prospects. Front. Plant Sci. 2018, 9, 1912. [Google Scholar]
- Weiss, M.; Sniezko, R.A.; Puiu, D.; Crepeau, M.W.; Stevens, K.; Salzberg, S.L.; Langley, C.H.; Neale, D.B.; De La Torre, A.R. Genomic basis of white pine blister rust quantitative disease resistance and its relationship with qualitative resistance. Plant J. 2020, 104, 365–376. [Google Scholar] [CrossRef]
- Chen, Z.-Q.; Zan, Y.; Milesi, P.; Zhou, L.; Chen, J.; Li, L.; Cui, B.; Niu, S.; Westin, J.; Karlsson, B.; et al. Leveraging breeding programs and genomic data in Norway spruce (Picea abies L. Karst) for GWAS analysis. Genome Biol. 2021, 22, 179. [Google Scholar] [CrossRef]
- Howe, L.J.; Nivard, M.G.; Morris, T.T.; Hansen, A.F. Within-sibship genome-wide association analyses decrease bias in estimates of direct genetic effects. Nat. Genet. 2022, 54, 581–592. [Google Scholar] [PubMed]
- Sumitomo, K.; Shirasawa, K.; Isobe, S.; Hirakawa, H.; Harata, A.; Nakano, M.; Nakano, Y.; Yagi, M.; Hisamatsu, T.; Yamaguchi, H.; et al. A genome-wide association and fine-mapping study of white rust resistance in hexaploid chrysanthemum cultivars with a wild diploid reference genome. Hortic. Res. 2022, 9, uhac170. [Google Scholar] [PubMed]
- Ghosh Dasgupta, M.; Abdul Bari, M.P.; Shanmugavel, S.; Dharanishanthi, V.; Muthupandi, M.; Kumar, N.; Chauhan, S.S.; Kalaivanan, J.; Mohan, H.; Krutovsky, K.V.; et al. Targeted re-sequencing and genome-wide association analysis for wood property traits in breeding population of Eucalyptus tereticornis × E. grandis. Genomics 2021, 113, 4276–4292. [Google Scholar] [PubMed]
- Park, S.; Kumar, P.; Shi, A.; Mou, B. Population genetics and genome-wide association studies provide insights into the influence of selective breeding on genetic variation in lettuce. Plant Genome 2021, 14, e20086. [Google Scholar] [PubMed]
- Wang, J.; Raskin, L.; Samuels, D.C.; Shyr, Y.; Guo, Y. Genome measures used for quality control are dependent on gene function and ancestry. Bioinformatics 2015, 31, 318–323. [Google Scholar] [PubMed] [Green Version]
- Guo, M.; Yue, W.; Samuels, D.C.; Yu, H.; He, J.; Zhao, Y.-Y.; Guo, Y. Quality and concordance of genotyping array data of 12,064 samples from 5840 cancer patients. Genomics 2019, 111, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Long, J.; He, J.; Li, C.-I.; Cai, Q.; Shu, X.-O.; Zheng, W.; Li, C. Exome sequencing generates high quality data in non-target regions. BMC Genom. 2012, 13, 194. [Google Scholar]
- Yang, G.; Luo, W.; Zhang, J.; Yan, X.; Du, Y.; Zhou, L.; Li, W.; Wang, H.; Chen, Z.; Guo, T. Genome-Wide Comparisons of Mutations Induced by Carbon-Ion Beam and Gamma-Rays Irradiation in Rice via Resequencing Multiple Mutants. Front. Plant Sci. 2019, 10, 1514. [Google Scholar]
- Han, W.; Xue, Q.; Li, G.; Yin, J.; Zhang, H.; Zhu, Y.; Xing, W.; Cao, Y.; Su, Y.; Wang, K.; et al. Genome-wide analysis of the role of DNA methylation in inbreeding depression of reproduction in Langshan chicken. Genomics 2020, 112, 2677–2687. [Google Scholar]
- Thami, P.; Choga, W.; Mulisa, D.; Dandara, C.; Shevchenko, A.; Leteane, M.; Novitsky, V.; O’Brien, S.; Essex, M.; Gaseitsiwe, S.; et al. Whole Genome Sequencing-based Characterization of Human Genome Variation and Mutation Burden in Botswana. bioRxiv 2020, 1, 1–34. [Google Scholar] [CrossRef]
- Halkett, F.; Simon, J.-C.; Balloux, F. Tackling the population genetics of clonal and partially clonal organisms. Trends Ecol. Evol. 2005, 20, 194–201. [Google Scholar] [PubMed]
- Meng, B.; Wang, T.; Luo, Y.; Guo, Y.; Xu, D.; Liu, C.; Zou, J.; Li, L.; Diao, Y.; Gao, Z.; et al. Identification and Allele Combination Analysis of Rice Grain Shape-Related Genes by Genome-Wide Association Study. Int. J. Mol. Sci. 2022, 23, 1065. [Google Scholar] [PubMed]
- Jia, H.; Liu, G.; Li, J.; Zhang, J.; Sun, P.; Zhao, S.; Zhou, X.; Lu, M.; Hu, J. Genome resequencing reveals demographic history and genetic architecture of seed salinity tolerance in Populus euphratica. J. Exp. Bot. 2020, 71, 4308–4320. [Google Scholar] [PubMed]
- Alqudah, A.M.; Sallam, A.; Stephen Baenziger, P.; Börner, A. GWAS: Fast-forwarding gene identification and characterization in temperate Cereals: Lessons from Barley—A review. J. Adv. Res. 2020, 22, 119–135. [Google Scholar] [PubMed]
- Tibbs Cortes, L.; Zhang, Z.; Yu, J. Status and prospects of genome-wide association studies in plants. Plant Genome 2021, 14, e20077. [Google Scholar] [PubMed]
- Monnot, S.; Desaint, H.; Mary-Huard, T.; Moreau, L.; Schurdi-Levraud, V.; Boissot, N. Deciphering the Genetic Architecture of Plant Virus Resistance by GWAS, State of the Art and Potential Advances. Cells 2021, 10, 3080. [Google Scholar]
- Li, X.; Nie, C.; Liu, Y.; Chen, Y.; Lv, X.; Wang, L.; Zhang, J.; Li, K.; Jia, Y.; Ban, L.; et al. A genome-wide association study explores the genetic determinism of host resistance to Salmonella pullorum infection in chickens. Genet. Sel. Evol. GSE 2019, 51, 1–12. [Google Scholar]
- Korte, A.; Farlow, A. The advantages and limitations of trait analysis with GWAS: A review. Plant Methods 2013, 9, 29. [Google Scholar]
- Zhang, X.F.; Li, H.G.; You, L.X.; Xao, J. Variation and genetic stability of two-year-old liriodendron seedling growth for 39 mating combinations. J. Zhejiang A F Univ. 2011, 28, 103–108. [Google Scholar]
- Chen, Y.; Wu, H.; Yang, W.; Zhao, W.; Tong, C. Multivariate linear mixed model enhanced the power of identifying genome-wide association to poplar tree heights in a randomized complete block design. G3 Genes Genomes Genet. 2021, 11, jkaa053. [Google Scholar]
- Lu, N.; Zhang, M.; Xiao, Y.; Han, D.; Liu, Y.; Zhang, Y.; Yi, F.; Zhu, T.; Ma, W.; Fan, E.; et al. Construction of a high-density genetic map and QTL mapping of leaf traits and plant growth in an interspecific F1 population of Catalpa bungei × Catalpa duclouxii Dode. BMC Plant Biol. 2019, 19, 596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Yao, D.; Wu, H.; Zhao, W.; Chen, Y.; Tong, C. Multivariate genome-wide association study of leaf shape in a Populus deltoides and P. simonii F1 pedigree. PLoS ONE 2021, 16, e0259278. [Google Scholar]
- Li, W.; Boer, M.P.; Zheng, C.; Joosen, R.V.L.; van Eeuwijk, F.A. An IBD-based mixed model approach for QTL mapping in multiparental populations. Theor. Appl. Genet. 2021, 134, 3643–3660. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, Z.; Guo, Y.; Zhang, S.; Jiang, L.; Wu, R. Genome-wide association studies of callus differentiation for the desert tree, Populus euphratica. Tree Physiol. 2020, 40, 1762–1777. [Google Scholar] [CrossRef] [PubMed]
- Nyaga, C.; Gowda, M.; Beyene, Y.; Muriithi, W.T.; Makumbi, D.; Olsen, M.S.; Suresh, L.M.; Bright, J.M.; Das, B.; Prasanna, B.M. Genome-Wide Analyses and Prediction of Resistance to MLN in Large Tropical Maize Germplasm. Genes 2020, 11, 16. [Google Scholar]
- Fahrenkrog, A.M.; Neves, L.G.; Resende, M.F.R., Jr.; Dervinis, C.; Davenport, R.; Barbazuk, W.B.; Kirst, M. Population genomics of the eastern cottonwood (Populus deltoides). Ecol. Evol. 2017, 7, 9426–9440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Shah, T.; Hao, Z.; Taba, S.; Zhang, S.; Gao, S.; Liu, J.; Cao, M.; Wang, J.; Prakash, A.B.; et al. Comparative SNP and Haplotype Analysis Reveals a Higher Genetic Diversity and Rapider LD Decay in Tropical than Temperate Germplasm in Maize. PLoS ONE 2011, 6, e24861. [Google Scholar]
- George, A.W.; Cavanagh, C. Genome-wide association mapping in plants. Theor. Appl. Genet. 2015, 128, 1163–1174. [Google Scholar]
- Vogt, F.; Shirsekar, G.; Weigel, D. vcf2gwas: Python API for comprehensive GWAS analysis using GEMMA. Bioinformatics 2022, 38, 839–840. [Google Scholar]
- Hoffman, G.E. Correcting for Population Structure and Kinship Using the Linear Mixed Model: Theory and Extensions. PLoS ONE 2013, 8, e75707. [Google Scholar]
- Wang, X.; Niu, Q.W.; Teng, C.; Li, C.; Mu, J.; Chua, N.H.; Zuo, J. Overexpression of PGA37/MYB118 and MYB115 promotes vegetative-to-embryonic transition in Arabidopsis. Cell Res. 2009, 19, 224–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, Y. MYB transcription factors orchestrating the developmental program of xylem vessels in Arabidopsis roots. Plant Biotechnol. 2010, 27, 267–272. [Google Scholar]
- Oh, S.; Park, S.; Han, K.H. Transcriptional regulation of secondary growth in Arabidopsis thaliana. J. Exp. Bot. 2003, 54, 2709–2722. [Google Scholar] [PubMed] [Green Version]
- McCarthy, R.L.; Zhong, R.; Fowler, S.; Lyskowski, D.; Piyasena, H.; Carleton, K.; Spicer, C.; Ye, Z.H. The poplar MYB transcription factors, PtrMYB3 and PtrMYB20, are involved in the regulation of secondary wall biosynthesis. Plant Cell Physiol. 2010, 51, 1084–1090. [Google Scholar] [PubMed] [Green Version]
- Liu, Y.; Man, J.; Wang, Y.; Yuan, C.; Shi, Y.; Liu, B.; Hu, X.; Wu, S.; Zhang, T.; Lian, C. Overexpression of PtrMYB121 Positively Regulates the Formation of Secondary Cell Wall in Arabidopsis thaliana. Int. J. Mol. Sci. 2020, 21, 7734. [Google Scholar] [PubMed]
- Li, C.; Wang, X.; Ran, L.; Tian, Q.; Fan, D.; Luo, K. PtoMYB92 is a Transcriptional Activator of the Lignin Biosynthetic Pathway During Secondary Cell Wall Formation in Populus tomentosa. Plant Cell Physiol. 2015, 56, 2436–2446. [Google Scholar] [PubMed] [Green Version]
- Xu, C.; Fu, X.; Liu, R.; Guo, L.; Ran, L.; Li, C.; Tian, Q.; Jiao, B.; Wang, B.; Luo, K. PtoMYB170 positively regulates lignin deposition during wood formation in poplar and confers drought tolerance in transgenic Arabidopsis. Tree Physiol. 2017, 37, 1713–1726. [Google Scholar] [PubMed] [Green Version]
- Tian, Q.; Wang, X.; Li, C.; Lu, W.; Yang, L.; Jiang, Y.; Luo, K. Functional characterization of the poplar R2R3-MYB transcription factor PtoMYB216 involved in the regulation of lignin biosynthesis during wood formation. PLoS ONE 2013, 8, e76369. [Google Scholar]
- Wang, L.; Lu, W.; Ran, L.; Dou, L.; Yao, S.; Hu, J.; Fan, D.; Li, C.; Luo, K. R2R3-MYB transcription factor MYB6 promotes anthocyanin and proanthocyanidin biosynthesis but inhibits secondary cell wall formation in Populus tomentosa. Plant J. 2019, 99, 733–751. [Google Scholar]
- Yang, L.; Zhao, X.; Ran, L.; Li, C.; Fan, D.; Luo, K. PtoMYB156 is involved in negative regulation of phenylpropanoid metabolism and secondary cell wall biosynthesis during wood formation in poplar. Sci. Rep. 2017, 7, 41209. [Google Scholar] [CrossRef] [Green Version]
- Jiao, B.; Zhao, X.; Lu, W.; Guo, L.; Luo, K. The R2R3 MYB transcription factor MYB189 negatively regulates secondary cell wall biosynthesis in Populus. Tree Physiol. 2019, 39, 1187–1200. [Google Scholar] [PubMed]
- Tang, X.; Zhuang, Y.; Qi, G.; Wang, D.; Liu, H.; Wang, K.; Chai, G.; Zhou, G. Poplar PdMYB221 is involved in the direct and indirect regulation of secondary wall biosynthesis during wood formation. Sci. Rep. 2015, 5, 12240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.S.; Li, H.L.; Grierson, D.; Fu, D.Q. NAC Transcription Factor Family Regulation of Fruit Ripening and Quality: A Review. Cells 2022, 11, 525. [Google Scholar] [PubMed]
- Ding, Y.; Yu, S.; Wang, J.; Li, M.; Qu, C.; Li, J.; Liu, L. Comparative transcriptomic analysis of seed coats with high and low lignin contents reveals lignin and flavonoid biosynthesis in Brassica napus. BMC Plant Biol. 2021, 21, 246. [Google Scholar]
- Tonfack, L.B.; Hussey, S.G.; Veale, A.; Myburg, A.A.; Mizrachi, E. Analysis of orthologous Secondary wall-associated NAC Domain1(SND1) promotor activity in herbaceous and woody angiosperms. Int. J. Mol. Sci. 2019, 20, 4623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaschek, L.; Murozuka, E.; Serk, H.; Menard, D.; Pesquet, E. Different combinations of laccase paralogs nonredundantly control the amount and composition of lignin in specific cell types and cell wall layers in Arabidopsis. Plant Cell 2023, 35, 889–909. [Google Scholar] [PubMed]
- Voelker, S.L.; Lachenbruch, B.; Meinzer, F.C.; Jourdes, M.; Ki, C.; Patten, A.M.; Davin, L.B.; Lewis, N.G.; Tuskan, G.A.; Gunter, L.; et al. Antisense down-regulation of 4CL expression alters lignification, tree growth, and saccharification potential of field-grown poplar. Plant Physiol. 2010, 154, 874–886. [Google Scholar]
- Sun, Q.; Liu, X.; Yang, J.; Liu, W.; Du, Q.; Wang, H.; Fu, C.; Li, W.-X. MicroRNA528 Affects Lodging Resistance of Maize by Regulating Lignin Biosynthesis under Nitrogen-Luxury Conditions. Mol. Plant 2018, 11, 806–814. [Google Scholar]
- Wang, C.-Y.; Zhang, S.; Yu, Y.; Luo, Y.-C.; Liu, Q.; Ju, C.; Zhang, Y.-C.; Qu, L.-H.; Lucas, W.J.; Wang, X.; et al. MiR397b regulates both lignin content and seed number in Arabidopsis via modulating a laccase involved in lignin biosynthesis. Plant Biotechnol. J. 2014, 12, 1132–1142. [Google Scholar]
- Lu, S.; Li, Q.; Wei, H.; Chang, M.-J.; Tunlaya-Anukit, S.; Kim, H.; Liu, J.; Song, J.; Sun, Y.-H.; Yuan, L.; et al. Ptr-miR397a is a negative regulator of laccase genes affecting lignin content in Populus trichocarpa. Proc. Natl. Acad. Sci. USA 2013, 110, 10848–10853. [Google Scholar]
- Wang, M.H.M. Trait analysis of three new clones in Aigeiros. For. Sci. 1979, 1, 9–14. [Google Scholar]
- Franklin, G.L. Preparation of Thin Sections of Synthetic Resins and Wood-Resin Composites, and a New Macerating Method for Wood. Nature 1945, 155, 51. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Genome Project Data Processing, S. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Dong, S.-S.; Xu, J.-Y.; He, W.-M.; Yang, T.-L. PopLDdecay: A fast and effective tool for linkage disequilibrium decay analysis based on variant call format files. Bioinformatics 2019, 35, 1786–1788. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Z. GAPIT Version 3: Boosting Power and Accuracy for Genomic Association and Prediction. Genom. Proteom. Bioinform. 2021, 19, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Stephens, M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 2012, 44, 821–824. [Google Scholar]
- Yang, J.; Lee, S.H.; Goddard, M.E.; Visscher, P.M. GCTA: A Tool for Genome-wide Complex Trait Analysis. Am. J. Hum. Genet. 2011, 88, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef] [Green Version]
- Emrani, H.; Masoudi, A.A.; Vaez Torshizi, R.; Ehsani, A. Genome-wide association study of shank length and diameter at different developmental stages in chicken F2 resource population. Anim. Genet. 2020, 51, 722–730. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. Clusterprofiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Ding, C.; Shen, T.; Ran, N.; Zhang, H.; Pan, H.; Su, X.; Xu, M. Integrated Degradome and Srna Sequencing Revealed miRNA-mRNA Regulatory Networks between the Phloem and Developing Xylem of Poplar. Int. J. Mol. Sci. 2022, 23, 4537. [Google Scholar] [CrossRef]
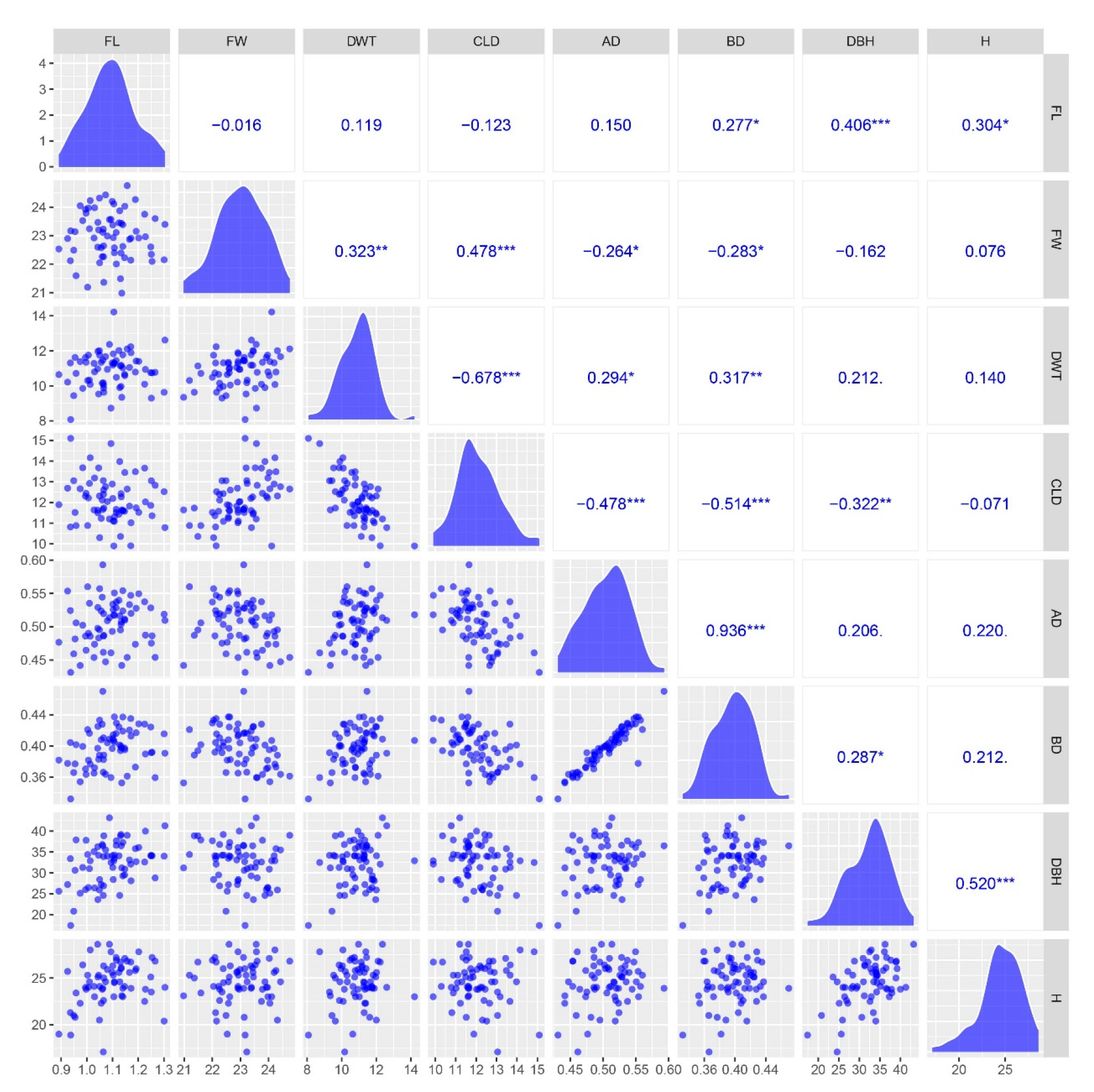

| Traits | Male | Female | F1 Population | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Min | Max | CV (%) | |||
| FL (mm) | 0.94 | 1.14 | 1.09 ± 0.09 | 0.89 | 1.30 | 8.55 |
| FW (μm) | 23.17 | 23.38 | 23.00 ± 0.85 | 20.98 | 24.75 | 3.69 |
| DWT (μm) | 8.07 | 11.77 | 10.93 ± 0.95 | 8.72 | 14.21 | 8.65 |
| CLD (μm) | 15.09 | 11.61 | 12.07 ± 1.03 | 9.90 | 14.84 | 8.51 |
| AD (g/cm3) | 0.43 | 0.52 | 0.51 ± 0.03 | 0.44 | 0.59 | 6.59 |
| BD (g/cm3) | 0.33 | 0.42 | 0.40 ± 0.2 | 0.35 | 0.47 | 6.23 |
| DBH (cm) | 17.40 | 29.30 | 32.61 ± 4.81 | 20.80 | 43.20 | 14.74 |
| H (m) | 18.90 | 23.90 | 24.59 ± 2.28 | 17.10 | 28.60 | 9.89 |
| GAPIT.GLM | GCTA | GEMMA | PLINK | |
|---|---|---|---|---|
| FL | 1.116 | 1.144 | 1.153 | 1.131 |
| FW | 1.060 | 1.093 | 1.062 | 1.080 |
| DWT | 1.484 | 1.507 | 1.024 | 1.504 |
| CLD | 1.737 | 1.855 | 1.000 | 1.866 |
| AD | 1.177 | 1.201 | 1.009 | 1.187 |
| BD | 1.181 | 1.422 | 1.047 | 1.407 |
| DBH | 1.498 | 1.379 | 1.028 | 1.376 |
| H | 1.101 | 1.068 | 0.981 | 1.071 |
| Average λ | 1.301 | 1.340 | 1.040 | 1.334 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, H.; Zhu, S.; Shen, T.; Pan, H.; Xu, M. Association Mapping and Expression Analysis of the Genes Involved in the Wood Formation of Poplar. Int. J. Mol. Sci. 2023, 24, 12662. https://doi.org/10.3390/ijms241612662
Wang Y, Zhang H, Zhu S, Shen T, Pan H, Xu M. Association Mapping and Expression Analysis of the Genes Involved in the Wood Formation of Poplar. International Journal of Molecular Sciences. 2023; 24(16):12662. https://doi.org/10.3390/ijms241612662
Chicago/Turabian StyleWang, Yaolin, Heng Zhang, Sheng Zhu, Tengfei Shen, Huixin Pan, and Meng Xu. 2023. "Association Mapping and Expression Analysis of the Genes Involved in the Wood Formation of Poplar" International Journal of Molecular Sciences 24, no. 16: 12662. https://doi.org/10.3390/ijms241612662





