Characterization of Biogenic PbS Quantum Dots
Abstract
:1. Introduction
2. Results and Discussion
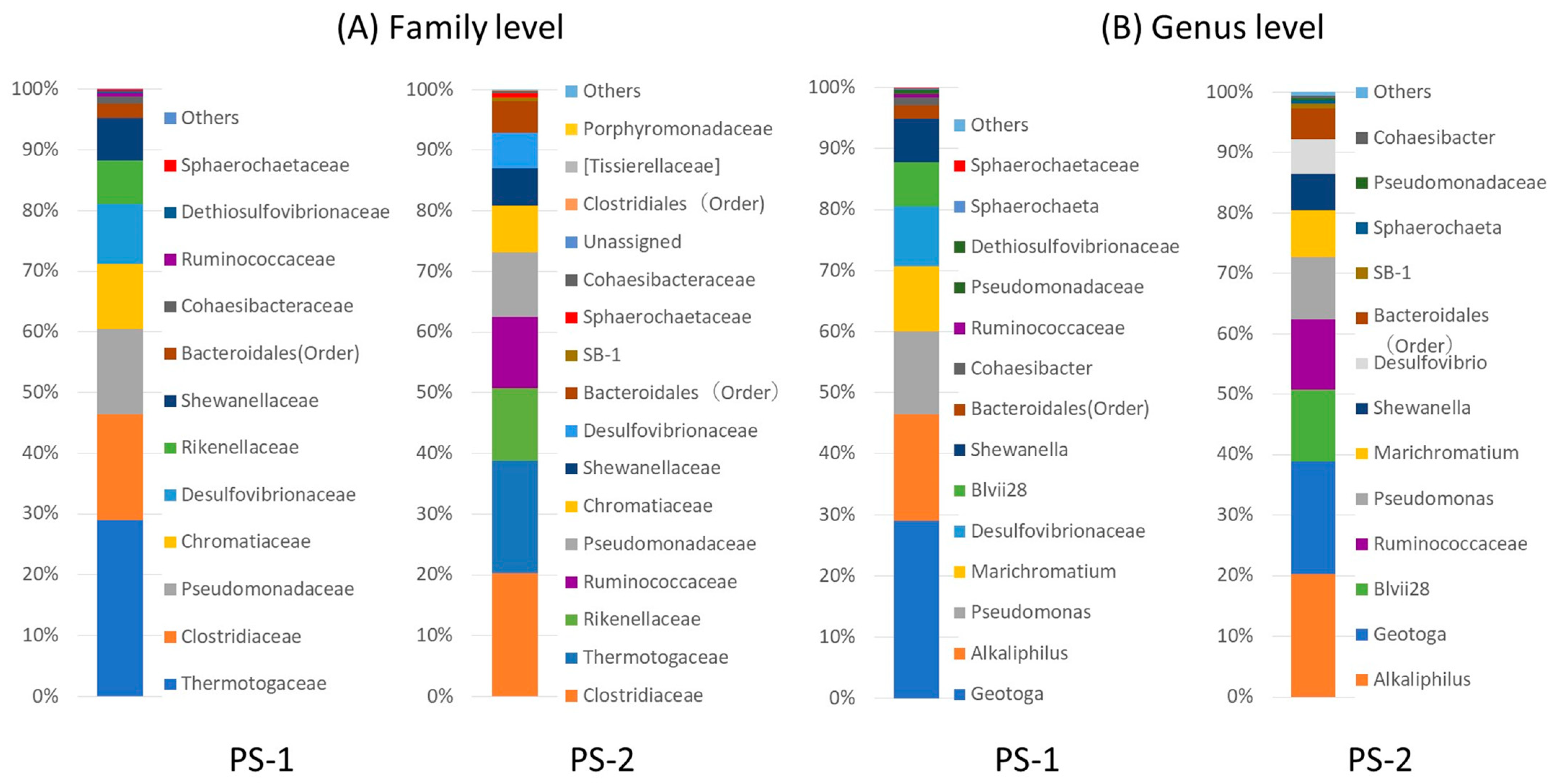
2.1. Bacterial Compositions of Enrichment Cultures PS-1 and PS-2
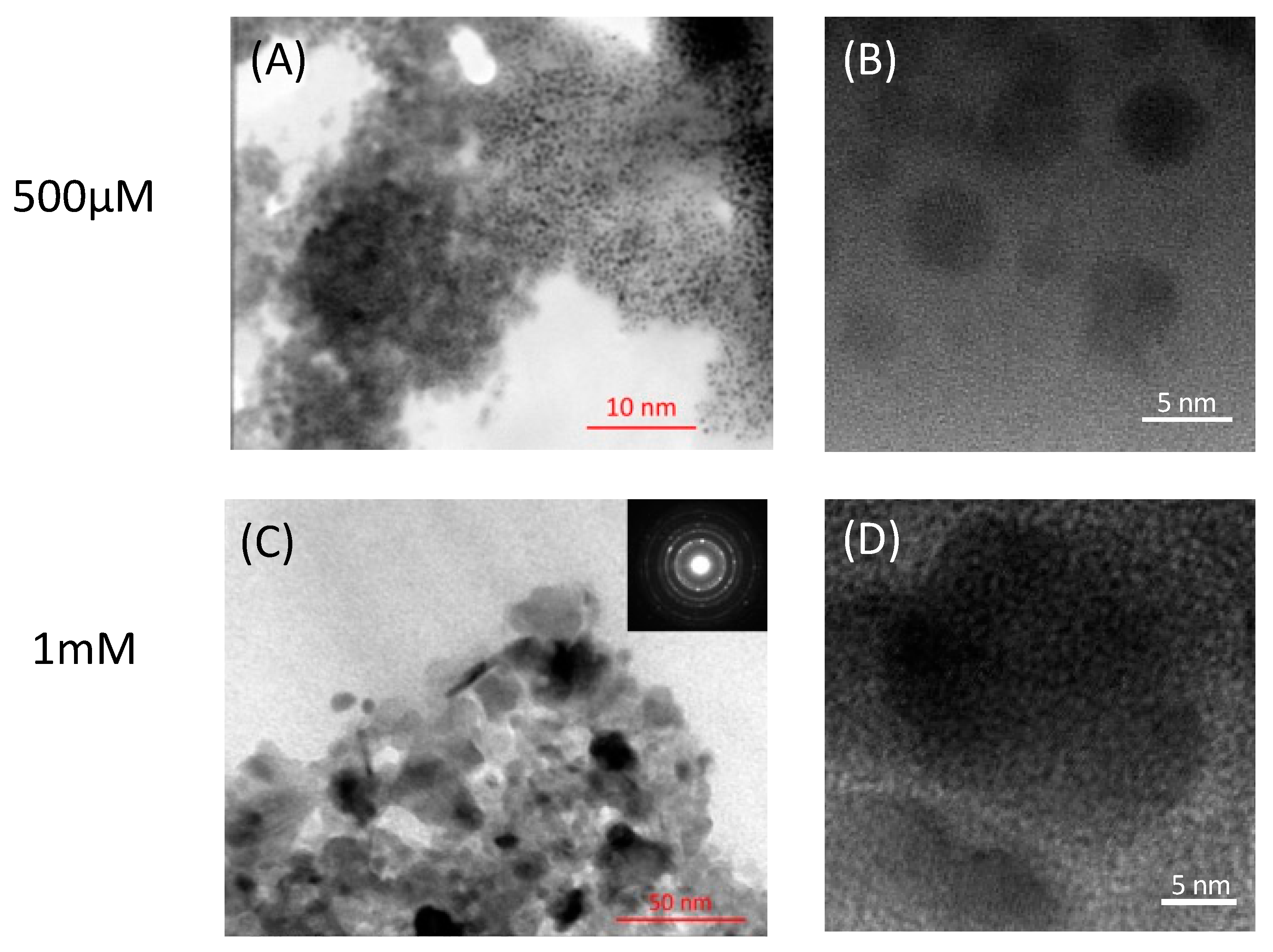
2.2. Localization and Morphology of PbS Nanoparticles
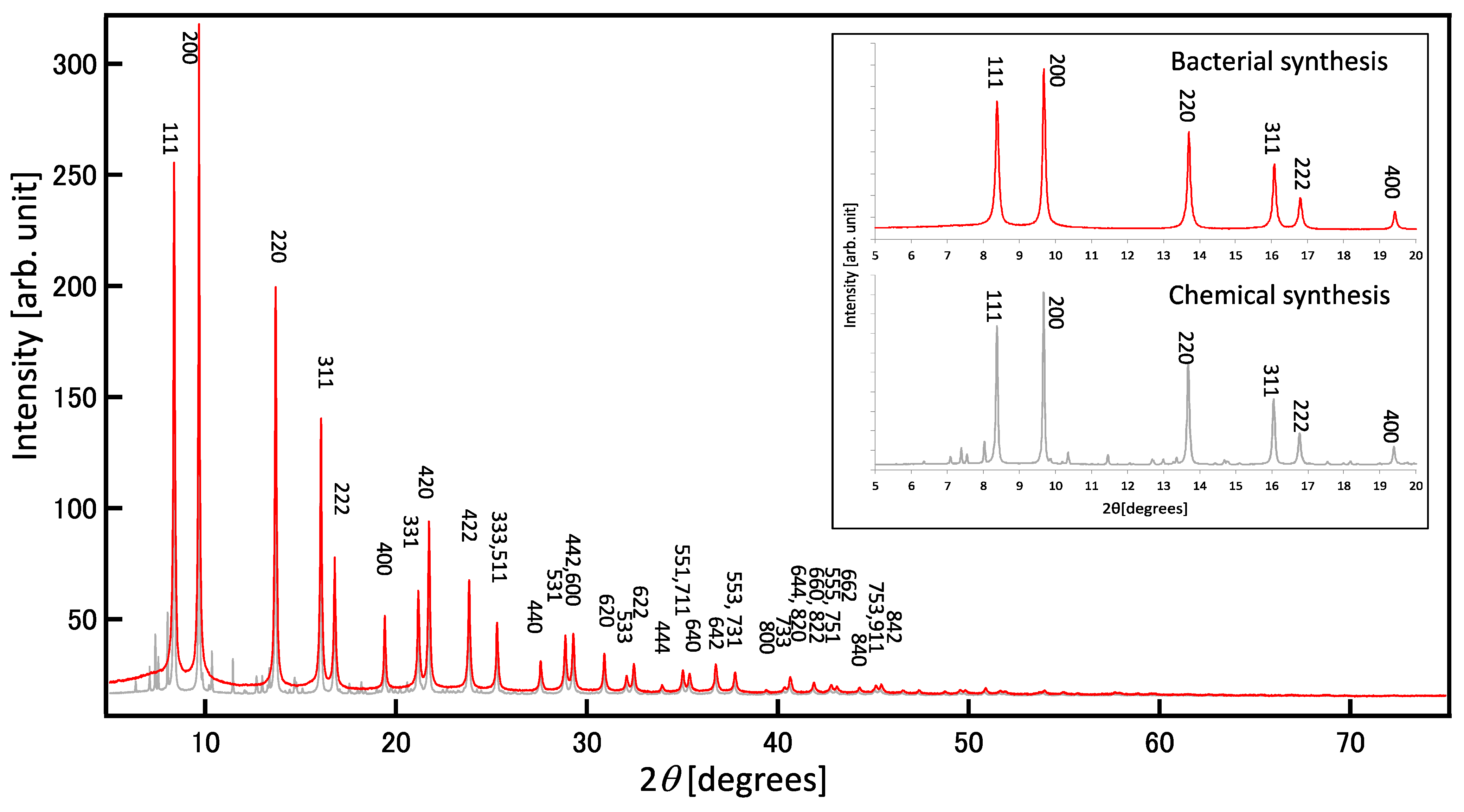
2.3. Crystallinity of Bacterial PbS Nanoparticles Evaluated Using XRD
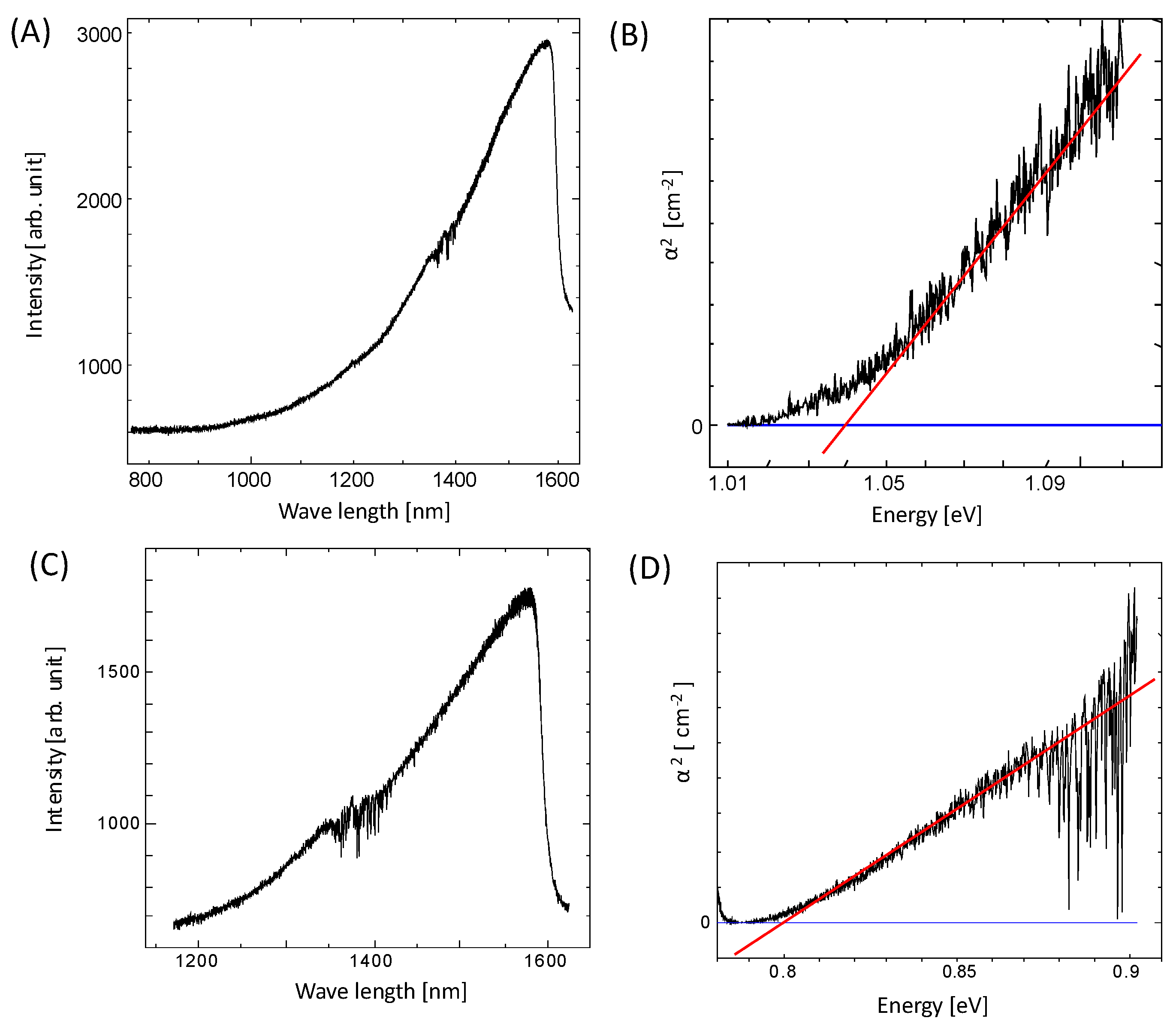
2.4. Optical Characteristics of Bacterial PbS Nanoparticles Evaluated with Optical Absorption Measurements
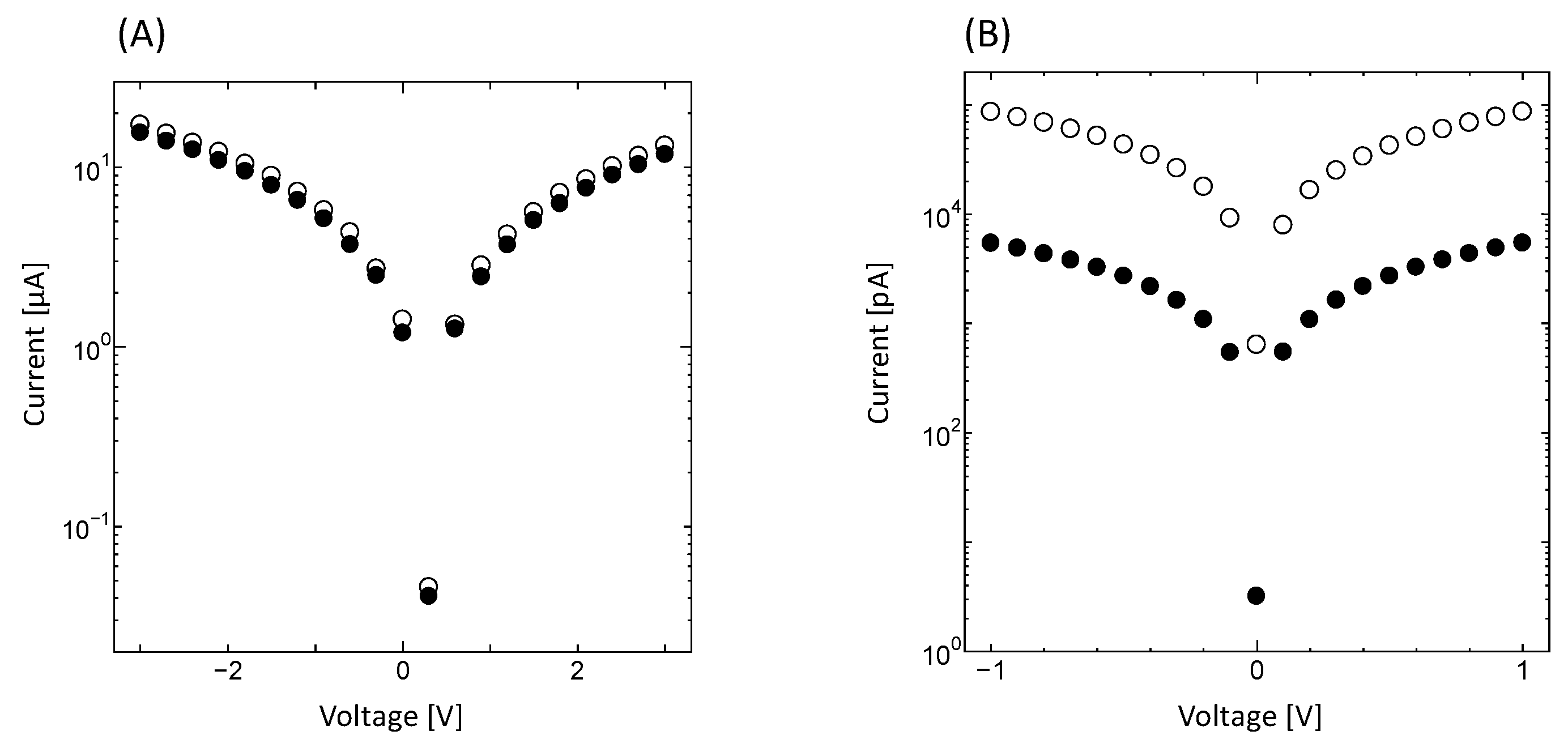
2.5. Electric Characteristics of Bacterial PbS Nanoparticles Evaluated Using Current–Voltage Measurements
3. Materials and Methods
3.1. Enrichment Culture
3.2. Bacterial Analysis Based on 16S rDNA Sequence
3.3. PbS Nanoparticle Formation and Extraction
3.4. Transmission Electron Microscopy and Energy-Dispersive Spectroscopy
3.5. X-ray Diffraction
3.6. Optical Absorption Measurements
3.7. Energy Band Gap (Eg) Calculation
3.8. Current–Voltage Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Utgikar, V.P.; Harmon, S.M.; Chaudhary, N.; Tabak, H.H.; Govind, R.; Haines, J.R. Inhibition of sulfate-reducing bacteria by metal sulfide formation in bioremediation of acid mine drainage. Environ. Toxicol. 2002, 17, 40–48. [Google Scholar] [CrossRef]
- Hosseini, M.R.; Sarvi, M.N. Recent achievements in the microbial synthesis of semiconductor metal sulfide nanoparticles. Mater. Sci. Semicond. Process. 2015, 40, 293–301. [Google Scholar] [CrossRef]
- Costa, J.; Girão, A.; Trindade, T.; Costa, M.; Duarte, A.; Rocha-Santos, T. Biological synthesis of nanosized sulfide semiconductors: Current status and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 8283–8302. [Google Scholar] [CrossRef]
- Dameron, C.T.; Reese, R.N.; Mehra, R.K.; Kortan, A.R.; Carroll, P.J.; Steigerwald, M.L.; Brus, L.E.; Winge, D.R. Biosynthesis of cadmium sulphide quantum semiconductor crystallites. Nature 1989, 338, 596. [Google Scholar] [CrossRef]
- Kowshik, M.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Microbial synthesis of semiconductor PbS nanocrystallites. Adv. Mater. 2002, 14, 815–818. [Google Scholar] [CrossRef]
- Wei, S.; Guo, C.; Wang, L.; Xu, J.; Dong, H. Bacterial synthesis of PbS nanocrystallites in one-step with L-cysteine serving as both sulfur source and capping ligand. Sci. Rep. 2021, 11, 1216. [Google Scholar] [CrossRef]
- Sweeney, R.Y.; Mao, C.; Gao, X.; Burt, J.L.; Belcher, A.M.; Georgiou, G.; Iverson, B.L. Bacterial biosynthesis of cadmium sulfide nanocrystals. Chem. Biol. 2004, 11, 1553–1559. [Google Scholar] [CrossRef]
- Moon, J.W.; Ivanov, I.N.; Joshi, P.C.; Armstrong, B.L.; Wang, W.; Jung, H.; Rondinone, A.J.; Jellison, G.E., Jr.; Meyer, H.M., III; Jang, G.G.; et al. Scalable production of microbially mediated zinc sulfide nanoparticles and application to functional thin films. Acta Biomater. 2014, 10, 4474–4483. [Google Scholar] [CrossRef]
- Zhou, N.Q.; Tian, L.J.; Wang, Y.C.; Li, D.B.; Li, P.P.; Zhang, X.; Yu, H.Q. Extracellular biosynthesis of copper sulfide nanoparticles by Shewanella oneidensis MR-1 as a photothermal agent. Enzym. Microb. Technol. 2016, 95, 230–235. [Google Scholar] [CrossRef]
- Querejeta-Fernández, A.; Hernández-Garrido, J.; Yang, H.; Zhou, Y.; Varela, A.; Parras, M.; Calvino-Gámez, J.; González-Calbet, J.; Green, P.; Kotov, N. Unknown Aspects of Self-Assembly of PbS Microscale Superstructures. Am. Chem. Soc. Nano 2012, 6, 3800–3812. [Google Scholar] [CrossRef]
- Wilson, I.; Zheng, N.; Knipping, U.; Tsong, I. Scanning tunneling microscopy of an ion-bombarded PbS (001) surface. Appl. Physiscs Lett. 1988, 53, 2039–2041. [Google Scholar] [CrossRef]
- Lan, X.; Voznyy, O.; Kiani, A.; Arquer, F.; Abbas, A.; Kim, G.; Liu, M.; Yang, Z.; Walters, G.; Xu, J.; et al. Passivation using molecular halides increases quantum dot solar cell performance. Adv. Mater. 2015, 28, 299–304. [Google Scholar] [CrossRef]
- Kim, G.; Arquer, F.; Yoon, Y.; Lan, X.; Liu, M.; Voznyy, O.; Yang, Z.; Fan, F.; Ip, A.; Kanjanaboos, P.; et al. High-efficiency colloidal quantum dot photovoltaics via robust self-assembled monolayers. Nano Lett. 2015, 15, 7691–7696. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Zhang, Z. Microbial synthesis of semiconductor lead sulfide nanoparticles using immobilitized Rhodobacter sphaeroides. Mater. Lett. 2009, 63, 764–766. [Google Scholar] [CrossRef]
- Davey, M.E.; Wood, W.A.; Key, R.; Nakamura, K.; Stahl, D.A. Isolation of three species of Geotoga and Petrotoga: Two new genera, representing a new lineage in the bacterial line of descent distantly related to the “Thermotogales”. Syst. Appl. Microbiol. 1993, 16, 191–200. [Google Scholar] [CrossRef]
- Takai, K.; Moser, D.P.; Onstott, T.C.; Spoelstra, N.; Pfiffner, S.M.; Dohnalkova, A.; Fredrickson, J.K. Alkaliphilus transvaalensis gen. nov., sp. nov., an extremely alkaliphilic bacterium isolated from a deep South African gold mine. Int. J. Syst. Evol. Microbiol. 2001, 51, 1245–1256. [Google Scholar] [CrossRef]
- Su, X.L.; Tian, Q.; Zhang, J.; Yuan, X.Z.; Shi, X.S.; Guo, R.B.; Qiu, Y.L. Acetobacteroides hydrogenigenes gen. nov., sp. nov., an anaerobic hydrogen-producing bacterium in the family Rikenellaceae isolated from a reed swamp. Int. J. Syst. Evol. Microbiol. 2014, 64, 2986–2991. [Google Scholar] [CrossRef]
- Loubinoux, J.; Valente, F.M.A.; Pereira, I.A.C.; Costa, A.; Grimont, P.A.D.; Le Faou, A.E. Reclassification of the only species of the genus Desulfomonas, Desulfomonas pigra, as Desulfovibrio piger comb. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 1305–1308. [Google Scholar]
- Imhoff, J.F.; Süling, J.; Petri, R. Phylogenetic relationships among the Chromatiaceae, their taxonomic reclassification and description of the new genera Allochromatium, Halochromatium, Isochromatium, Marichromatium, Thiococcus, Thiohalocapsa and Thermochromatium. Int. J. Syst. Bacteriol. 1998, 48, 1129–1143. [Google Scholar] [CrossRef]
- Thorell, K.; Meier-Kolthoff, J.P.; Sjöling, Å.; Martín-Rodríguez, A.J. Whole-genome sequencing redefines Shewanella taxonomy. Front. Microbiol. 2019, 10, 1861. [Google Scholar] [CrossRef]
- Hwang, C.Y.; Cho, B.C. Cohaesibacter gelatinilyticus gen. nov., sp. nov., a marine bacterium that forms a distinct branch in the order Rhizobiales, and proposal of Cohaesibacteraceae fam. nov. Int. J. Syst. Evol. Microbiol. 2008, 58, 267–277. [Google Scholar] [CrossRef]
- Ravot, G.; Ollivier, B.; Magot, M.; Patel, B.; Crolet, J.; Garcia, J. Thiosulfate reduction, an important physiological feature shared by members of the Order Thermotogales. Appl. Environ. Microbiol. 1995, 61, 2053–2055. [Google Scholar] [CrossRef]
- Arunasri, K.; Sasikala, C.; Ramana, C.; Suling, J.; Imhoff, J. Marichromatium indicum sp. nov., a novel purple sulfur gammaproteobacterium from mangrove soil of Goa, India. Int. J. Syst. Evol. Microbiol. 2005, 55, 673–679. [Google Scholar] [CrossRef]
- Thar, R.; Kuhl, M. Motility of Marichromatium gracilein response to light, oxygen, and sulfide. Appl. Environ. Microbiol. 2001, 67, 5410–5419. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Jyothsna, T.; Srinivas, T.; Sasikala, C.; Ramana, C.; Imhoff, J. Marichromatium bheemlicum sp. nov., a non-diazotrophic, photosynthetic gammaproteobacterium from a marine aquaculture pond. Int. J. Syst. Evol. Microbiol. 2007, 57, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Heidelberg, J.F.; Seshadri, R.; Fraser, C.M. The genome sequence of the anaerobic, sulfate-reducing bacterium Desulfovibrio vulgaris Hildenborough. Nat. Biotechnol. 2004, 22, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Uhler, A.D.; Helz, G.R. Solubility product of galena at 298°K: A possible explanation for apparent supersaturation in nature. Geochim. Et Cosmochim. Acta 1984, 48, 1155–1160. [Google Scholar] [CrossRef]
- Valls, M.; de Lorenzo, V. Exploiting the genetic and biochemical capacities of bacteria for the remediation of heavy metal pollution. FEMS Microbiol. Rev. 2002, 26, 327–338. [Google Scholar] [CrossRef]
- Ekimov, A.; Efros, A.; Onushchenko, A. Quantum size effect in semiconductor microcrystals. Solid State Commun. 1985, 56, 921–924. [Google Scholar] [CrossRef]
- Beatty, J.T.; Gest, H. Generation of succinyl-coenzyme A in photosynthetic bacteria. Arch. Microbiol. 1981, 129, 335–340. [Google Scholar] [CrossRef]
- Hannon, G.J. FASTX-Toolkit: FASTQ/A Short-Reads Pre-Processing Tools. 2010. Available online: http://hannonlab.Cshl.Edu/fastx_toolkit (accessed on 29 January 2018).
- Magoc, T.; Salzberg, L.S. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okamura, Y.; Shimizu, R.; Tominaga, Y.; Maki, S.; Aki, T.; Matsumura, Y.; Nakashimada, Y. Characterization of Biogenic PbS Quantum Dots. Int. J. Mol. Sci. 2023, 24, 14149. https://doi.org/10.3390/ijms241814149
Okamura Y, Shimizu R, Tominaga Y, Maki S, Aki T, Matsumura Y, Nakashimada Y. Characterization of Biogenic PbS Quantum Dots. International Journal of Molecular Sciences. 2023; 24(18):14149. https://doi.org/10.3390/ijms241814149
Chicago/Turabian StyleOkamura, Yoshiko, Ryo Shimizu, Yoriko Tominaga, Sachiko Maki, Tsunehiro Aki, Yukihiko Matsumura, and Yutaka Nakashimada. 2023. "Characterization of Biogenic PbS Quantum Dots" International Journal of Molecular Sciences 24, no. 18: 14149. https://doi.org/10.3390/ijms241814149





