Oil-in-Water Pickering Emulsions Stabilized with Nanostructured Biopolymers: A Venue for Templating Bacterial Cellulose
Abstract
:1. Introduction
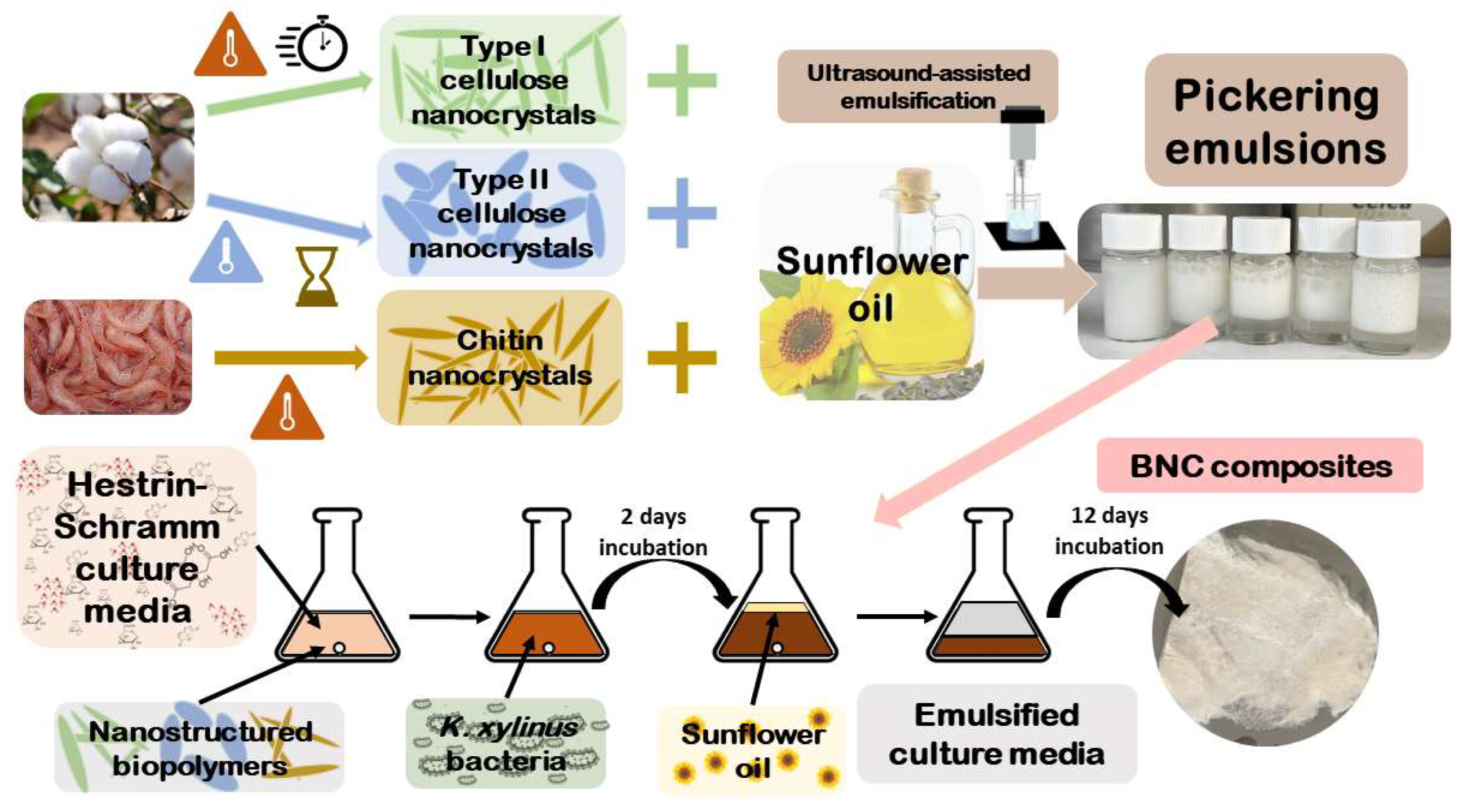
2. Results and Discussion
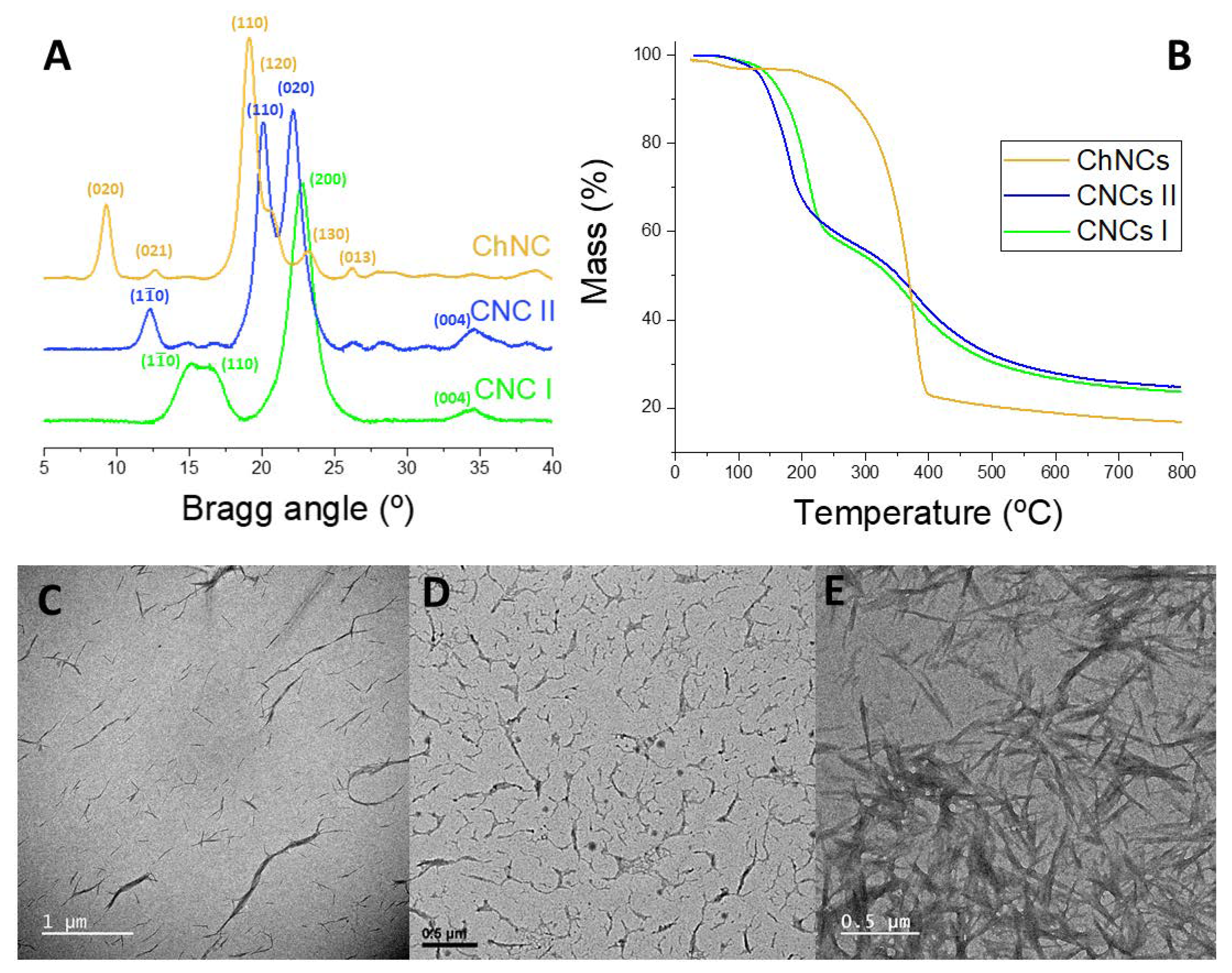
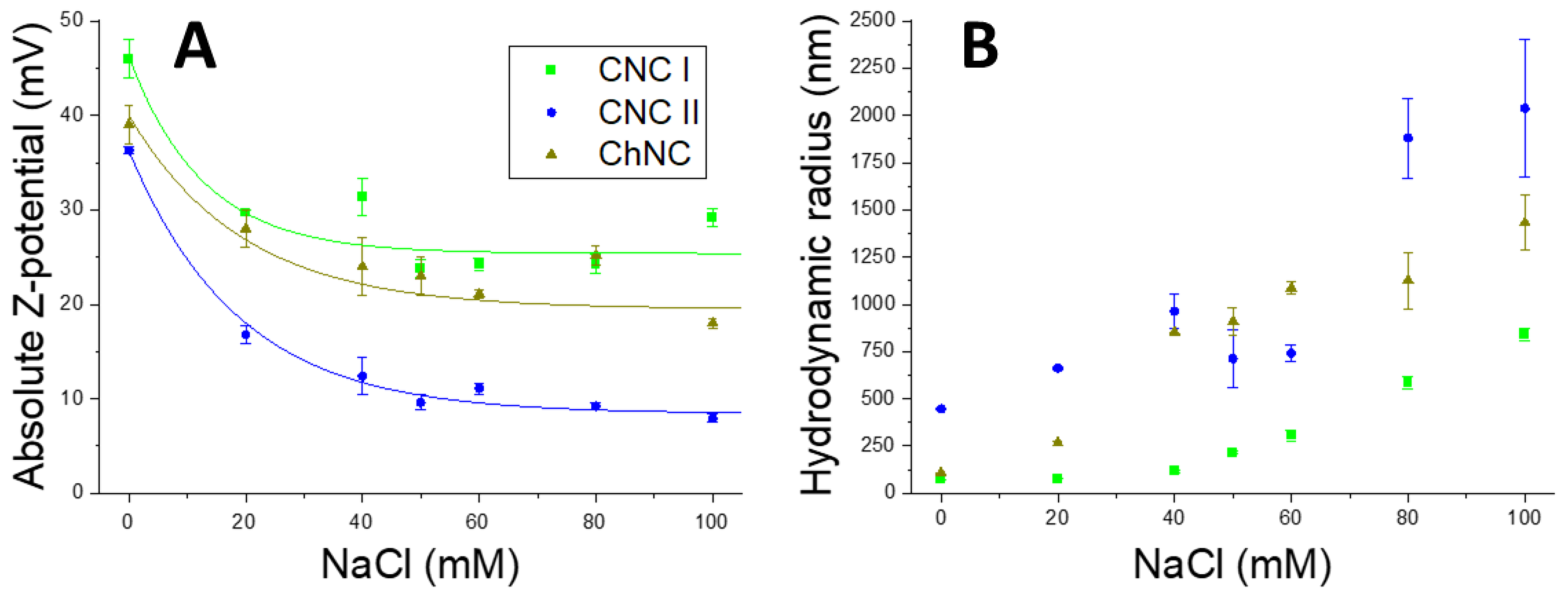
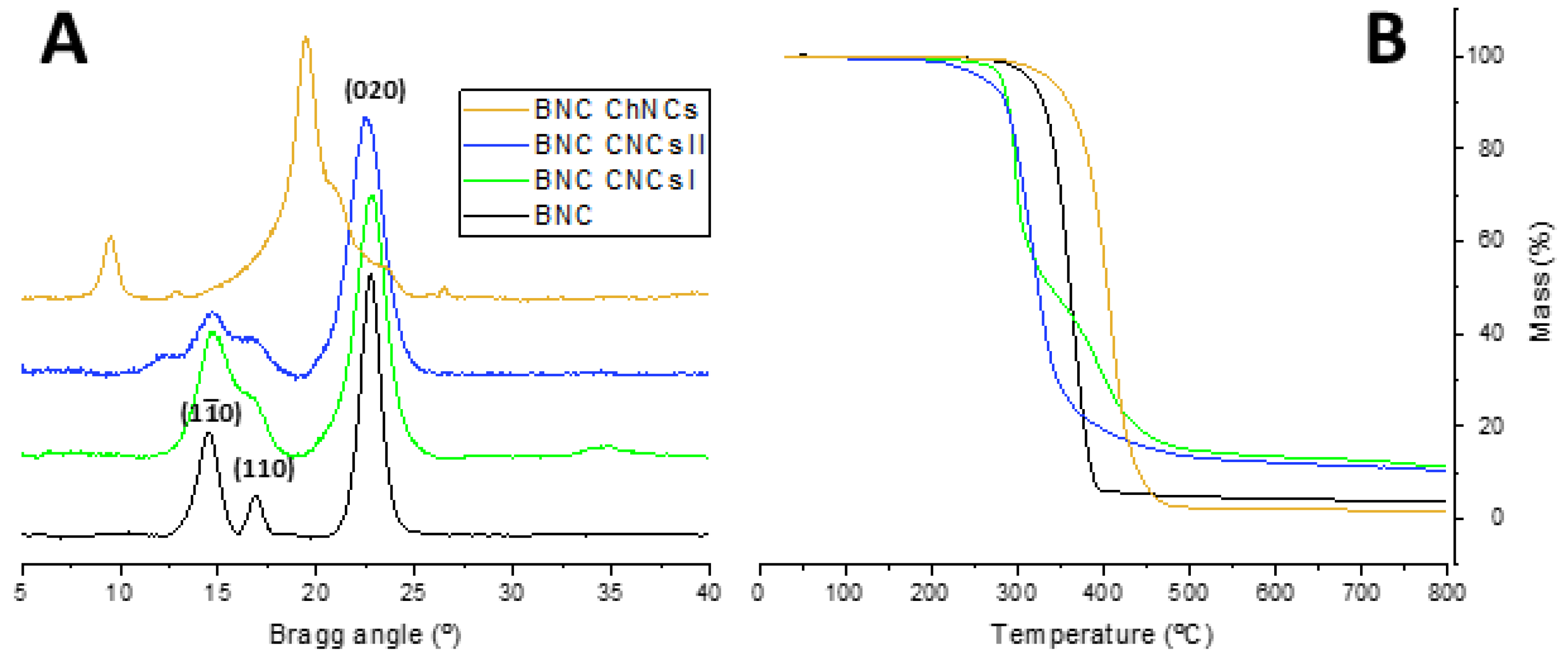
2.1. Synthesis and Characterization of CNCs and ChNCs
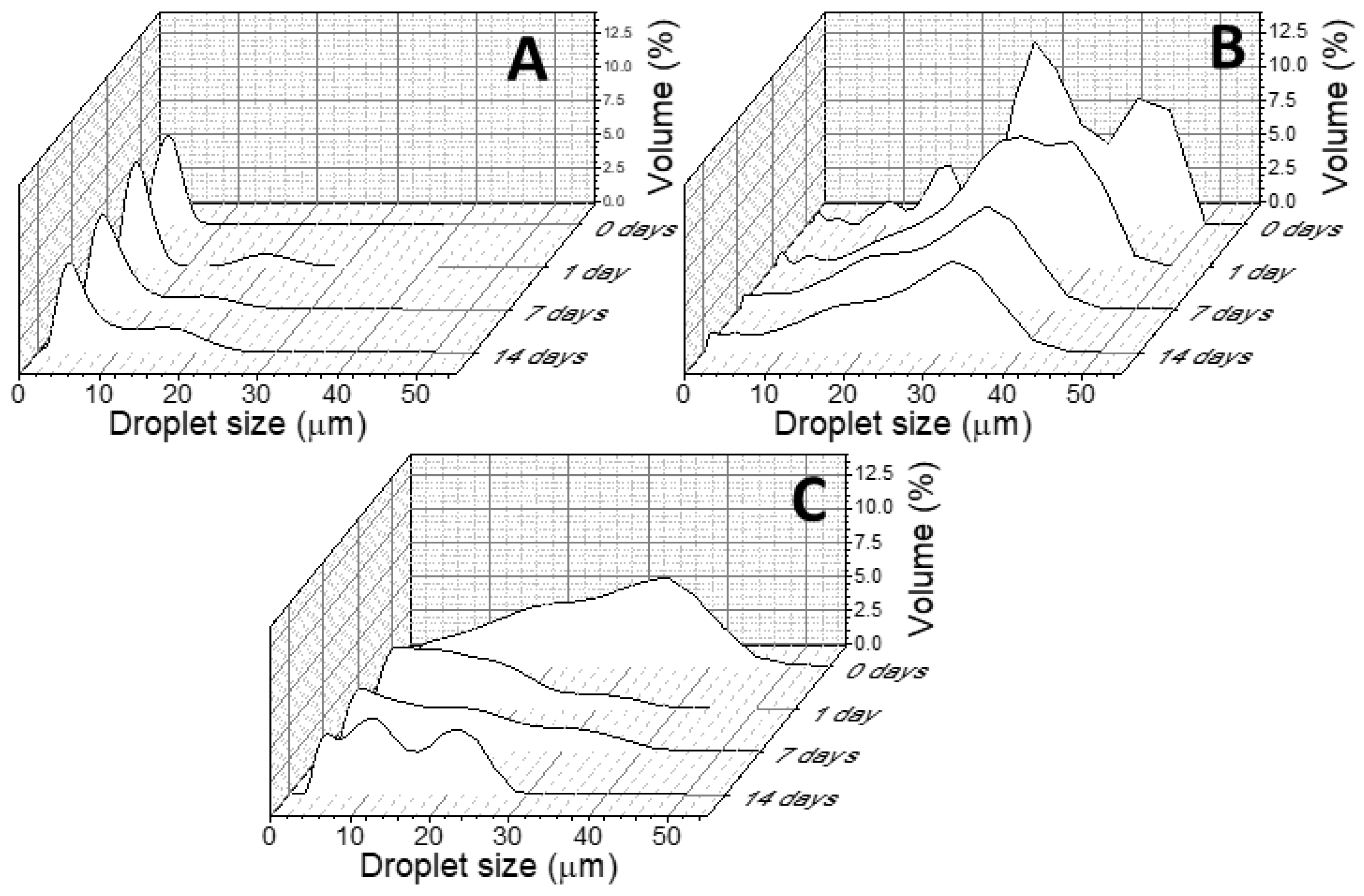
2.2. PEs Preparation, Optimization and Characterization
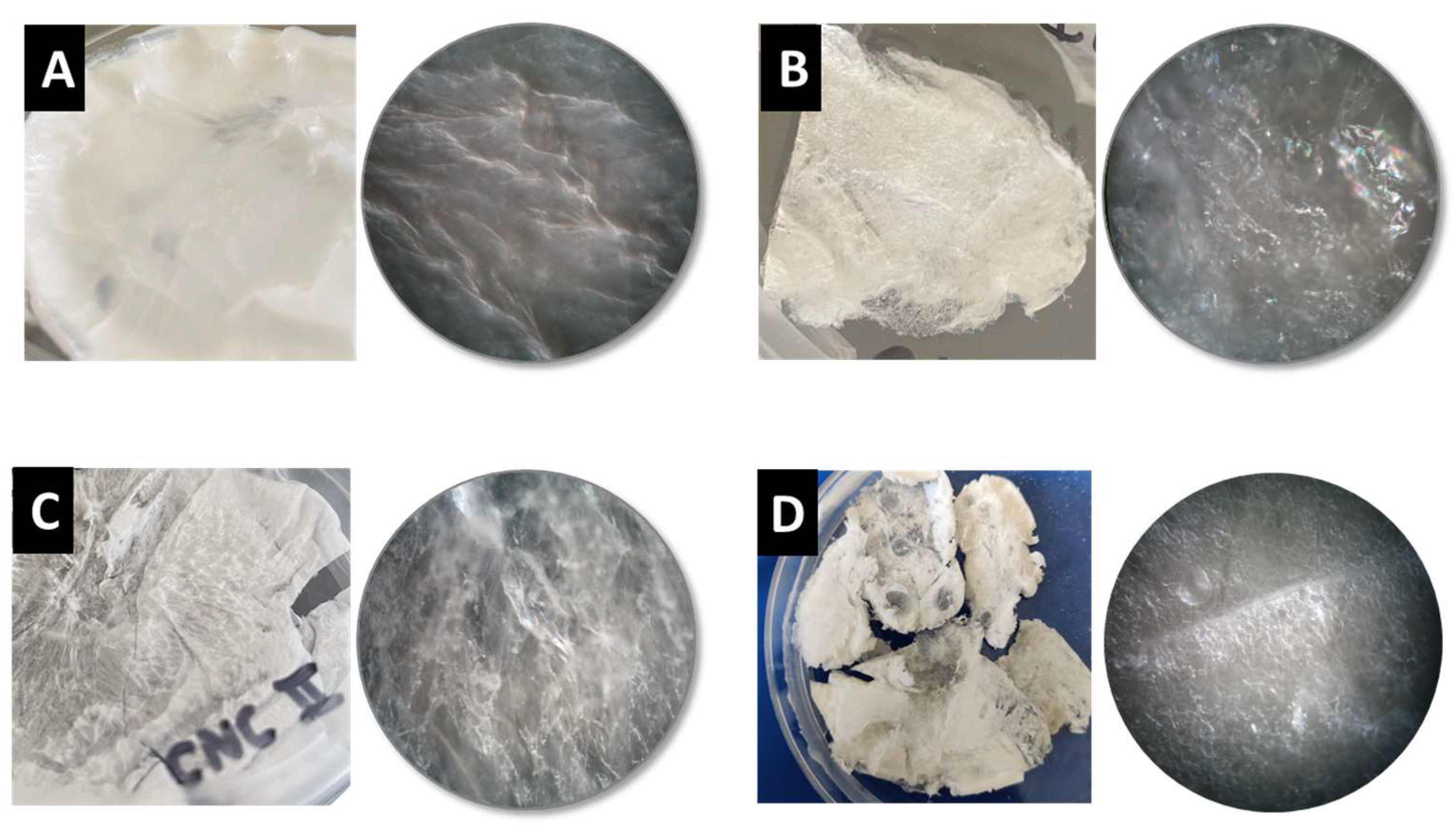
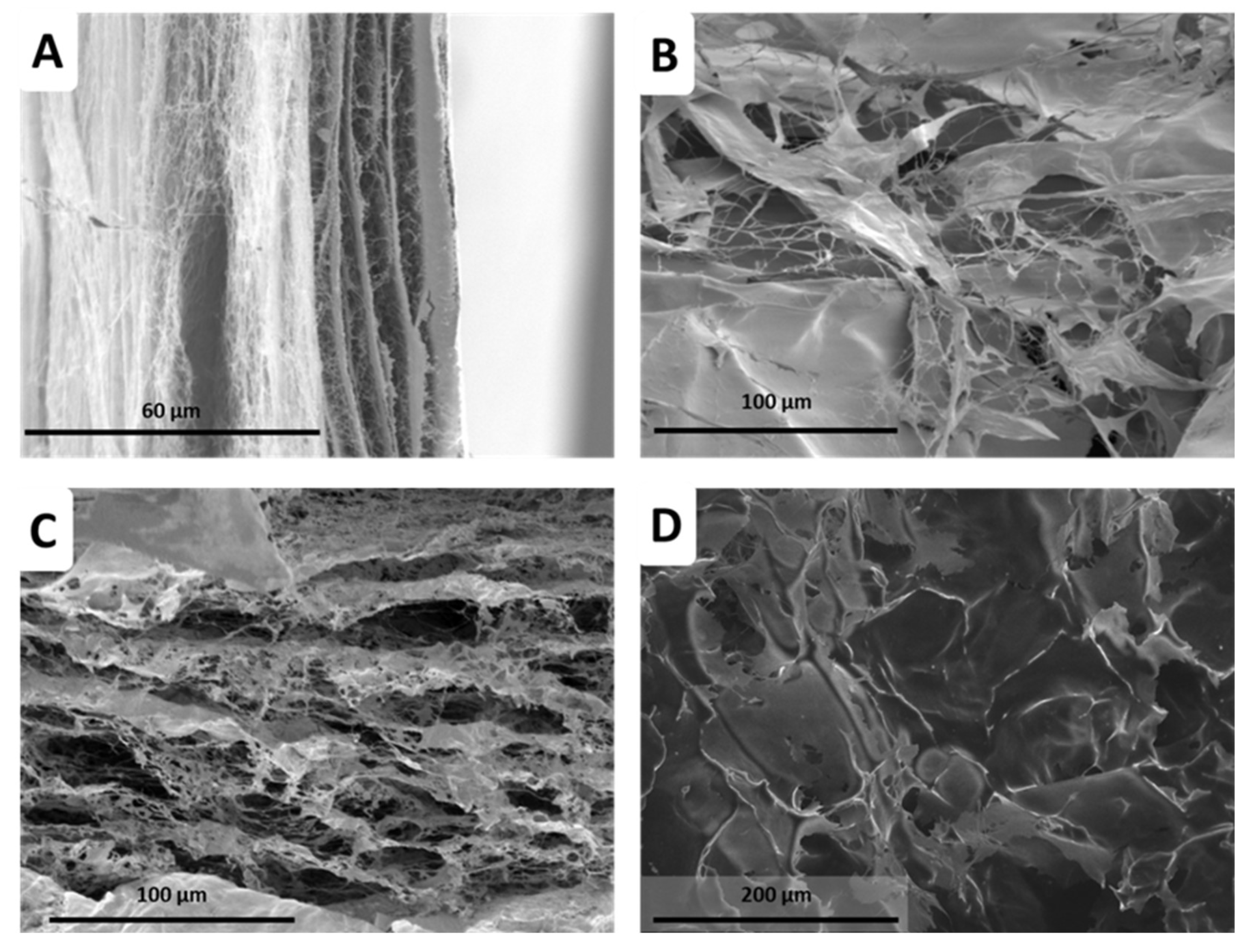
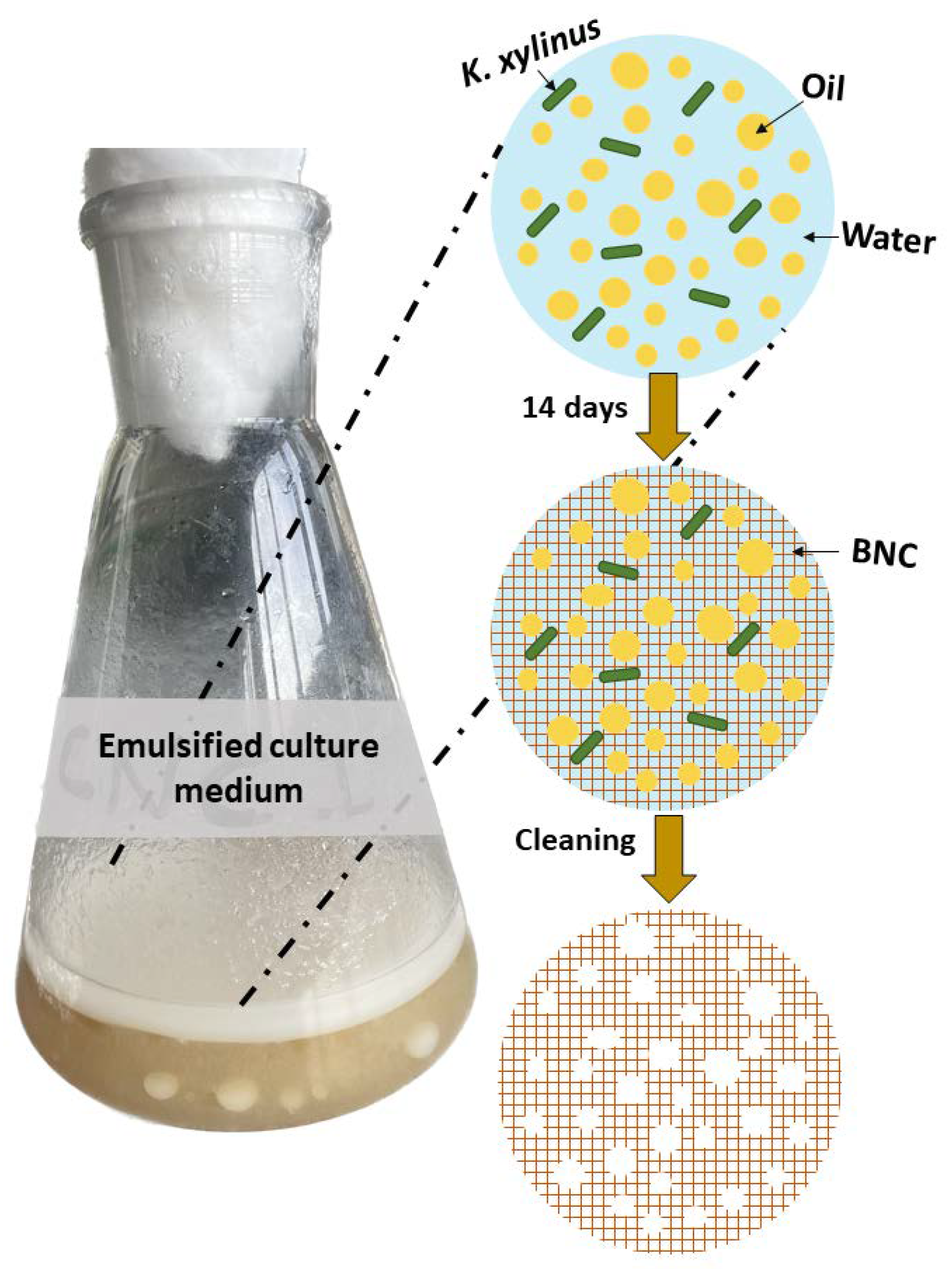
2.3. BNC Production using PEs as Template
3. Materials and Methods
3.1. Materials and Reagents
3.2. Synthesis of NBs
3.3. Characterization of NBs
3.4. Preparation of Oil-in-Water PEs
3.5. Characterization of Oil-in-Water PEs
3.6. Production of BNC Composites
3.7. Characterization of BNC Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Carvalho-Guimarães, F.B.; Correa, K.L.; de Souza, T.P.; Rodríguez Amado, J.R.; Ribeiro-Costa, R.M.; Silva-Júnior, J.O.C. A Review of Pickering Emulsions: Perspectives and Applications. Pharmaceuticals 2022, 15, 1413. [Google Scholar] [CrossRef]
- Low, L.E.; Siva, S.P.; Ho, Y.K.; Chan, E.S.; Tey, B.T. Recent Advances of Characterization Techniques for the Formation, Physical Properties and Stability of Pickering Emulsion. Adv. Colloid Interface Sci. 2020, 277, 102117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, D.; Liu, S.; Tang, J. Bacterial Cellulose Nanofibril-Based Pickering Emulsions: Recent Trends and Applications in the Food Industry. Foods 2022, 11, 4064. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wu, J.; Zhang, H.; Chen, Y.; Ma, L.; Huang, H.; Huang, Y.; Zhang, Y. Recent Advances on Cellulose Nanocrystals for Pickering Emulsions: Development and Challenge. Trends Food Sci. Technol. 2020, 102, 16–29. [Google Scholar] [CrossRef]
- Ma, T.; Cui, R.; Lu, S.; Hu, X.; Xu, B.; Song, Y.; Hu, X. High Internal Phase Pickering Emulsions Stabilized by Cellulose Nanocrystals for 3D Printing. Food Hydrocoll. 2022, 125, 107418. [Google Scholar] [CrossRef]
- Xie, Y.; Lei, Y.; Rong, J.; Zhang, X.; Li, J.; Chen, Y.; Liang, H.; Li, Y.; Li, B.; Fang, Z.; et al. Physico-Chemical Properties of Reduced-Fat Biscuits Prepared Using O/W Cellulose-Based Pickering Emulsion. LWT 2021, 148, 111745. [Google Scholar] [CrossRef]
- Mougel, J.B.; Bertoncini, P.; Cathala, B.; Chauvet, O.; Capron, I. Macroporous Hybrid Pickering Foams Based on Carbon Nanotubes and Cellulose Nanocrystals. J. Colloid Interface Sci. 2019, 544, 78–87. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a Natural Source for Groundbreaking Applications in Materials Science: Today’s State. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Gopakumar, D.; Ahmad, I.; Thomas, S.; Dufresne, A.; Huang, J.; Lin, N. Advances in Cellulose Nanomaterials. Cellulose 2018, 25, 2151–2189. [Google Scholar] [CrossRef]
- Benselfelt, T.; Kummer, N.; Nordenström, M.; Fall, A.B.; Nyström, G.; Wågberg, L. The Colloidal Properties of Nanocellulose. ChemSusChem 2023, 16, e202201955. [Google Scholar] [CrossRef]
- Dortez, S.; Sierra, T.; Álvarez-Sánchez, M.Á.; González-Domínguez, J.M.; Benito, A.M.; Maser, W.K.; Crevillen, A.G.; Escarpa, A. Effect of Nanocellulose Polymorphism on Electrochemical Analytical Performance in Hybrid Nanocomposites with Non-Oxidized Single-Walled Carbon Nanotubes. Microchim. Acta 2022, 189, 62. [Google Scholar] [CrossRef]
- González-Domínguez, J.M.; Ansón-Casaos, A.; Grasa, L.; Abenia, L.; Salvador, A.; Colom, E.; Mesonero, J.E.; García-Bordejé, J.E.; Benito, A.M.; Maser, W.K. Unique Properties and Behavior of Nonmercerized Type-II Cellulose Nanocrystals as Carbon Nanotube Biocompatible Dispersants. Biomacromolecules 2019, 20, 3147–3160. [Google Scholar] [CrossRef]
- González-Domínguez, J.M.; Grasa, L.; Frontiñán-Rubio, J.; Abás, E.; Domínguez-Alfaro, A.; Mesonero, J.E.; Criado, A.; Ansón-Casaos, A. Intrinsic and Selective Activity of Functionalized Carbon Nanotube/Nanocellulose Platforms against Colon Cancer Cells. Colloids Surf. B Biointerfaces 2022, 212, 112363. [Google Scholar] [CrossRef]
- Calvo, V.; Álvarez Sánchez, M.Á.; Güemes, L.; Martínez-Barón, C.; Baúlde, S.; Criado, A.; González-Domínguez, J.M.; Maser, W.K.; Benito, A.M.; Miguel, A.A.; et al. Preparation of Cellulose Nanocrystals: Controlling the Crystalline Type by One-Pot Acid Hydrolysis. ACS Macro Lett. 2023, 12, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Wolcotta, M.P.; Liua, H.; Wang, J. Developing chitin nanocrystals for flexible packaging coatings. Carbohydr. Polym. 2019, 226, 115276. [Google Scholar] [CrossRef]
- Yang, T.; Qi, H.; Liu, P.; Zhang, K. Selective Isolation Methods for Cellulose and Chitin Nanocrystals. ChemPlusChem 2020, 85, 1081–1088. [Google Scholar] [CrossRef]
- Bai, L.; Liu, L.; Esquivel, M.; Tardy, B.L.; Huan, S.; Niu, X.; Liu, S.; Yang, G.; Fan, Y.; Rojas, O.J. Nanochitin: Chemistry, Structure, Assembly, and Applications. Chem. Rev. 2022, 122, 11604–11674. [Google Scholar] [CrossRef]
- Peng, G.; Wu, D. Insight into Different Roles of Chitin Nanocrystals and Cellulose Nanocrystals towards Stabilizing Pickering Emulsions. Food Hydrocoll. 2022, 131, 107808. [Google Scholar] [CrossRef]
- Tzoumaki, M.V.; Moschakis, T.; Kiosseoglou, V.; Biliaderis, C.G. Oil-in-Water Emulsions Stabilized by Chitin Nanocrystal Particles. Food Hydrocoll. 2011, 25, 1521–1529. [Google Scholar] [CrossRef]
- Li, J.; Revol, J.F.; Marchessault, R.H. Effect of Degree of Deacetylation of Chitin on the Properties of Chitin Crystallites. J. Appl. Polym. Sci. 1997, 65, 373–380. [Google Scholar] [CrossRef]
- Xing, L.; Gu, J.; Zhang, W.; Tu, D.; Hu, C. Cellulose I and II Nanocrystals Produced by Sulfuric Acid Hydrolysis of Tetra Pak Cellulose, I. Carbohydr. Polym. 2018, 192, 184–192. [Google Scholar] [CrossRef]
- Han, J.; Zhou, C.; Wu, Y.; Liu, F.; Wu, Q. Self-Assembling Behavior of Cellulose Nanoparticles during Freeze-Drying: Effect of Suspension Concentration, Particle Size, Crystal Structure, and Surface Charge. Biomacromolecules 2013, 14, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. New Pickering Emulsions Stabilized by Bacterial Cellulose Nanocrystals. Langmuir 2011, 27, 7471–7479. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, J.; Gong, J.; Kuang, Y.; Mo, L.; Song, T. Cellulose Nanocrystals (CNCs) with Different Crystalline Allomorph for Oil in Water Pickering Emulsions. Carbohydr. Polym. 2018, 183, 303–310. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Surface Chemistry, Morphological Analysis and Properties of Cellulose Nanocrystals with Gradiented Sulfation Degrees. Nanoscale 2014, 6, 5384–5393. [Google Scholar] [CrossRef]
- Flauzino Neto, W.P.; Putaux, J.L.; Mariano, M.; Ogawa, Y.; Otaguro, H.; Pasquini, D.; Dufresne, A. Comprehensive Morphological and Structural Investigation of Cellulose I and II Nanocrystals Prepared by Sulphuric Acid Hydrolysis. RSC Adv. 2016, 6, 76017–76027. [Google Scholar] [CrossRef]
- Calvo, V.; González-Domínguez, J.M.; Benito, A.M.; Maser, W.K. Synthesis and Processing of Nanomaterials Mediated by Living Organisms. Angew. Chem. Int. Ed. 2022, 61, e202113286. [Google Scholar] [CrossRef]
- Esa, F.; Tasirin, S.M.; Rahman, N.A. Overview of Bacterial Cellulose Production and Application. Agric. Agric. Sci. Procedia 2014, 2, 113–119. [Google Scholar] [CrossRef]
- Calvo, V.; Torrubia, J.; Blanco, D.; García-Bordeje, E.; Maser, W.K.; Benito, A.M.; González-Domínguez, J.M. Optimizing Bacterial Cellulose Production towards Materials for Water Remediation. In NATO Science for Peace and Security Series B: Physics and Biophysics; Springer: Berlin/Heidelberg, Germany, 2020; pp. 391–403. [Google Scholar]
- Żywickaa, A.; Junka, A.F.; Szymczykc, P.; Chodaczekd, G.; Grzesiake, J.; Sedghizadehf, P.P.; Fijałkowski, K. Bacterial cellulose yield increased over 500% by supplementation of medium with vegetable oil. Carbohydr. Polym. 2018, 199, 294–303. [Google Scholar] [CrossRef]
- Stumpf, T.R.; Yang, X.; Zhang, J.; Cao, X. In Situ and Ex Situ Modifications of Bacterial Cellulose for Applications in Tissue Engineering. Mater. Sci. Eng. C 2018, 82, 372–383. [Google Scholar] [CrossRef]
- Park, S.; Park, J.; Jo, I.; Cho, S.P.; Sung, D.; Ryu, S.; Park, M.; Min, K.A.; Kim, J.; Hong, S.; et al. In Situ Hybridization of Carbon Nanotubes with Bacterial Cellulose for Three-Dimensional Hybrid Bioscaffolds. Biomaterials 2015, 58, 93–102. [Google Scholar] [CrossRef]
- Piaia, L.; Pittella, C.Q.P.; De Souza, S.S.; Berti, F.V.; Porto, L.M. Incorporation of Aloe Vera Extract in Bacterial Nanocellulose Membranes. Polimeros 2022, 32, e2022002. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.; Kim, H.; Kim, H.J.; Yang, Y.-H.; Kim, Y.H.; Jung, S.-K.; Kan, E.; Lee, S.H. Alginate/Bacterial Cellulose Nanocomposite Beads Prepared Using Gluconacetobacter xylinus and Their Application in Lipase Immobilization. Carbohydr. Polym. 2017, 157, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Schaffner, M.; Rühs, P.A.; Coulter, F.; Kilcher, S.; Studart, A.R. 3D Printing of Bacteria into Functional Complex Materials. Sci. Adv. 2017, 3, eaao6804. [Google Scholar] [CrossRef]
- Pepicelli, M.; Binelli, M.R.; Studart, A.R.; Rühs, P.A.; Fischer, P. Self-Grown Bacterial Cellulose Capsules Made through Emulsion Templating. ACS Biomater. Sci. Eng. 2021, 7, 3221–3228. [Google Scholar] [CrossRef]
- Lu, H.; Sun, S.; Sun, J.; Peng, X.; Li, N.; Ullah, M.W.; Zhang, Y.; Chen, L.; Zhou, J. Sustainable production of flocculant-containing bacterial cellulose composite for removal of PET nano-plastics. Chem. Eng. J. 2023, 469, 143848. [Google Scholar] [CrossRef]
- Żywickaa, A.; Wenelska, K.; Junka, A.; Czajkowska, J.; Fijałkowski, K. An efficient method of Yarrowia lipolytica immobilization using oil-and emulsion-modified bacterial cellulose carriers. Electron. J. Biotechnol. 2019, 41, 30–36. [Google Scholar] [CrossRef]
- Pongjinapeth, T.; Sudying, P.; Jaturapiree, P. The utilization of wastewater of Thai fermented rice noodle (Kanom-jeen) manufacturing process for the production of bacterial cellulose by Acetobacter xylinim TISTR 975. IOP Conf. Ser. Mater. Sci. Eng. 2020, 773, 012039. [Google Scholar] [CrossRef]
- Zeng, M.; Laromaine, A.; Roig, A. Bacterial Cellulose Films: Influence of Bacterial Strain and Drying Route on Film Properties. Cellulose 2014, 21, 4455–4469. [Google Scholar] [CrossRef]
- Kanno, T.; Uyama, H. Unique Ivy-like Morphology Composed of Poly(Lactic Acid) and Bacterial Cellulose Cryogel. ACS Omega 2018, 3, 631–635. [Google Scholar] [CrossRef]
- Stoica-Guzun, A.; Stroescu, M.; Jinga, S.I.; Mihalache, N.; Botez, A.; Matei, C.; Berger, D.; Damian, C.M.; Ionita, V. Box-Behnken experimental design for chromium(VI) ions removal by bacterial cellulose-magnetite composites. Int. J. Biol. Macromol. 2016, 91, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Critical Review of Techniques and Methodologies for Characterization of Emulsion Stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef] [PubMed]
- Hestrin, S.; Schramm, M. Synthesis of Cellulose by Acetobacter xylinum. 2. Preparation of Freeze-Dried Cells Capable of Polymerizing Glucose to Cellulose. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo, V.; Fuentes, L.; Berdejo, D.; González-Domínguez, J.M.; Maser, W.K.; Benito, A.M. Oil-in-Water Pickering Emulsions Stabilized with Nanostructured Biopolymers: A Venue for Templating Bacterial Cellulose. Int. J. Mol. Sci. 2023, 24, 13141. https://doi.org/10.3390/ijms241713141
Calvo V, Fuentes L, Berdejo D, González-Domínguez JM, Maser WK, Benito AM. Oil-in-Water Pickering Emulsions Stabilized with Nanostructured Biopolymers: A Venue for Templating Bacterial Cellulose. International Journal of Molecular Sciences. 2023; 24(17):13141. https://doi.org/10.3390/ijms241713141
Chicago/Turabian StyleCalvo, Víctor, Laura Fuentes, Daniel Berdejo, José M. González-Domínguez, Wolfgang K. Maser, and Ana M. Benito. 2023. "Oil-in-Water Pickering Emulsions Stabilized with Nanostructured Biopolymers: A Venue for Templating Bacterial Cellulose" International Journal of Molecular Sciences 24, no. 17: 13141. https://doi.org/10.3390/ijms241713141




