Are Antimicrobial Peptides a 21st-Century Solution for Atopic Dermatitis?
Abstract
1. Introduction
2. Atopic Dermatitis
3. Antimicrobial Peptides
3.1. Human AMPs
3.2. Microbial AMPs
3.3. Animal-Sourced AMPs
4. AMPs in Clinical Trials
5. Limitations to AMPs Usage
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moniz, T.; Costa Lima, S.A.; Reis, S. Human skin models: From healthy to disease-mimetic systems; characteristics and applications. Br. J. Pharmacol. 2020, 177, 4314–4329. [Google Scholar] [CrossRef]
- Lima, S.C.; Reis, S. Nanoparticles in Life Sciences and Biomedicine; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Chinnappan, M.; Harris-Tryon, T.A. Novel mechanisms of microbial crosstalk with skin innate immunity. Exp. Dermatol. 2021, 30, 1484–1495. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Park, K.; Kabashima, K. Immunology of the Skin: Basic and Clinical Sciences in Skin Immune Responses; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Lynch, B.; Pageon, H.; Le Blay, H.; Brizion, S.; Bastien, P.; Bornschlögl, T.; Domanov, Y. A mechanistic view on the aging human skin through ex vivo layer-by-layer analysis of mechanics and microstructure of facial and mammary dermis. Sci. Rep. 2022, 12, 849. [Google Scholar] [CrossRef]
- Asher, M.I.; Montefort, S.; Björkstén, B.; Lai, C.K.W.; Strachan, D.P.; Weiland, S.K.; Williams, H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Nutten, S. Atopic Dermatitis: Global Epidemiology and Risk Factors. Ann. Nutr. Metab. 2015, 66 (Suppl. S1), 8–16. [Google Scholar] [CrossRef]
- Barbarot, S.; Auziere, S.; Gadkari, A.; Girolomoni, G.; Puig, L.; Simpson, E.L.; Margolis, D.J.; de Bruin-Weller, M.; Eckert, L. Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy 2018, 73, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Kowalska-Olędzka, E.; Czarnecka, M.; Baran, A. Epidemiology of atopic dermatitis in Europe. J. Drug Assess. 2019, 8, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Mallol, J.; Crane, J.; von Mutius, E.; Odhiambo, J.; Keil, U.; Stewart, A. The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three: A global synthesis. Allergol. Immunopathol. 2013, 41, 73–85. [Google Scholar] [CrossRef]
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular Mechanisms of Atopic Dermatitis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4130. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.A.; Vocanson, M.; Nicolas, J.-F.; Wolf, P.; Patra, V. Microbial derived antimicrobial peptides as potential therapeutics in atopic dermatitis. Front. Immunol. 2023, 14, 1125635. [Google Scholar] [CrossRef] [PubMed]
- Kulthanan, K.; Samutrapong, P.; Jiamton, S.; Tuchinda, P. Adult-onset atopic dermatitis: A cross-sectional study of natural history and clinical manifestation. Asian Pac. J. Allergy Immunol. 2007, 25, 207. [Google Scholar] [PubMed]
- Silverberg, J.I. Atopic dermatitis in adults. Med. Clin. 2020, 104, 157–176. [Google Scholar] [CrossRef]
- Silverberg, J.I. Comorbidities and the impact of atopic dermatitis. Ann. Allergy Asthma Immunol. 2019, 123, 144–151. [Google Scholar] [CrossRef]
- Nguyen, H.L.T.; Peng, G.; Trujillo-Paez, J.V.; Yue, H.; Ikutama, R.; Takahashi, M.; Umehara, Y.; Okumura, K.; Ogawa, H.; Ikeda, S.; et al. The Antimicrobial Peptide AMP-IBP5 Suppresses Dermatitis-like Lesions in a Mouse Model of Atopic Dermatitis through the Low-Density Lipoprotein Receptor-Related Protein-1 Receptor. Int. J. Mol. Sci. 2023, 24, 5200. [Google Scholar] [CrossRef] [PubMed]
- Morelli, P.; Gaspari, M.; Gabriele, C.; Dastoli, S.; Bennardo, L.; Pavel, A.B.; Patruno, C.; Del Duca, E.; Nisticò, S.P. Proteomic analysis from skin swabs reveals a new set of proteins identifying skin impairment in atopic dermatitis. Exp. Dermatol. 2021, 30, 811–819. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Skov, L.; Hamann, C.R.; Gislason, G.H.; Egeberg, A. Assessment of major comorbidities in adults with atopic dermatitis using the Charlson comorbidity index. J. Am. Acad. Dermatol. 2017, 76, 1088–1092.e1081. [Google Scholar] [CrossRef]
- Andersen, Y.M.; Egeberg, A.; Skov, L.; Thyssen, J.P. Comorbidities of atopic dermatitis: Beyond rhinitis and asthma. Curr. Dermatol. Rep. 2017, 6, 35–41. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Gelfand, J.; Margolis, D.; Boguniewicz, M.; Fonacier, L.S.; Grayson, M.H.; Ong, P.Y.; Fuxench, Z.C.; Simpson, E.L. Measurement properties of Hospital Anxiety and Depression Scale used in atopic dermatitis in adults. J. Allergy Clin. Immunol. 2019, 143, AB130. [Google Scholar] [CrossRef]
- Bieber, T. Atopic dermatitis: An expanding therapeutic pipeline for a complex disease. Nat. Rev. Drug Discov. 2022, 21, 21–40. [Google Scholar] [CrossRef]
- Sasson, E.; Anzi, S.; Bell, B.; Yakovian, O.; Zorsky, M.; Deutsch, U.; Engelhardt, B.; Sherman, E.; Vatine, G.; Dzikowski, R. Nano-scale architecture of blood-brain barrier tight-junctions. Elife 2021, 10, e63253. [Google Scholar] [CrossRef]
- Paradis, T.; Bègue, H.; Basmaciyan, L.; Dalle, F.; Bon, F. Tight junctions as a key for pathogens invasion in intestinal epithelial cells. Int. J. Mol. Sci. 2021, 22, 2506. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Niyonsaba, F.; Kiatsurayanon, C.; Ushio, H.; Fujimura, T.; Ueno, T.; Okumura, K.; Ogawa, H.; Ikeda, S. The human cathelicidin LL-37 host defense peptide upregulates tight junction-related proteins and increases human epidermal keratinocyte barrier function. J. Innate Immun. 2014, 6, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, S.; Makris, M.; Vakirlis, E.; Gregoriou, S. The Role of Tight Junctions in Atopic Dermatitis: A Systematic Review. J. Clin. Med. 2023, 12, 1538. [Google Scholar] [CrossRef] [PubMed]
- Yuki, T.; Komiya, A.; Kusaka, A.; Kuze, T.; Sugiyama, Y.; Inoue, S. Impaired tight junctions obstruct stratum corneum formation by altering polar lipid and profilaggrin processing. J. Dermatol. Sci. 2013, 69, 148–158. [Google Scholar] [CrossRef]
- Yokouchi, M.; Kubo, A.; Kawasaki, H.; Yoshida, K.; Ishii, K.; Furuse, M.; Amagai, M. Epidermal tight junction barrier function is altered by skin inflammation, but not by filaggrin-deficient stratum corneum. J. Dermatol. Sci. 2015, 77, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Seguchi, T.; Chang-Yi, C.; Kusuda, S.; Takahashi, M.; Aisu, K.; Tezuka, T. Decreased expression of filaggrin in atopic skin. Arch. Dermatol. Res. 1996, 288, 442–446. [Google Scholar] [CrossRef]
- Jensen, J.-M.; Fölster-Holst, R.; Baranowsky, A.; Schunck, M.; Winoto-Morbach, S.; Neumann, C.; Schütze, S.; Proksch, E. Impaired sphingomyelinase activity and epidermal differentiation in atopic dermatitis. J. Investig. Dermatol. 2004, 122, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.E.; Leung, D.Y.; Boguniewicz, M.; Howell, M.D. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin. Immunol. 2008, 126, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Common, J.; Haines, R.; Balakrishnan, A.; Brown, S.; Goh, C.; Cordell, H.; Sandilands, A.; Campbell, L.; Kroboth, K. Wide spectrum of filaggrin-null mutations in atopic dermatitis highlights differences between Singaporean Chinese and European populations. Br. J. Dermatol. 2011, 165, 106–114. [Google Scholar] [CrossRef]
- Elias, P.; Schmuth, M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr. Allergy Asthma Rep. 2009, 9, 265–272. [Google Scholar] [CrossRef]
- Palmer, C.N.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef]
- Jurakic Toncic, R.; Kezic, S.; Jakasa, I.; Ljubojevic Hadzavdic, S.; Balic, A.; Petkovic, M.; Pavicic, B.; Zuzul, K.; Marinovic, B. Filaggrin loss-of-function mutations and levels of filaggrin degradation products in adult patients with atopic dermatitis in Croatia. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Clausen, M.L.; Edslev, S.; Andersen, P.; Clemmensen, K.; Krogfelt, K.; Agner, T. Staphylococcus aureus colonization in atopic eczema and its association with filaggrin gene mutations. Br. J. Dermatol. 2017, 177, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef]
- Williams, M.R.; Costa, S.K.; Zaramela, L.S.; Khalil, S.; Todd, D.A.; Winter, H.L.; Sanford, J.A.; O’Neill, A.M.; Liggins, M.C.; Nakatsuji, T. Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis. Sci. Transl. Med. 2019, 11, eaat8329. [Google Scholar] [CrossRef] [PubMed]
- Rerknimitr, P.; Otsuka, A.; Nakashima, C.; Kabashima, K. The etiopathogenesis of atopic dermatitis: Barrier disruption, immunological derangement, and pruritus. Inflamm. Regen. 2017, 37, 14. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, N.; Flohr, C.; Irvine, A.D. The exposome in atopic dermatitis. Allergy 2020, 75, 63–74. [Google Scholar] [CrossRef]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; Program, N.C.S.; et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef]
- Geoghegan, J.A.; Irvine, A.D.; Foster, T.J. Staphylococcus aureus and Atopic Dermatitis: A Complex and Evolving Relationship. Trends Microbiol. 2018, 26, 484–497. [Google Scholar] [CrossRef]
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Perrett, K.P.; Peters, R.L. Emollients for prevention of atopic dermatitis in infancy. Lancet 2020, 395, 923–924. [Google Scholar] [CrossRef] [PubMed]
- Hadi, H.A.; Tarmizi, A.I.; Khalid, K.A.; Gajdács, M.; Aslam, A.; Jamshed, S. The Epidemiology and Global Burden of Atopic Dermatitis: A Narrative Review. Life 2021, 11, 936. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, T. Immune dysregulation in the pathogenesis of atopic dermatitis. Dermatitis 2018, 29, 57–62. [Google Scholar] [CrossRef]
- Behzadi, P.; Gajdács, M. Dos and don’ts of a successfully peer-reviewed publication: From A–Z. Eur. J. Microbiol. Immunol. 2020, 10, 125–130. [Google Scholar] [CrossRef]
- Thyssen, J.; Vestergaard, C.; Deleuran, M.; de Bruin-Weller, M.; Bieber, T.; Taieb, A.; Seneschal, J.; Cork, M.; Paul, C.; Flohr, C. European Task Force on Atopic Dermatitis (ETFAD): Treatment targets and treatable traits in atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e839–e842. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, C.C.; Reich, P.; Fritz, S.; Coughlin, C.C. Staphylococcus aureus antibiotic susceptibility patterns in pediatric atopic dermatitis. Pediatr. Dermatol. 2019, 36, 482–485. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 16, 843–862. [Google Scholar] [CrossRef]
- Otero, M.E.; van den Reek, J.M.; Seyger, M.M.; van de Kerkhof, P.C.; Kievit, W.; de Jong, E.M. Determinants for drug survival of methotrexate in patients with psoriasis, split according to different reasons for discontinuation: Results of the prospective MTX-CAPTURE. Br. J. Dermatol. 2017, 177, 497–504. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, Y.; Liu, H.; Qiu, F.; Liang, C.L.; Zhang, Q.; Huang, R.Y.; Han, L.; Lu, C.; Dai, Z. Dihydroartemisinin ameliorates psoriatic skin inflammation and its relapse by diminishing CD8(+) T-cell memory in wild-type and humanized mice. Theranostics 2020, 10, 10466–10482. [Google Scholar] [CrossRef]
- Gutiérrez-Vázquez, C.; Quintana, F.J. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity 2018, 48, 19–33. [Google Scholar] [CrossRef]
- Schlessinger, J.; Shepard, J.S.; Gower, R.; Su, J.C.; Lynde, C.; Cha, A.; Ports, W.C.; Purohit, V.; Takiya, L.; Werth, J.L. Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to< 24 months with mild-to-moderate atopic dermatitis: A phase IV open-label study (CrisADe CARE 1). Am. J. Clin. Dermatol. 2020, 21, 275–284. [Google Scholar]
- Lin, C.M.; Cooles, F.A.; Isaacs, J.D. Basic mechanisms of JAK inhibition. Mediterr. J. Rheumatol. 2020, 31, 100. [Google Scholar] [CrossRef]
- Blair, H.A. Tralokinumab in Atopic Dermatitis: A Profile of Its Use. Clin. Drug Investig. 2022, 42, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Agboola, F.; Atlas, S.J.; Brouwer, E.; Carlson, J.J.; Hansen, R.N.; Herron-Smith, S.; Nhan, E.; Rind, D.M.; Pearson, S.D. JAK inhibitors and monoclonal antibodies for the treatment of atopic dermatitis: Effectiveness and value. J. Manag. Care Spec. Pharm. 2022, 28, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; Maurelli, M.; Costanzo, A.; Esposito, M.; Girolomoni, G. The Combination of Dupilumab with Other Monoclonal Antibodies. Dermatol. Ther. 2023, 13, 7–12. [Google Scholar] [CrossRef]
- Li, X.; Zuo, S.; Wang, B.; Zhang, K.; Wang, Y. Antimicrobial Mechanisms and Clinical Application Prospects of Antimicrobial Peptides. Molecules 2022, 27, 2675. [Google Scholar] [CrossRef]
- Thakur, A.; Sharma, A.; Alajangi, H.K.; Jaiswal, P.K.; Lim, Y.-B.; Singh, G.; Barnwal, R.P. In pursuit of next-generation therapeutics: Antimicrobial peptides against superbugs, their sources, mechanism of action, nanotechnology-based delivery, and clinical applications. Int. J. Biol. Macromol. 2022, 218, 135–156. [Google Scholar] [CrossRef]
- Diamond, G.; Beckloff, N.; Weinberg, A.; Kisich, K.O. The roles of antimicrobial peptides in innate host defense. Curr. Pharm. Des. 2009, 15, 2377–2392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-j.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14–R19. [Google Scholar] [CrossRef]
- Klubthawee, N.; Adisakwattana, P.; Hanpithakpong, W.; Somsri, S.; Aunpad, R. A novel, rationally designed, hybrid antimicrobial peptide, inspired by cathelicidin and aurein, exhibits membrane-active mechanisms against Pseudomonas aeruginosa. Sci. Rep. 2020, 10, 9117. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial peptides: Diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef]
- Nakamura, T.; Furunaka, H.; Miyata, T.; Tokunaga, F.; Muta, T.; Iwanaga, S.; Niwa, M.; Takao, T.; Shimonishi, Y. Tachyplesin, a class of antimicrobial peptide from the hemocytes of the horseshoe crab (Tachypleus tridentatus). Isolation and chemical structure. J. Biol. Chem. 1988, 263, 16709–16713. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.-P.S.; Hancock, R.E. The relationship between peptide structure and antibacterial activity. Peptides 2003, 24, 1681–1691. [Google Scholar] [CrossRef]
- Dash, R.; Bhattacharjya, S. Thanatin: An emerging host defense antimicrobial peptide with multiple modes of action. Int. J. Mol. Sci. 2021, 22, 1522. [Google Scholar] [CrossRef]
- Fehlbaum, P.; Bulet, P.; Chernysh, S.; Briand, J.-P.; Roussel, J.-P.; Letellier, L.; Hetru, C.; Hoffmann, J.A. Structure-activity analysis of thanatin, a 21-residue inducible insect defense peptide with sequence homology to frog skin antimicrobial peptides. Proc. Natl. Acad. Sci. USA 1996, 93, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. The antimicrobial peptide database provides a platform for decoding the design principles of naturally occurring antimicrobial peptides. Protein Sci. 2020, 29, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Hanchi, H.; Hammami, R.; Fernandez, B.; Kourda, R.; Ben Hamida, J.; Fliss, I. Simultaneous production of formylated and nonformylated enterocins L50A and L50B as well as 61A, a new glycosylated durancin, by Enterococcus durans 61A, a strain isolated from artisanal fermented milk in Tunisia. J. Agric. Food Chem. 2016, 64, 3584–3590. [Google Scholar] [CrossRef]
- Garg, N.; Tang, W.; Goto, Y.; Nair, S.K.; Van Der Donk, W.A. Lantibiotics from Geobacillus thermodenitrificans. Proc. Natl. Acad. Sci. USA 2012, 109, 5241–5246. [Google Scholar] [CrossRef]
- Elsayed, S.S.; Trusch, F.; Deng, H.; Raab, A.; Prokes, I.; Busarakam, K.; Asenjo, J.A.; Andrews, B.A.; Van West, P.; Bull, A.T. Chaxapeptin, a lasso peptide from extremotolerant Streptomyces leeuwenhoekii strain C58 from the hyperarid Atacama Desert. J. Org. Chem. 2015, 80, 10252–10260. [Google Scholar] [CrossRef]
- Hudson, G.A.; Burkhart, B.J.; DiCaprio, A.J.; Schwalen, C.J.; Kille, B.; Pogorelov, T.V.; Mitchell, D.A. Bioinformatic mapping of radical S-adenosylmethionine-dependent ribosomally synthesized and post-translationally modified peptides identifies new Cα, Cβ, and Cγ-linked thioether-containing peptides. J. Am. Chem. Soc. 2019, 141, 8228–8238. [Google Scholar] [CrossRef]
- Tang, Y.-Q.; Yuan, J.; Osapay, G.; Osapay, K.; Tran, D.; Miller, C.J.; Ouellette, A.J.; Selsted, M.E. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated α-defensins. Science 1999, 286, 498–502. [Google Scholar] [CrossRef]
- Nguyen, H.L.T.; Trujillo-Paez, J.V.; Umehara, Y.; Yue, H.; Peng, G.; Kiatsurayanon, C.; Chieosilapatham, P.; Song, P.; Okumura, K.; Ogawa, H.; et al. Role of Antimicrobial Peptides in Skin Barrier Repair in Individuals with Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 7607. [Google Scholar] [CrossRef]
- Clausen, M.-L.; Slotved, H.-C.; Krogfelt, K.A.; Andersen, P.S.; Agner, T. In vivo expression of antimicrobial peptides in atopic dermatitis. Exp. Dermatol. 2016, 25, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Gläser, R.; Meyer-Hoffert, U.; Harder, J.; Cordes, J.; Wittersheim, M.; Kobliakova, J.; Fölster-Holst, R.; Proksch, E.; Schröder, J.-M.; Schwarz, T. The antimicrobial protein psoriasin (S100A7) is upregulated in atopic dermatitis and after experimental skin barrier disruption. J. Investig. Dermatol. 2009, 129, 641–649. [Google Scholar] [CrossRef]
- Saville, C.R.; Metris, A.; Humphreys, G.J.; O’Neill, C.; Barrett, P.; Fernandez-Piquer, J.; McBain, A.J. Transitory Shifts in Skin Microbiota Composition and Reductions in Bacterial Load and Psoriasin following Ethanol Perturbation. Msphere 2022, 7, e00171–e00122. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Skrygan, M.; Tomi, N.S.; Othlinghaus, N.; Brockmeyer, N.H.; Altmeyer, P.; Kreuter, A. Differential mRNA expression of antimicrobial peptides and proteins in atopic dermatitis as compared to psoriasis vulgaris and healthy skin. Int. Arch. Allergy Immunol. 2008, 147, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, F.; Dreyer, S.; Kopfnagel, V.; Gläser, R.; Werfel, T.; Harder, J. The antimicrobial and immunomodulatory function of RNase 7 in skin. Front. Immunol. 2019, 10, 2553. [Google Scholar] [CrossRef]
- Suwanchote, S.; Waitayangkoon, P.; Chancheewa, B.; Inthanachai, T.; Niwetbowornchai, N.; Edwards, S.W.; Virakul, S.; Thammahong, A.; Kiatsurayanon, C.; Rerknimitr, P. Role of antimicrobial peptides in atopic dermatitis. Int. J. Dermatol. 2022, 61, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Alatas, E.T.; Kara Polat, A.; Kalayci, M.; Dogan, G.; Akin Belli, A. Plasma dermcidin levels in acne patients, and the effect of isotretinoin treatment on dermcidin levels. Dermatol. Ther. 2019, 32, e13044. [Google Scholar] [CrossRef]
- Kanda, N.; Hau, C.; Tada, Y.; Sato, S.; Watanabe, S. Decreased serum LL-37 and vitamin D 3 levels in atopic dermatitis: Relationship between IL-31 and oncostatin M. Allergy 2012, 67, 804–812. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Björn, C.; Ekblom, J. Antimicrobial peptides as therapeutic agents: Opportunities and challenges. Crit. Rev. Biotechnol. 2020, 40, 978–992. [Google Scholar] [CrossRef] [PubMed]
- Răileanu, M.; Borlan, R.; Campu, A.; Janosi, L.; Turcu, I.; Focsan, M.; Bacalum, M. No country for old antibiotics! Antimicrobial peptides (AMPs) as next-generation treatment for skin and soft tissue infection. Int. J. Pharm. 2023, 642, 123169. [Google Scholar] [CrossRef] [PubMed]
- Do, N.; Weindl, G.; Grohmann, L.; Salwiczek, M.; Koksch, B.; Korting, H.C.; Schäfer-Korting, M. Cationic membrane-active peptides–anticancer and antifungal activity as well as penetration into human skin. Exp. Dermatol. 2014, 23, 326–331. [Google Scholar] [CrossRef]
- Pfalzgraff, A.; Brandenburg, K.; Weindl, G. Antimicrobial Peptides and Their Therapeutic Potential for Bacterial Skin Infections and Wounds. Front. Pharmacol. 2018, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.; Sasaki, K.; Minamino, N. Peptidomics-based discovery of an antimicrobial peptide derived from insulin-like growth factor-binding protein 5. J. Proteome Res. 2011, 10, 1870–1880. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Kiatsurayanon, C.; Chieosilapatham, P.; Ogawa, H. Friends or Foes? Host defense (antimicrobial) peptides and proteins in human skin diseases. Exp. Dermatol. 2017, 26, 989–998. [Google Scholar] [CrossRef]
- Yue, H.; Song, P.; Sutthammikorn, N.; Umehara, Y.; Trujillo-Paez, J.V.; Nguyen, H.L.T.; Takahashi, M.; Peng, G.; Ikutama, R.; Okumura, K. Antimicrobial peptide derived from insulin-like growth factor-binding protein 5 improves diabetic wound healing. Wound Repair Regen. 2022, 30, 232–244. [Google Scholar] [CrossRef]
- Håkansson, J.; Ringstad, L.; Umerska, A.; Johansson, J.; Andersson, T.; Boge, L.; Rozenbaum, R.T.; Sharma, P.K.; Tollbäck, P.; Björn, C.; et al. Characterization of the in vitro, ex vivo, and in vivo Efficacy of the Antimicrobial Peptide DPK-060 Used for Topical Treatment. Front. Cell. Infect. Microbiol. 2019, 9, 174. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef]
- Acedo, J.Z.; Chiorean, S.; Vederas, J.C.; van Belkum, M.J. The expanding structural variety among bacteriocins from Gram-positive bacteria. FEMS Microbiol. Rev. 2018, 42, 805–828. [Google Scholar] [CrossRef] [PubMed]
- Shelburne, C.E.; An, F.Y.; Dholpe, V.; Ramamoorthy, A.; Lopatin, D.E.; Lantz, M.S. The spectrum of antimicrobial activity of the bacteriocin subtilosin A. J. Antimicrob. Chemother. 2007, 59, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Fabbretti, A.; He, C.-G.; Gaspari, E.; Maffioli, S.; Brandi, L.; Spurio, R.; Sosio, M.; Jabes, D.; Donadio, S. A derivative of the thiopeptide GE2270A highly selective against Propionibacterium acnes. Antimicrob. Agents Chemother. 2015, 59, 4560–4568. [Google Scholar] [CrossRef] [PubMed]
- Donia, M.S.; Cimermancic, P.; Schulze, C.J.; Brown, L.C.W.; Martin, J.; Mitreva, M.; Clardy, J.; Linington, R.G.; Fischbach, M.A. A systematic analysis of biosynthetic gene clusters in the human microbiome reveals a common family of antibiotics. Cell 2014, 158, 1402–1414. [Google Scholar] [CrossRef]
- Panina, I.; Taldaev, A.; Efremov, R.; Chugunov, A. Molecular dynamics insight into the lipid II recognition by type A lantibiotics: Nisin, epidermin, and gallidermin. Micromachines 2021, 12, 1169. [Google Scholar] [CrossRef]
- Kim, P.I.; Sohng, J.K.; Sung, C.; Joo, H.-S.; Kim, E.-M.; Yamaguchi, T.; Park, D.; Kim, B.-G. Characterization and structure identification of an antimicrobial peptide, hominicin, produced by Staphylococcus hominis MBBL 2–9. Biochem. Biophys. Res. Commun. 2010, 399, 133–138. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, N. Quorum Sensing, Bioluminescence and Chemotaxis. In Fundamentals of Bacterial Physiology and Metabolism; Gupta, R., Gupta, N., Eds.; Springer: Singapore, 2021; pp. 633–652. [Google Scholar]
- Jenul, C.; Horswill, A.R. Regulation of Staphylococcus aureus virulence. Microbiol. Spectr. 2019, 7, 29. [Google Scholar] [CrossRef]
- Brown, M.M.; Kwiecinski, J.M.; Cruz, L.M.; Shahbandi, A.; Todd, D.A.; Cech, N.B.; Horswill, A.R. Novel peptide from commensal Staphylococcus simulans blocks methicillin-resistant Staphylococcus aureus quorum sensing and protects host skin from damage. Antimicrob. Agents Chemother. 2020, 64, e00172-20. [Google Scholar] [CrossRef]
- Van Hemert, S.; Meijerink, M.; Molenaar, D.; Bron, P.A.; De Vos, P.; Kleerebezem, M.; Wells, J.M.; Marco, M.L. Identification of Lactobacillus plantarum genes modulating the cytokine response of human peripheral blood mononuclear cells. BMC Microbiol. 2010, 10, 293. [Google Scholar] [CrossRef]
- Sierra, J.M.; Fusté, E.; Rabanal, F.; Vinuesa, T.; Viñas, M. An overview of antimicrobial peptides and the latest advances in their development. Expert Opin. Biol. Ther. 2017, 17, 663–676. [Google Scholar] [CrossRef]
- Niemeyer-van der Kolk, T.; van der Wall, H.; Hogendoorn, G.K.; Rijneveld, R.; Luijten, S.; van Alewijk, D.C.; van den Munckhof, E.H.; de Kam, M.L.; Feiss, G.L.; Prens, E.P.; et al. Pharmacodynamic effects of topical omiganan in patients with mild to moderate atopic dermatitis in a randomized, placebo-controlled, phase II trial. Clin. Transl. Sci. 2020, 13, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Fei, F.; Wang, T.; Jiang, Y.; Chen, X.; Ma, C.; Zhou, M.; Wu, Q.; Cao, P.; Duan, J.; Chen, T.; et al. A frog-derived antimicrobial peptide as a potential anti-biofilm agent in combating Staphylococcus aureus skin infection. J. Cell. Mol. Med. 2023, 27, 1565–1579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, M. Antimicrobial Peptides: From Design to Clinical Application. Antibiotics 2022, 11, 349. [Google Scholar] [CrossRef]
- Koo, H.B.; Seo, J. Antimicrobial peptides under clinical investigation. Pept. Sci. 2019, 111, e24122. [Google Scholar] [CrossRef]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Review: Lessons Learned From Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef]
- Divyashree, M.; Mani, M.K.; Reddy, D.; Kumavath, R.; Ghosh, P.; Azevedo, V.; Barh, D. Clinical Applications of Antimicrobial Peptides (AMPs): Where do we Stand Now? Protein Pept. Lett. 2020, 27, 120–134. [Google Scholar] [CrossRef]
- Zhu, Y.; Hao, W.; Wang, X.; Ouyang, J.; Deng, X.; Yu, H.; Wang, Y. Antimicrobial peptides, conventional antibiotics, and their synergistic utility for the treatment of drug-resistant infections. Med. Res. Rev. 2022, 42, 1377–1422. [Google Scholar] [CrossRef]
- Guo, L.; McLean, J.S.; Yang, Y.; Eckert, R.; Kaplan, C.W.; Kyme, P.; Sheikh, O.; Varnum, B.; Lux, R.; Shi, W. Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc. Natl. Acad. Sci. USA 2015, 112, 7569–7574. [Google Scholar] [CrossRef] [PubMed]
- Van Groenendael, R.; Beunders, R.; Kox, M.; van Eijk, L.T.; Pickkers, P. The Human Chorionic Gonadotropin Derivate EA-230 Modulates the Immune Response and Exerts Renal Protective Properties: Therapeutic Potential in Humans. Semin. Nephrol. 2019, 39, 496–504. [Google Scholar] [CrossRef]
- Huang, H.W. DAPTOMYCIN, its membrane-active mechanism vs. that of other antimicrobial peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183395. [Google Scholar] [CrossRef]
- Travis, S.; Yap, L.M.; Hawkey, C.; Warren, B.; Lazarov, M.; Fong, T.; Tesi, R. RDP58 is a novel and potentially effective oral therapy for ulcerative colitis. Inflamm. Bowel Dis. 2005, 11, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Gries, K.; Josten, M.; Wiedemann, I.; Pelzer, S.; Labischinski, H.; Sahl, H.-G. The lipopeptide antibiotic Friulimicin B inhibits cell wall biosynthesis through complex formation with bactoprenol phosphate. Antimicrob. Agents Chemother. 2009, 53, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Miki, K.; Kitada, S.; Miki, M.; Hui, S.-P.; Shrestha, R.; Yoshimura, K.; Tsujino, K.; Kagawa, H.; Oshitani, Y.; Kida, H.; et al. A phase II, open-label clinical trial on the combination therapy with medium-chain triglycerides and ghrelin in patients with chronic obstructive pulmonary disease. J. Physiol. Sci. 2019, 69, 969–979. [Google Scholar] [CrossRef]
- Gualillo, O.; Lago, F.; Gómez-Reino, J.; Casanueva, F.F.; Dieguez, C. Ghrelin, a widespread hormone: Insights into molecular and cellular regulation of its expression and mechanism of action. FEBS Lett. 2003, 552, 105–109. [Google Scholar] [CrossRef]
- Pavithrra, G.; Rajasekaran, R. Gramicidin peptide to combat antibiotic resistance: A review. Int. J. Pept. Res. Ther. 2020, 26, 191–199. [Google Scholar] [CrossRef]
- Corey, R.; Naderer, O.J.; O’Riordan, W.D.; Dumont, E.; Jones, L.S.; Kurtinecz, M.; Zhu, J.Z. Safety, tolerability, and efficacy of GSK1322322 in the treatment of acute bacterial skin and skin structure infections. Antimicrob. Agents Chemother. 2014, 58, 6518–6527. [Google Scholar] [CrossRef]
- Nibbering, P.; Ravensbergen, E.; Welling, M.; Van Berkel, L.; Van Berkel, P.; Pauwels, E.; Nuijens, J. Human lactoferrin and peptides derived from its N terminus are highly effective against infections with antibiotic-resistant bacteria. Infect. Immun. 2001, 69, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, C.; Roscini, L.; Cardinali, G.; Corte, L.; Pierantoni, D.C.; Robert, V.; Rahman, M.; Welling, M.M. Structure-activity relationship study of synthetic variants derived from the highly potent human antimicrobial peptide hLF (1-11). Cohesive J. Microbiol. Infect. Dis 2018, 1, 1–19. [Google Scholar] [CrossRef]
- Papazian, L.; Donati, S.Y. Chapter 28—Hospital-acquired pneumonia. In Infectious Diseases, 3rd ed.; Cohen, J., Opal, S.M., Powderly, W.G., Eds.; Mosby: London, UK, 2010; pp. 294–299. [Google Scholar]
- Mullane, K.; Lee, C.; Bressler, A.; Buitrago, M.; Weiss, K.; Dabovic, K.; Praestgaard, J.; Leeds, J.A.; Blais, J.; Pertel, P. Multicenter, randomized clinical trial to compare the safety and efficacy of LFF571 and vancomycin for Clostridium difficile infections. Antimicrob. Agents Chemother. 2015, 59, 1435–1440. [Google Scholar] [CrossRef]
- Brown, K.L.; Poon, G.F.; Birkenhead, D.; Pena, O.M.; Falsafi, R.; Dahlgren, C.; Karlsson, A.; Bylund, J.; Hancock, R.E.; Johnson, P. Host defense peptide LL-37 selectively reduces proinflammatory macrophage responses. J. Immunol. 2011, 186, 5497–5505. [Google Scholar]
- AS, P.H. A Double-Blind, Placebo-Controlled, Interventional Parallel Group Study to Evaluate the Antiviral Effect of a Single Nasal Application of LTX-109 3% Gel, in Comparison to Placebo Gel, in Subjects with COVID-19 Infection; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Yasir, M.; Dutta, D.; Willcox, M.D. Mode of action of the antimicrobial peptide Mel4 is independent of Staphylococcus aureus cell membrane permeability. PLoS ONE 2019, 14, e0215703. [Google Scholar] [CrossRef]
- Askari, P.; Namaei, M.H.; Ghazvini, K.; Hosseini, M. In vitro and in vivo toxicity and antibacterial efficacy of melittin against clinical extensively drug-resistant bacteria. BMC Pharmacol. Toxicol. 2021, 22, 42. [Google Scholar] [CrossRef] [PubMed]
- Martin-Loeches, I.; Dale, G.E.; Torres, A. Murepavadin: A new antibiotic class in the pipeline. Expert Rev. Anti-Infect. Ther. 2018, 16, 259–268. [Google Scholar] [CrossRef]
- Wang, H.; Ai, L.; Zhang, Y.; Cheng, J.; Yu, H.; Li, C.; Zhang, D.; Pan, Y.; Lin, L. The Effects of Antimicrobial Peptide Nal-P-113 on Inhibiting Periodontal Pathogens and Improving Periodontal Status. BioMed Res. Int. 2018, 2018, 1805793. [Google Scholar] [CrossRef] [PubMed]
- Campion, A.; Casey, P.G.; Field, D.; Cotter, P.D.; Hill, C.; Ross, R.P. In vivo activity of Nisin A and Nisin V against Listeria monocytogenesin mice. BMC Microbiol. 2013, 13, 23. [Google Scholar] [CrossRef]
- Mercer, D.K.; Robertson, J.C.; Miller, L.; Stewart, C.S.; O’Neil, D.A. NP213 (Novexatin®): A unique therapy candidate for onychomycosis with a differentiated safety and efficacy profile. Med. Mycol. 2020, 58, 1064–1072. [Google Scholar] [CrossRef]
- Malanovic, N.; Leber, R.; Schmuck, M.; Kriechbaum, M.; Cordfunke, R.A.; Drijfhout, J.W.; de Breij, A.; Nibbering, P.H.; Kolb, D.; Lohner, K. Phospholipid-driven differences determine the action of the synthetic antimicrobial peptide OP-145 on Gram-positive bacterial and mammalian membrane model systems. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 2437–2447. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.-T.; Wu, C.-L.; Yip, B.-S.; Chih, Y.-H.; Peng, K.-L.; Hsu, S.-Y.; Yu, H.-Y.; Cheng, J.-W. The interactions between the antimicrobial peptide P-113 and living candida albicans cells shed light on mechanisms of antifungal activity and resistance. Int. J. Mol. Sci. 2020, 21, 2654. [Google Scholar] [CrossRef]
- Jang, W.S.; Li, X.S.; Sun, J.N.; Edgerton, M. The P-113 fragment of histatin 5 requires a specific peptide sequence for intracellular translocation in Candida albicans, which is independent of cell wall binding. Antimicrob. Agents Chemother. 2008, 52, 497–504. [Google Scholar] [CrossRef]
- Peek, N.F.A.W.; Nell, M.J.; Brand, R.; Jansen-Werkhoven, T.; van Hoogdalem, E.J.; Verrijk, R.; Vonk, M.J.; Wafelman, A.R.; Valentijn, A.R.P.M.; Frijns, J.H.M.; et al. Ototopical drops containing a novel antibacterial synthetic peptide: Safety and efficacy in adults with chronic suppurative otitis media. PLoS ONE 2020, 15, e0231573. [Google Scholar] [CrossRef]
- Cohen, H.; Wani, N.A.; Ben Hur, D.; Migliolo, L.; Cardoso, M.H.; Porat, Z.; Shimoni, E.; Franco, O.L.; Shai, Y. Interaction of Pexiganan (MSI-78)-Derived Analogues Reduces Inflammation and TLR4-Mediated Cytokine Secretion: A Comparative Study. ACS Omega 2023, 8, 17856–17868. [Google Scholar] [CrossRef] [PubMed]
- Scorciapino, M.A.; Rinaldi, A.C. Antimicrobial peptidomimetics: Reinterpreting nature to deliver innovative therapeutics. Front. Immunol. 2012, 3, 171. [Google Scholar] [CrossRef]
- Avedissian, S.N.; Liu, J.; Rhodes, N.J.; Lee, A.; Pais, G.M.; Hauser, A.R.; Scheetz, M.H. A review of the clinical pharmacokinetics of polymyxin B. Antibiotics 2019, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Al-Dulaimi, M.; Algburi, A.; Abdelhameed, A.; Mazanko, M.S.; Rudoy, D.V.; Ermakov, A.M.; Chikindas, M.L. Antimicrobial and Anti-Biofilm Activity of Polymyxin E Alone and in Combination with Probiotic Strains of Bacillus subtilis KATMIRA1933 and Bacillus amyloliquefaciens B-1895 against Clinical Isolates of Selected Acinetobacter spp.: A Preliminary Study. Pathogens 2021, 10, 1574. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.; Björn, C.; Sjöstrand, V.; Lindgren, K.; Münnich, M.; Mattsby-Baltzer, I.; Ivarsson, M.-L.; Olmarker, K.; Mahlapuu, M. A novel polypeptide derived from human lactoferrin in sodium hyaluronate prevents postsurgical adhesion formation in the rat. Ann. Surg. 2009, 250, 1021–1028. [Google Scholar] [CrossRef]
- Wiig, M.; Dahlin, L.B.; Fridèn, J.; Hagberg, L.; Larsen, S.; Mahlapuu, M. PXL01 in Sodium Hyaluronate for Improvement of Hand Recovery After Flexor Tendon Repair Surgery: Randomized Controlled Trial: Level 1 Evidence. J. Hand Surg. 2014, 39, e45. [Google Scholar] [CrossRef][Green Version]
- Kudrimoti, M.; Curtis, A.; Azawi, S.; Worden, F.; Katz, S.; Adkins, D.; Bonomi, M.; Elder, J.; Sonis, S.T.; Straube, R. Dusquetide: A novel innate defense regulator demonstrating a significant and consistent reduction in the duration of oral mucositis in preclinical data and a randomized, placebo-controlled phase 2a clinical study. J. Biotechnol. 2016, 239, 115–125. [Google Scholar] [CrossRef]
- Kudrimoti, M.; Curtis, A.; Azawi, S.; Worden, F.; Katz, S.; Adkins, D.; Bonomi, M.; Scott, Z.; Elder, J.; Sonis, S.T. Dusquetide: Reduction in oral mucositis associated with enduring ancillary benefits in tumor resolution and decreased mortality in head and neck cancer patients. Biotechnol. Rep. 2017, 15, 24–26. [Google Scholar] [CrossRef]
- Martens, E.; Demain, A.L. The antibiotic resistance crisis, with a focus on the United States. J. Antibiot. 2017, 70, 520–526. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Lu, R.; Wang, S.; Fu, Z.; Yao, Z. Efficacy of a Novel Antibacterial Agent Exeporfinium Chloride,(XF-73), Against Antibiotic-Resistant Bacteria in Mouse Superficial Skin Infection Models. Infect. Drug Resist. 2023, 16, 4867–4879. [Google Scholar] [CrossRef]
- Maturana, P.; Martinez, M.; Noguera, M.E.; Santos, N.; Disalvo, E.A.; Semorile, L.; Maffia, P.C.; Hollmann, A. Lipid selectivity in novel antimicrobial peptides: Implication on antimicrobial and hemolytic activity. Colloids Surf. B Biointerfaces 2017, 153, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Cau, L.; Williams, M.R.; Butcher, A.M.; Nakatsuji, T.; Kavanaugh, J.S.; Cheng, J.Y.; Shafiq, F.; Higbee, K.; Hata, T.R.; Horswill, A.R. Staphylococcus epidermidis protease EcpA can be a deleterious component of the skin microbiome in atopic dermatitis. J. Allergy Clin. Immunol. 2021, 147, 955–966.e916. [Google Scholar] [CrossRef] [PubMed]
- Eichenfield, L.F.; DiBonaventura, M.; Xenakis, J.; Lafeuille, M.-H.; Duh, M.S.; Fakih, I.; Levenberg, M.; Cappelleri, J.C.; Sikirica, V. Costs and Treatment Patterns Among Patients with Atopic Dermatitis Using Advanced Therapies in the United States: Analysis of a Retrospective Claims Database. Dermatol. Ther. 2020, 10, 791–806. [Google Scholar] [CrossRef]
- Kuznik, A.; Bégo-Le-Bagousse, G.; Eckert, L.; Gadkari, A.; Simpson, E.; Graham, C.N.; Miles, L.; Mastey, V.; Mahajan, P.; Sullivan, S.D. Economic Evaluation of Dupilumab for the Treatment of Moderate-to-Severe Atopic Dermatitis in Adults. Dermatol. Ther. 2017, 7, 493–505. [Google Scholar] [CrossRef]
- Sidorczuk, K.; Gagat, P.; Pietluch, F.; Kała, J.; Rafacz, D.; Bąkała, L.; Słowik, J.; Kolenda, R.; Rödiger, S.; Fingerhut, L.C. Benchmarks in antimicrobial peptide prediction are biased due to the selection of negative data. Brief. Bioinform. 2022, 23, bbac343. [Google Scholar] [CrossRef]
- Talapko, J.; Meštrović, T.; Juzbašić, M.; Tomas, M.; Erić, S.; Horvat Aleksijević, L.; Bekić, S.; Schwarz, D.; Matić, S.; Neuberg, M.; et al. Antimicrobial Peptides—Mechanisms of Action, Antimicrobial Effects and Clinical Applications. Antibiotics 2022, 11, 1417. [Google Scholar] [PubMed]
- Deo, S.; Turton, K.L.; Kainth, T.; Kumar, A.; Wieden, H.-J. Strategies for improving antimicrobial peptide production. Biotechnol. Adv. 2022, 59, 107968. [Google Scholar] [CrossRef]
- Haney, E.F.; Straus, S.K.; Hancock, R.E.W. Reassessing the Host Defense Peptide Landscape. Front. Chem. 2019, 7, 43. [Google Scholar] [CrossRef]

| Layer | Major cellular Constituents | Major Functions | References |
|---|---|---|---|
| Hypodermis | Adipocytes, fibroblasts, endothelial and muscle cells | Insulation, mechanical integrity, support, conductance of vascular and neural signals | [1,2] |
| Dermis | Endothelial cells, fibroblasts, Langerhans and muscle cells | Mechanical integrity, support, thermal barrier, energy storage, protection from physical injury | [2,3] |
| Epidermis | Keratinocytes, melanocytes, Langerhans and Markel cells | Outermost barrier, immune function, protection from oxidative and mechanical stress | [4,5] |
| Structure | |||
|---|---|---|---|
| Class | Characteristics | Examples | References |
| α-helical | Contains <40 amino acids and forms an α-helical secondary structure in a non-polar environment. Antimicrobial activity correlates with α-helical content; these AMPs are mostly membrane disruptive and exhibit activity against fungi, viruses, bacteria, and even drug-resistant pathogens. | Cathelicidins and Magainins. | [60,61,62] |
| β-sheets | Contain conserved cysteine residues which form disulphide bonds between anti-parallel strands with helical fragments. Target Gram-positive and negative bacteria and complex with bacterial lipopolysaccharides. | Defensins and Tachyplesins. | [63,64,65] |
| extended peptides | The presence of glycine, histidine, arginine, and tryptophan instead of a structural pattern is the defining characteristic. Engage with membrane lipids and produce hydrogen bonds and van der Waals interactions. | Histatins and Indolicidin. | [61,66] |
| loop peptides | Distinguished by a single bond (disulphide or amide), which leads to a loop structure. Shows activity against a broad range of Gram-positive and Gram-negative bacteria. Only 1 AMP has been identified with this structure and is constituted by 21 amino acids. | Thanatin | [67,68] |
| Covalent Bond | |||
| Class | Characteristics | Examples | References |
| I | Linear or open chains with only one chain. No bonding registered. | Magainin and Cecropins. | [69] |
| II | Side-chain–side-chain with either one or two chains. Single chains with no bonding or two chains with disulphide bonds. | Enterocin L50 and Geobacillin I. | [70,71] |
| III | Side-chain–backbone bonding pattern with only a single chain present. Bonding is through either amide, ester or thioether bonds. | Capistruin and Huazacin. | [72,73] |
| IV | A backbone-to-backbone bonding pattern with only one chain present. Bonding through amide bonds. | RTD-1 and Kalata B1. | [74] |
| AMP | Expression Conditions | Major Functions | Crystaline Structure | References |
|---|---|---|---|---|
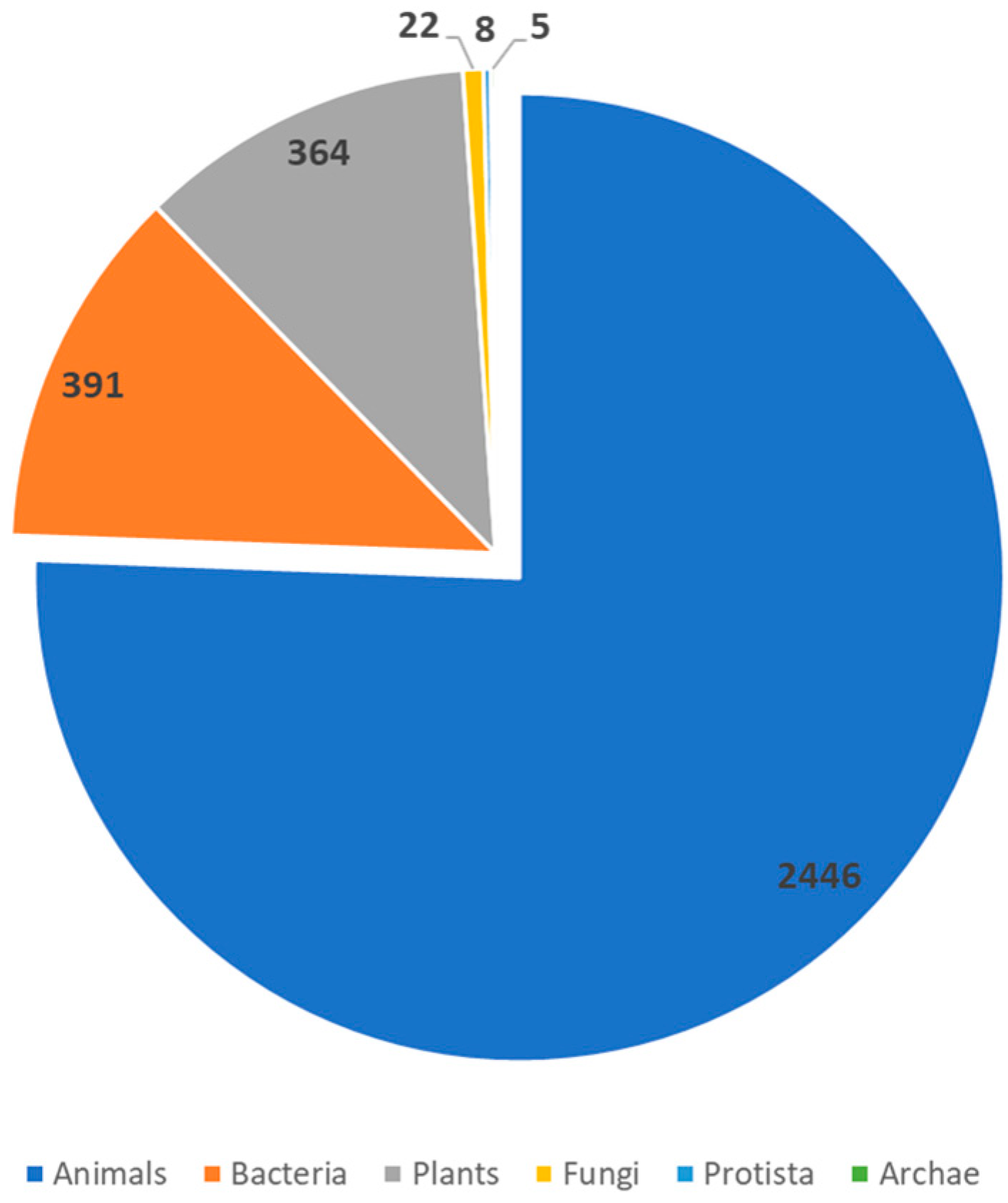
| Defensins | β-defensins expressed continuously; other defensins expressed by infection, injury, or pro-inflammatory cytokines | Antimicrobial defense of skin, synergistic effect with LL-37, potent Candida albicans and anaerobic pathogen inhibitor | 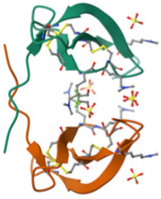 | [75,76] |
| LL-37 | Induced by injury and inflammation | Broad antimicrobial activity, chemotactic capacity to recruit neutrophils, T- and mast cells and monocytes. Promotes angiogenesis |  | [12,16] |
| Psoriasin | Induced by inflammatory conditions and barrier disruption | Immunomodulatory properties and strong inhibitor of Escherichia coli and other bacteria | 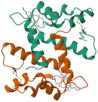 | [77,78] |
| Rnase7 | Induced by pro-inflammatory cytokines | Strong inhibitor of S. aureus |  | [79,80] |
| Dermcidin | Expressed in sweat glands; not found in keratinocytes | Active against S. aureus and C. albicans | 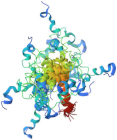 | [81,82] |
| AMP Name | Clinical Trial ID | Phase | Target | Reference |
|---|---|---|---|---|
| AP-214 | NCT00903604 | II a | Post-surgical organ failure | [110] |
| C16G2 | NCT03004365 | II c | Streptococcus mutans | [111] |
| CZEN-002 | NCT03145220 | II a | Antifungal | [112] |
| Daptomycin | NCT01922011; NCT00093067; NCT01104662; NCT02972983 | III/IV c | Skin infection/bacteremia | [113] |
| Delmitide (RDP58) | ISRCTN84220089 | II c | Inflammatory bowel disease | [114] |
| DPK-060 | NCT01447017; NCT01522391 | II c | Acute external otitis, topical treatment of microbial infections | [91] |
| EA-230 | NCT03145220 | II d | Sepsis/renal failure | [112] |
| Friulimicin | NCT00492271 | I a | MRSA/pneumonia | [115] |
| Ghrelin | NCT00763477 | II c | Chronic respiratory infection | [116,117] |
| Gramicidin | NCT00534391 | III d | Infected wounds and ulcers | [118] |
| GSK1322322 | NCT01209078 | II c | Bacterial skin infection | [119] |
| hLF1-11 | NCT00430469 | I/II a | Bacterial/fungal infections | [120,121] |
| Iseganan (IB-367) | NCT00118781; NCT00022373 | III a | Pneumonia/oral mucositis | [122] |
| LFF571 | NCT01232595 | II c | C. difficile | [123] |
| LL-37 | EUCTR2012-002100-41 | II a | Leg ulcers | [124] |
| LTX-109 | NCT01803035; NCT01158235 | I/II c | MRSA/impetigo, antiviral | [125] |
| Mel4 | ACTRN1261500072556 | II/III c | Contact lenses antimicrobial | [126] |
| Melittin | NCT02364349, NCT01526031 | I/II c | Inflammation | [127] |
| Murepavadin | EUCTR2017-003933-27-EE | II b | P. aeruginosa, K. pneumoniae | [128] |
| Nal-P-113 | ChiCTR-OIC-16010250 | III c | Periodontal disease | [129] |
| Neuprex® | NCT00462904 | III a | Pediatric meningococcemia | [107] |
| Nisin | NCT02928042; NCT02467972 | n.a. c | Gram-positive bacteria | [130] |
| Novexatin (NP213) | NCT02933879 | II a | Fungal nail infection | [131] |
| NVB-302 | ISRCTN40071144 | I a | C. difficile | [107] |
| Omiganan | NCT00231153; NCT02456480 | II/III c | Antisepsis/catheter, Atopic dermatitis | [104] |
| OP-145 | ISRCTN84220089 | I/II c | Chronic middle ear infection | [132] |
| PAC113 | NCT00659971 | II c | Oral candidiasis | [133,134] |
| P60.4Ac | ISRCTN12149720 | II c | Chronic ear infections | [135] |
| Pexiganan (MSI-78) | NCT00563394; NCT00563433; NCT01590758; NCT01594762 | III a | Diabetic foot ulcers | [136] |
| PMX-30063 | NCT01211470; NCT02052388 | II c | Acute bacterial skin infection | [137] |
| Polymyxin B | NCT00490477; NCT00534391 | III d | Gram-negative bacteria | [138] |
| Polymyxin E (Colistin) | NCT01292031; NCT02573064 | III c | A. baumannii/pneumonia | [139] |
| PXL01 | NCT01022242 | II/III c | Postsurgical adhesions | [140,141] |
| SGX942(Dusquetide) | NCT03237325 | III c | Oral mucositis | [142,143] |
| Surotomycin (CB-315) | NCT01597505 | III a | C. difficile | [144] |
| XF-73(Exeporfinium chloride) | NCT03915470 | II c | Staphylococcal infection | [145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, M.; Silva, S.; Costa, E.M. Are Antimicrobial Peptides a 21st-Century Solution for Atopic Dermatitis? Int. J. Mol. Sci. 2023, 24, 13460. https://doi.org/10.3390/ijms241713460
Machado M, Silva S, Costa EM. Are Antimicrobial Peptides a 21st-Century Solution for Atopic Dermatitis? International Journal of Molecular Sciences. 2023; 24(17):13460. https://doi.org/10.3390/ijms241713460
Chicago/Turabian StyleMachado, Manuela, Sara Silva, and Eduardo M. Costa. 2023. "Are Antimicrobial Peptides a 21st-Century Solution for Atopic Dermatitis?" International Journal of Molecular Sciences 24, no. 17: 13460. https://doi.org/10.3390/ijms241713460
APA StyleMachado, M., Silva, S., & Costa, E. M. (2023). Are Antimicrobial Peptides a 21st-Century Solution for Atopic Dermatitis? International Journal of Molecular Sciences, 24(17), 13460. https://doi.org/10.3390/ijms241713460








