Isolation of an Insulin-Like Receptor Involved in the Testicular Development of the Mud Crab Scylla paramamosain
Abstract
:1. Introduction
2. Results
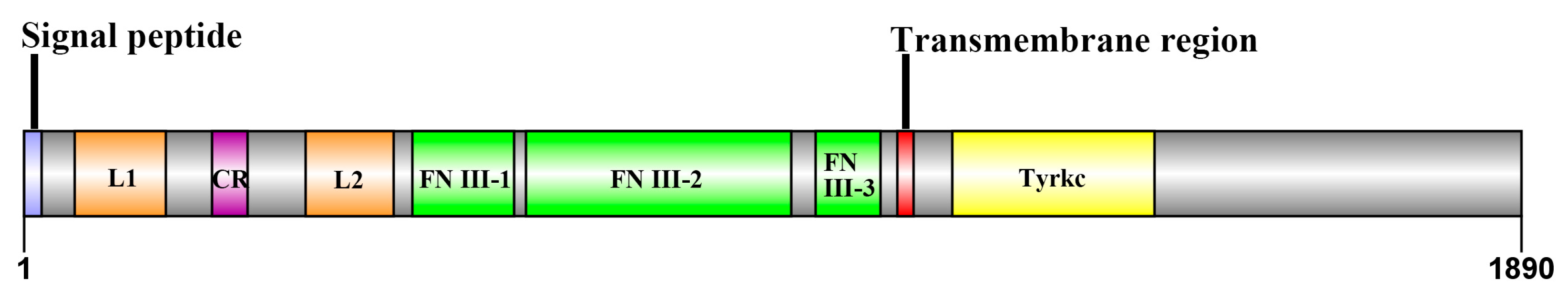
2.1. Cloning and Sequence Analysis of Sp-IR
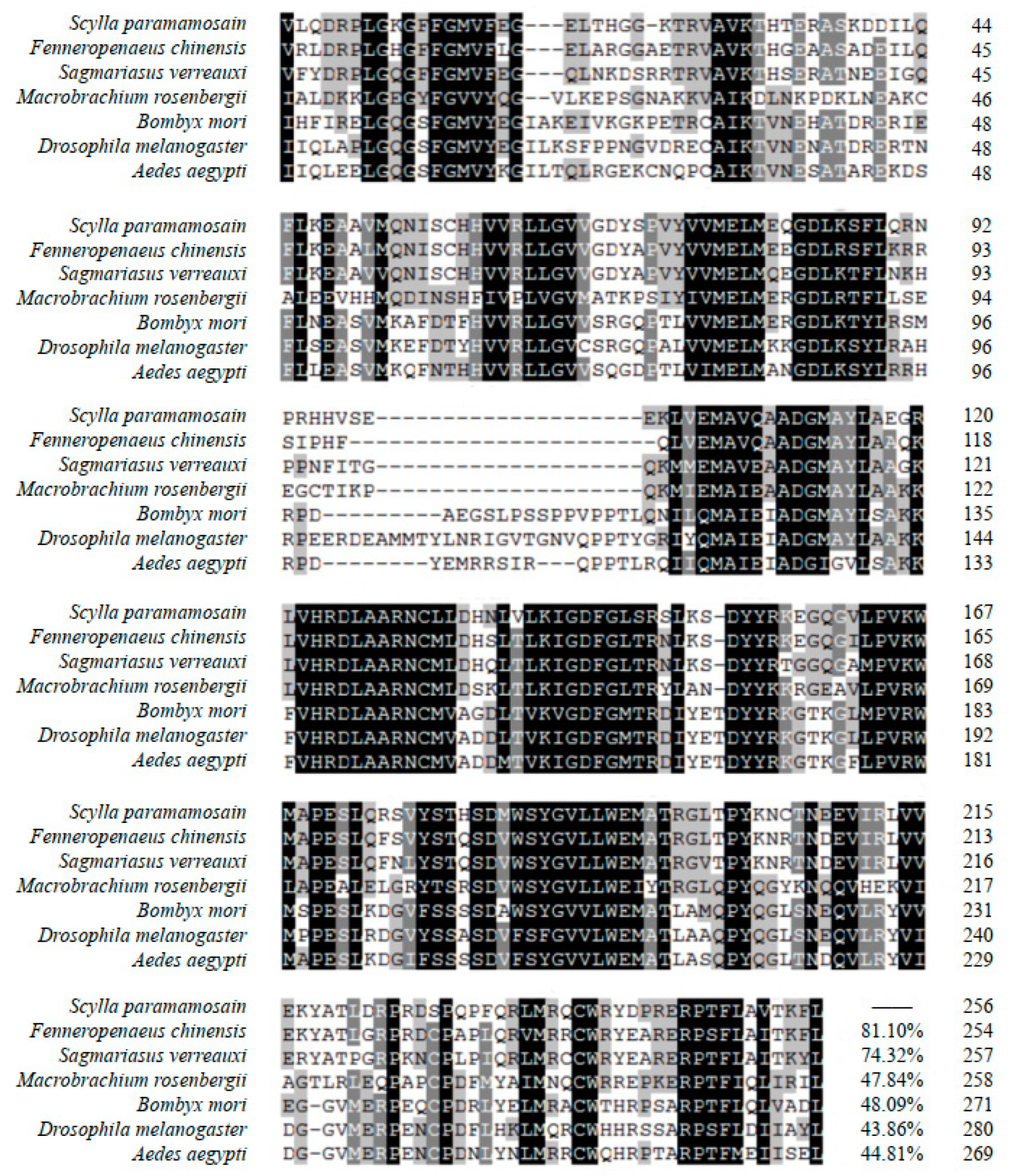
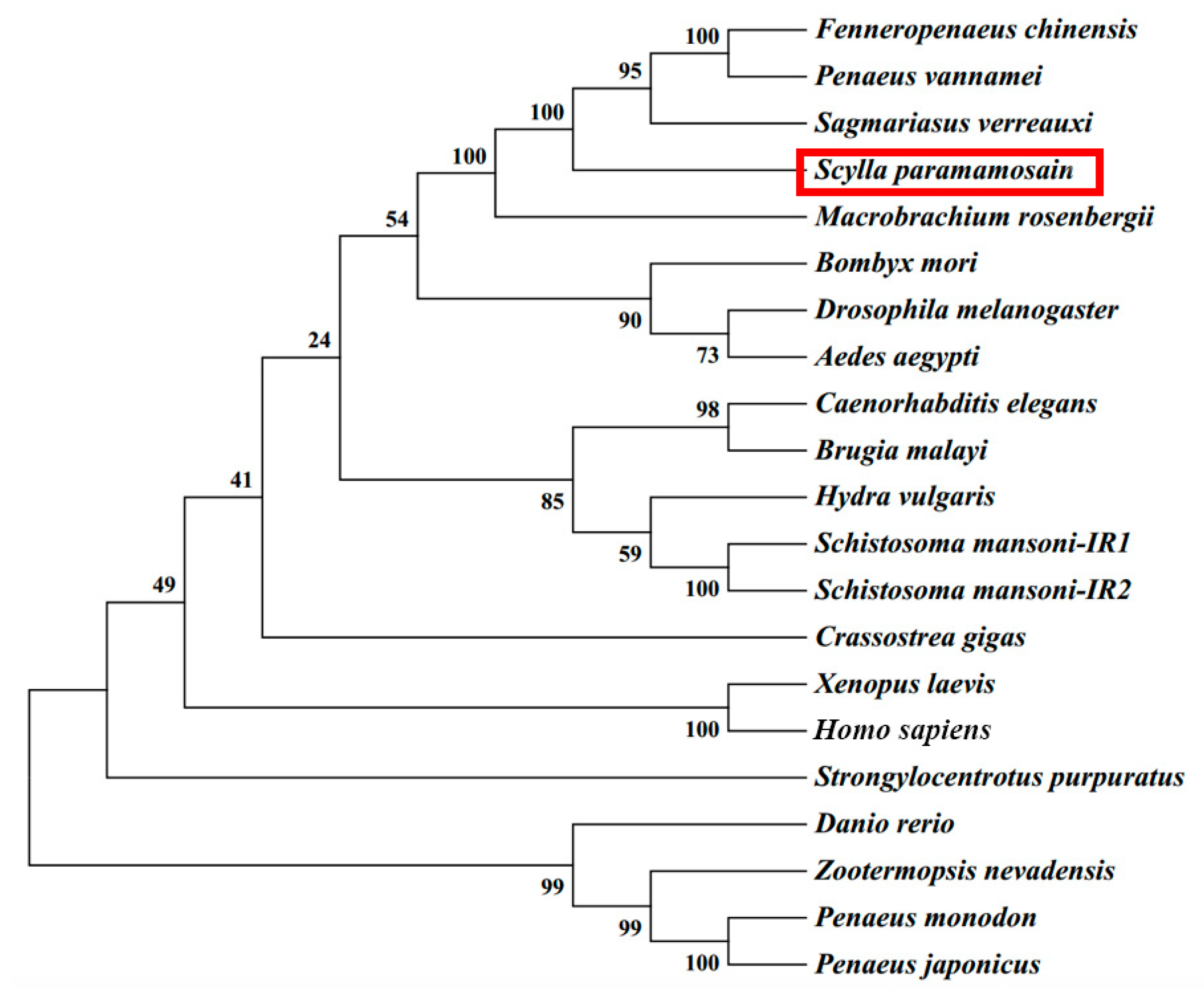
2.2. Multiple Sequence Alignment and Phylogenetic Tree Analysis
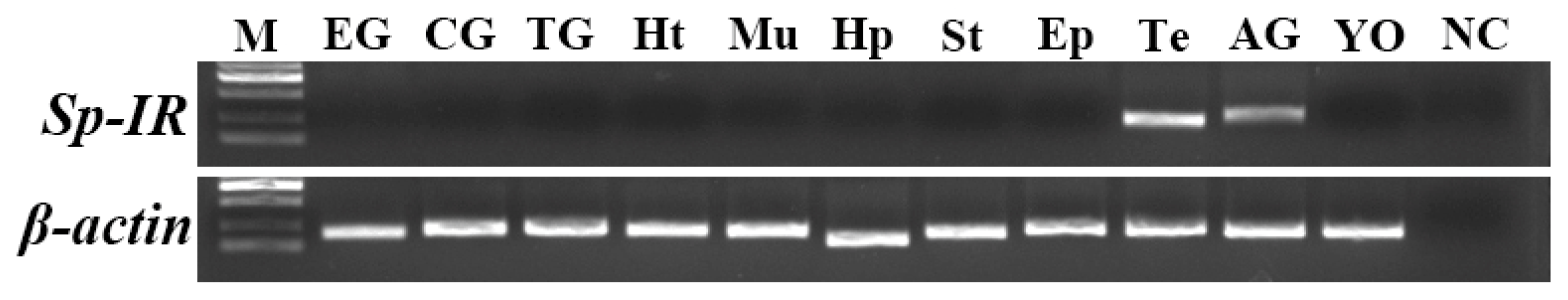
2.3. Tissue Distribution of Sp-IR in Male S. paramamosain
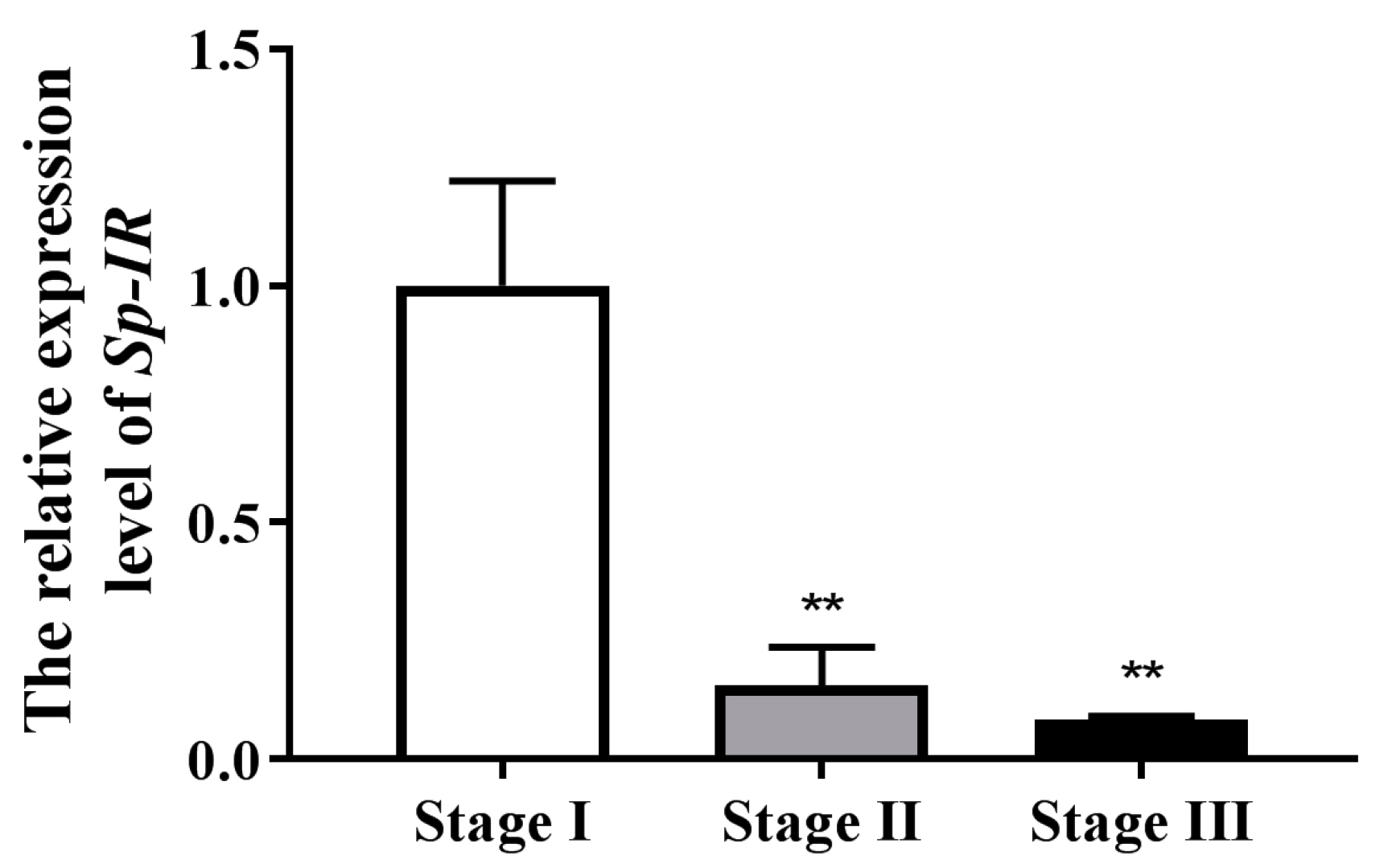
2.4. Profile Expression of Sp-IR in Testicular Development
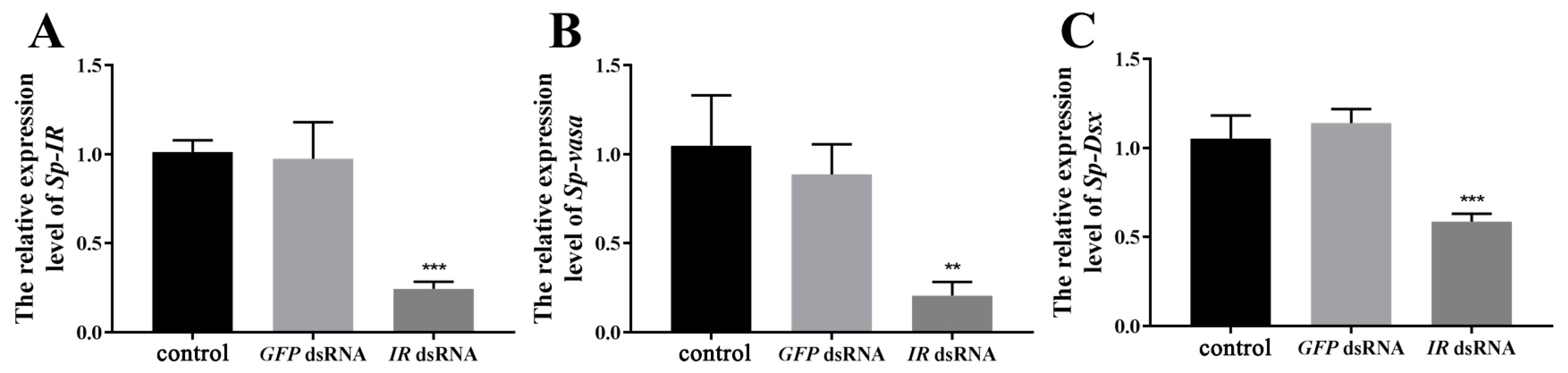
2.5. Expression of Sp-IR and Related Genes after Short-Term Injection of Sp-IR dsRNA
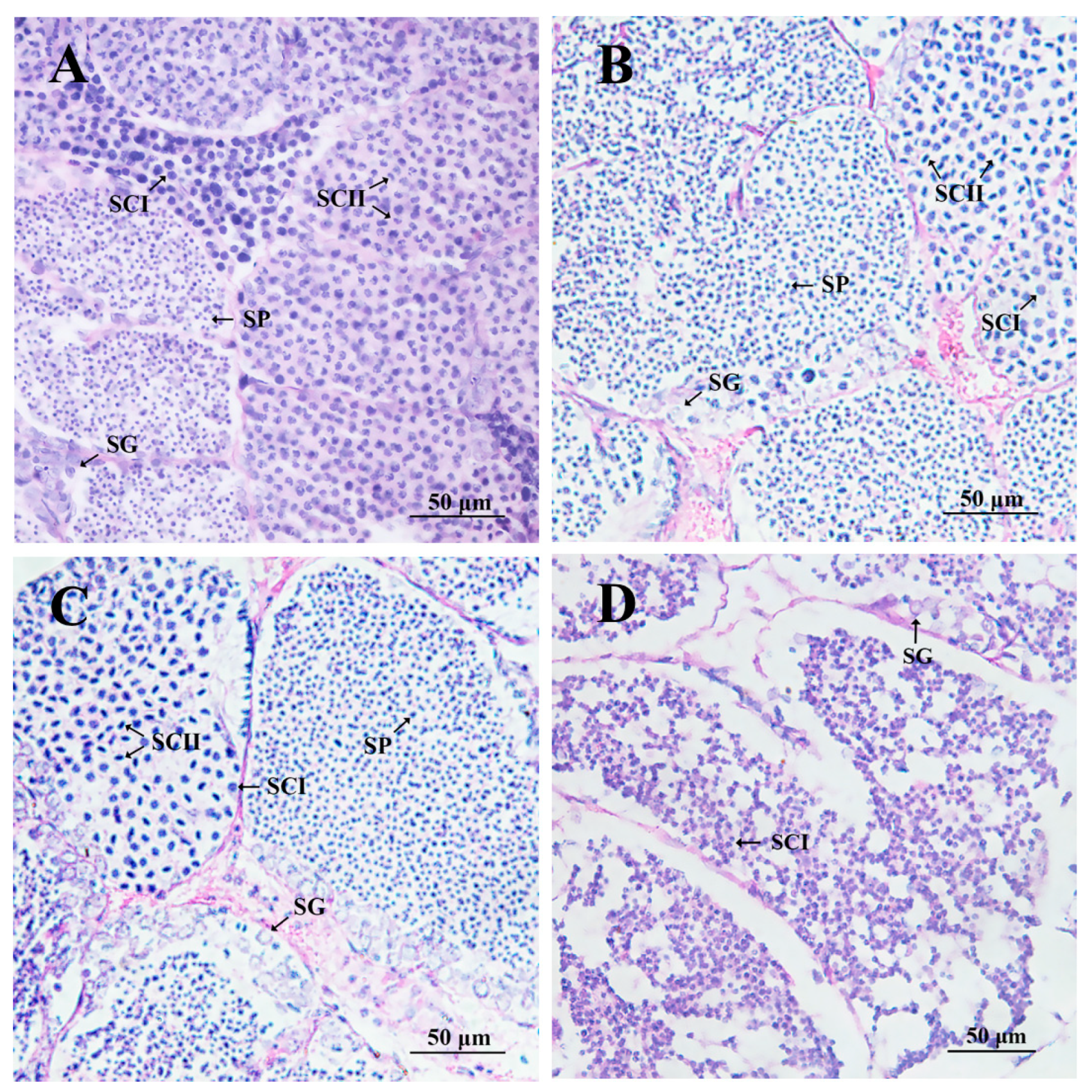
2.6. Effects on Related Genes and Testicular Development after Long-Term Injection of IR dsRNA
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Total RNA Extraction and Molecular Cloning
4.3. Tissue Distribution of Sp-IR in the Male Mud Crab
4.4. Short-Term RNA Interference Experiment In Vivo
4.4.1. dsRNA Preparation
4.4.2. RNA Interference
4.5. Long-Term RNA Interference Experiment In Vivo
4.6. Real-Time Quantitative PCR (qRT-PCR)
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sagi, A.; Aflalo, E.D. The androgenic gland and monosex culture of freshwater prawn Macrobrachium rosenbergii (De Man): A biotechnological perspective. Aquacult. Res. 2005, 36, 231–237. [Google Scholar] [CrossRef]
- Levy, T.; Sagi, A. The “IAG-switch”—A key controlling element in decapod crustacean sex differentiation. Front. Endocrinol. 2020, 11, 651. [Google Scholar] [CrossRef]
- Sagi, A.; Snir, E.; Khalaila, I. Sexual differentiation in decapod crustaceans: Role of the androgenic gland. Invertebr. Reprod. Dev. 1997, 31, 55–61. [Google Scholar] [CrossRef]
- Sagi, A.; Khalaila, I. The crustacean androgen: A hormone in an isopod and androgenic activity in decapods. Am. Zool. 2001, 41, 477–484. [Google Scholar] [CrossRef]
- Chang, E.S.; Brody, M.D. Crustacean organ and cell culture. Adv. Cell Cult. 1989, 7, 19–86. [Google Scholar]
- Okuno, A.; Hasegawa, Y.; Nagasawa, H. Purification and properties of androgenic gland hormone from the terrestrial isopod Armadillidium vulgare. Zool. Sci. 1997, 14, 837–842. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Okuno, A.; Nagasawa, H. Analyses of masculinizing process in the females of Armadillidium vulgare after implantation of the androgenic glands from mature males (Endocrinology) (Proceedings of the Seventy-Third Annual Meeting of the Zoological Society of Japan). Zool. Sci. 2002, 19, 1493. [Google Scholar]
- Ventura, T.; Rosen, O.; Sagi, A. From the discovery of the crustacean androgenic gland to the insulin-like hormone in six decades. Gen. Comp. Endocrinol. 2011, 173, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Rosen, O.; Manor, R.; Weil, S.; Gafni, O.; Linial, A.; Aflalo, E.D.; Ventura, T.; Sagi, A. A sexual shift induced by silencing of a single insulin-like gene in crayfish: Ovarian upregulation and testicular degeneration. PLoS ONE 2010, 5, e15281. [Google Scholar] [CrossRef]
- Liu, F.; Shi, W.; Ye, H.; Liu, A.; Zhu, Z. RNAi reveals role of insulin-like androgenic gland hormone 2 (IAG2) in sexual differentiation and growth in hermaphrodite shrimp. Front. Mar. Sci. 2021, 8, 666763. [Google Scholar] [CrossRef]
- Ventura, T.; Manor, R.; Aflalo, E.D.; Weil, S.; Raviv, S.; Glazer, L.; Sagi, A. Temporal silencing of an androgenic gland-specific insulin-like gene affecting phenotypical gender differences and spermatogenesis. Endocrinology 2009, 150, 1278–1286. [Google Scholar] [CrossRef]
- Ventura, T.; Manor, R.; Aflalo, E.D.; Weil, S.; Khalaila, I.; Rosen, O.; Sagi, A. Expression of an androgenic gland-specific insulin-like peptide during the course of prawn sexual and morphotypic differentiation. ISRN Endocrinol. 2011, 2011, e476283. [Google Scholar] [CrossRef]
- Aflalo, E.D.; Hoang, T.T.T.; Nguyen, V.H.; Lam, Q.; Nguyen, D.M.; Trinh, Q.S.; Raviv, S.; Sagi, A. A novel two-step procedure for mass production of all-male populations of the giant freshwater prawn Macrobrachium rosenbergii. Aquaculture 2006, 256, 468–478. [Google Scholar] [CrossRef]
- Ventura, T.; Manor, R.; Aflalo, E.D.; Weil, S.; Rosen, O.; Sagi, A. Timing sexual differentiation: Full functional sex reversal achieved through silencing of a single insulin-like gene in the prawn, Macrobrachium rosenbergii. Biol. Reprod. 2012, 86, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Ebina, Y.; Ellis, L.; Jarnagin, K.; Edery, M.; Graf, L.; Clauser, E.; Qu, J.H.; Masiarz, F.; Kan, Y.W.; Goldfine, I.D.; et al. The human insulin receptor cDNA: The structural basis for hormone-activated transmembrane signalling. Cell 1985, 40, 747–758. [Google Scholar] [CrossRef]
- Ullrich, A.; Bell, J.R.; Chen, E.Y.; Herrera, R.; Petruzzelli, L.M.; Dull, T.J.; Gray, A.; Coussens, L.; Liao, Y.C.; Tsubokawa, M.; et al. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature 1985, 313, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, O.; Manor, R.; Weil, S.; Aflalo, E.D.; Lezer, Y.; Levy, T.; Aizen, J.; Ventura, T.; Mather, P.B.; Khalaila, I.; et al. Identification and characterization of an insulin-like receptor involved in crustacean reproduction. Endocrinology 2016, 157, 928–941. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Li, Y.; Zhou, M.; Wang, W. siRNA knockdown of MrIR induces sex reversal in Macrobrachium rosenbergii. Aquaculture 2020, 523, 735172. [Google Scholar] [CrossRef]
- Aizen, J.; Chandler, J.C.; Fitzgibbon, Q.P.; Sagi, A.; Battaglene, S.C.; Elizur, A.; Ventura, T. Production of recombinant insulin-like androgenic gland hormones from three decapod species: In vitro testicular phosphorylation and activation of a newly identified tyrosine kinase receptor from the Eastern spiny lobster, Sagmariasus verreauxi. Gen. Comp. Endocrinol. 2016, 229, 8–18. [Google Scholar] [CrossRef]
- Guo, Q.; Li, S.; Lv, X.; Xiang, J.; Sagi, A.; Manor, R.; Li, F. A putative insulin-like androgenic gland hormone receptor gene specifically expressed in male Chinese shrimp. Endocrinology 2018, 159, 2173–2185. [Google Scholar] [CrossRef]
- Le-Vay, L. Ecology and management of mud crab Scylla spp. Asian Fish. Sci. 2001, 14, 101–112. [Google Scholar] [CrossRef]
- VinceCruz-Abeledo, C.C.; Solis, K.J.; Angeles, A.D.; Valdez, J.E.C.; Ngo, C.A.; Ablan-Lagman, M.C. Comparison of morphometric identification of species in juvenile mangrove crabs (Genus Scylla) by automated and local approaches. Aquaculture 2021, 531, 735917. [Google Scholar] [CrossRef]
- Chandler, J.C.; Aizen, J.; Fitzgibbon, Q.P.; Elizur, A.; Ventura, T. Applying the power of transcriptomics: Understanding male sexual development in decapod Crustacea. Integr. Comp. Biol. 2016, 56, 1144–1156. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kajiura-Kobayashi, H.; Nagahama, Y. Differential expression of vasa homologue gene in the germ cells during oogenesis and spermatogenesis in a teleost fish, tilapia, Oreochromis niloticus. Mech. Dev. 2000, 99, 139–142. [Google Scholar] [CrossRef]
- Xu, H.; Gui, J.; Hong, Y. Differential expression of vasa RNA and protein during spermatogenesis and oogenesis in the gibel carp (Carassius auratus gibelio), a bisexually and gynogenetically reproducing vertebrate. Dev. Dyn. 2005, 233, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Marracci, S.; Casola, C.; Bucci, S.; Ragghianti, M.; Ogielska, M.; Mancino, G. Differential expression of two vasa/PL10-related genes during gametogenesis in the special model system Rana. Dev. Genes Evol. 2007, 217, 395–402. [Google Scholar] [CrossRef]
- Nakkrasae, L.I.; Damrongphol, P. A vasa-like gene in the giant freshwater prawn, Macrobrachium rosenbergii. Mol. Reprod. Dev. 2007, 74, 835–842. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Han, K.; Zou, Z.; Zhang, Z. A vasa gene from green mud crab Scylla paramamosain and its expression during gonadal development and gametogenesis. Mol. Biol. Rep. 2012, 39, 4327–4335. [Google Scholar] [CrossRef]
- Li, S.; Li, F.; Yu, K.; Xiang, J. Identification and characterization of a doublesex gene which regulates the expression of insulin-like androgenic gland hormone in Fenneropenaeus chinensis. Gene 2018, 649, 1–7. [Google Scholar] [CrossRef]
- Martin, G.; Sorokine, O.; Moniatte, M.; Bulet, P.; Hetru, C.; Van, D.A. The structure of a glycosylated protein hormone responsible for sex determination in the isopod, Armadillidium vulgare. Eur. J. Biochem. 1999, 262, 727–736. [Google Scholar] [CrossRef]
- Chandler, J.C.; Elizur, A.; Ventura, T. The decapod researcher’s guide to the galaxy of sex determination. Hydrobiologia 2018, 825, 61–80. [Google Scholar] [CrossRef]
- Adams, T.E.; Epa, V.C.; Garrett, T.P.J. Structure and function of the type 1 insulin-like growth factor receptor. Cell. Mol. Life Sci. 2000, 57, 1050–1093. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Liu, J.; Liu, F.; Huang, Y.; Wang, G.; Ye, H. Crustacean female sex hormone from the mud crab Scylla paramamosain is highly expressed in prepubertal males and inhibits the development of androgenic gland. Front. Physiol. 2018, 9, 924. [Google Scholar] [CrossRef]
- Toyooka, Y.; Tsunekawa, N.; Takahashi, Y.; Matsui, Y.; Satoh, M.; Noce, T. Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development. Mech. Dev. 2000, 93, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.S.; Toyooka, Y.; Akasu, R.; Katoh-Fukui, Y.; Nakahara, Y.; Suzuki, R.; Yokoyama, M.; Noce, T. The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev. 2000, 14, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Griffeth, R.J.; Bianda, V.; Nef, S. The emerging role of insulin-like growth factors in testis development and function. Basic Clin. Androl. 2014, 24, 12. [Google Scholar] [CrossRef]
- Pitetti, J.L.; Calvel, P.; Romero, Y.; Conne, B.; Truong, V.; Papaioannou, M.D.; Schaad, O.; Docquier, M.; Herrera, L.P.; Wilhelm, D.; et al. Insulin and IGF1 receptors are essential for XX and XY gonadal differentiation and adrenal development in mice. PLoS Genet. 2013, 9, e1003160. [Google Scholar] [CrossRef]
- Ye, H.; Li, S.; Huang, H.; Wang, G. Histological studies on testes development of the mud crab, Scylla serrata. Zool. Res. 2002, 23, 141, (In Chinese, with English Abstract). [Google Scholar]
- Wang, Y.L.; Sun, W.J.; He, L.; Li, Q.; Wang, Q. Morphological alterations of all stages of spermatogenesis and acrosome reaction in Chinese mitten crab Eriocheir sinensis. Cell Tissue Res. 2015, 360, 401–412. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, A.; Hao, S.; Liu, F.; Huang, H.; Ye, H. Isolation of an Insulin-Like Receptor Involved in the Testicular Development of the Mud Crab Scylla paramamosain. Int. J. Mol. Sci. 2023, 24, 13639. https://doi.org/10.3390/ijms241713639
Liu A, Hao S, Liu F, Huang H, Ye H. Isolation of an Insulin-Like Receptor Involved in the Testicular Development of the Mud Crab Scylla paramamosain. International Journal of Molecular Sciences. 2023; 24(17):13639. https://doi.org/10.3390/ijms241713639
Chicago/Turabian StyleLiu, An, Shuang Hao, Fang Liu, Huiyang Huang, and Haihui Ye. 2023. "Isolation of an Insulin-Like Receptor Involved in the Testicular Development of the Mud Crab Scylla paramamosain" International Journal of Molecular Sciences 24, no. 17: 13639. https://doi.org/10.3390/ijms241713639




