A Study on the Use of the Phyto-Courier Technology in Tobacco Leaves Infected by Agrobacterium tumefaciens
Abstract
:1. Introduction
2. Results
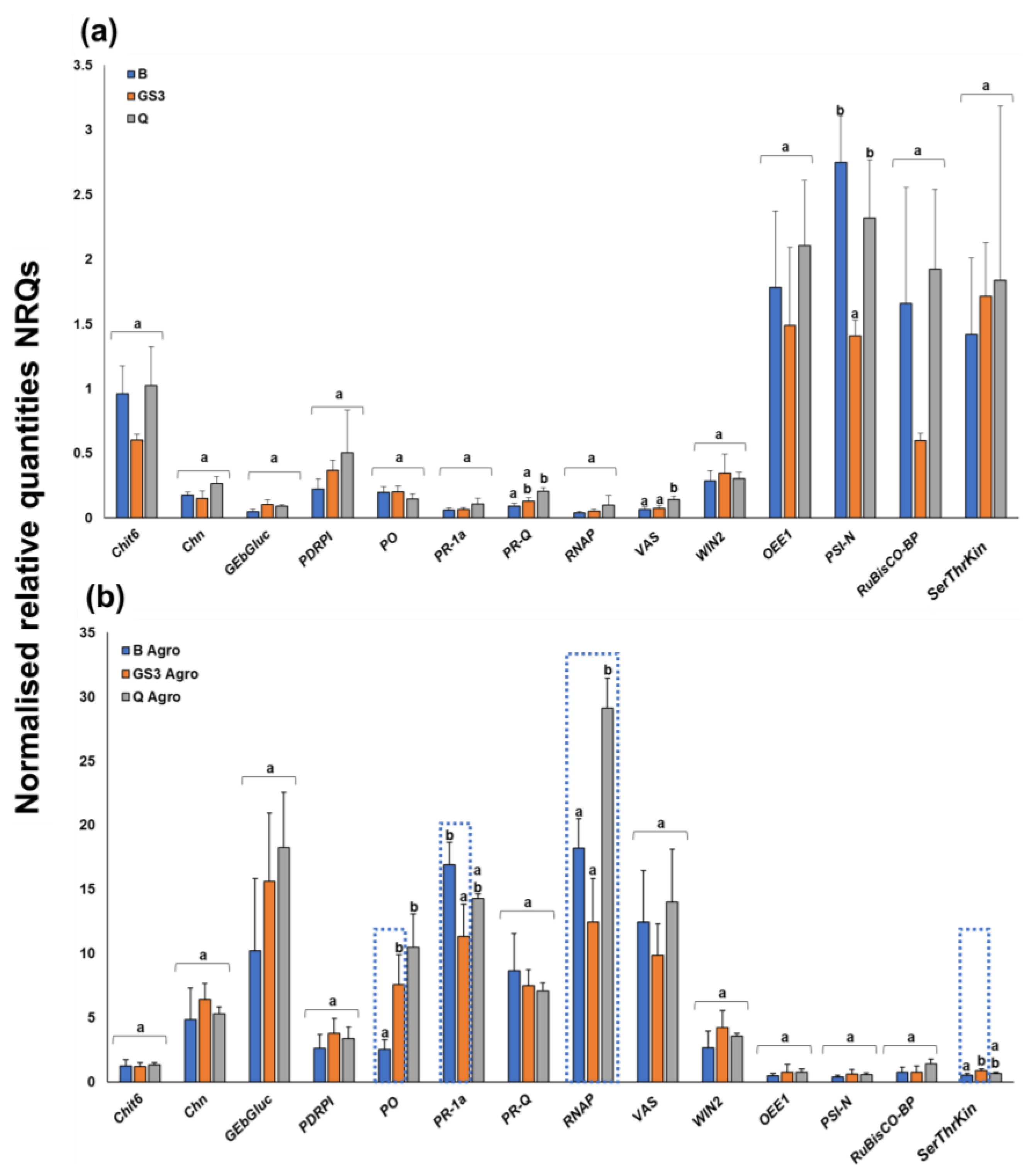
2.1. Agroinfiltration Stimulated Stress-Related Genes, but Priming with GS3 Induced Some Significant Changes
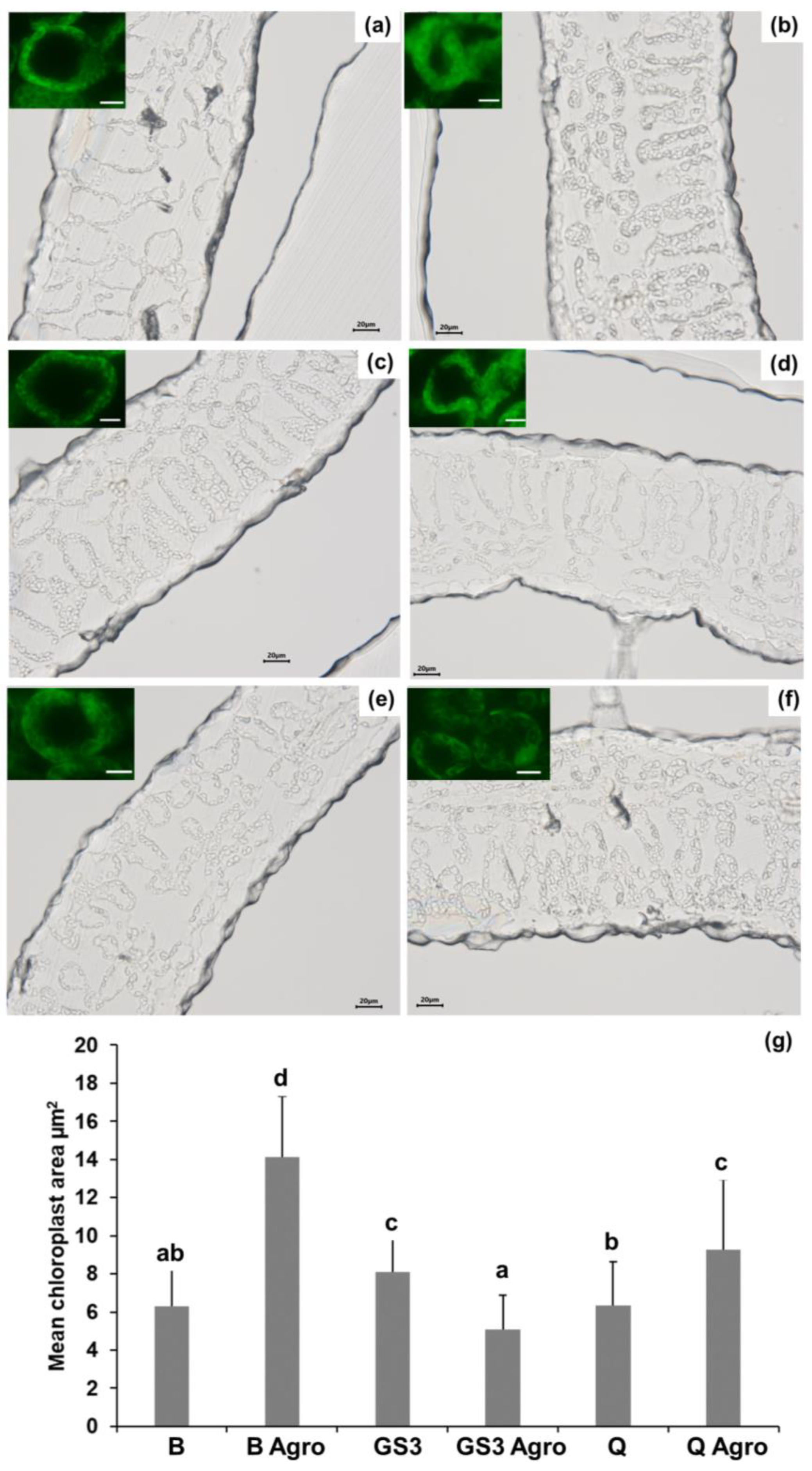
2.2. Agroinfiltration Increased the Chloroplasts’ Mean Area and GS3 Prevented This Effect
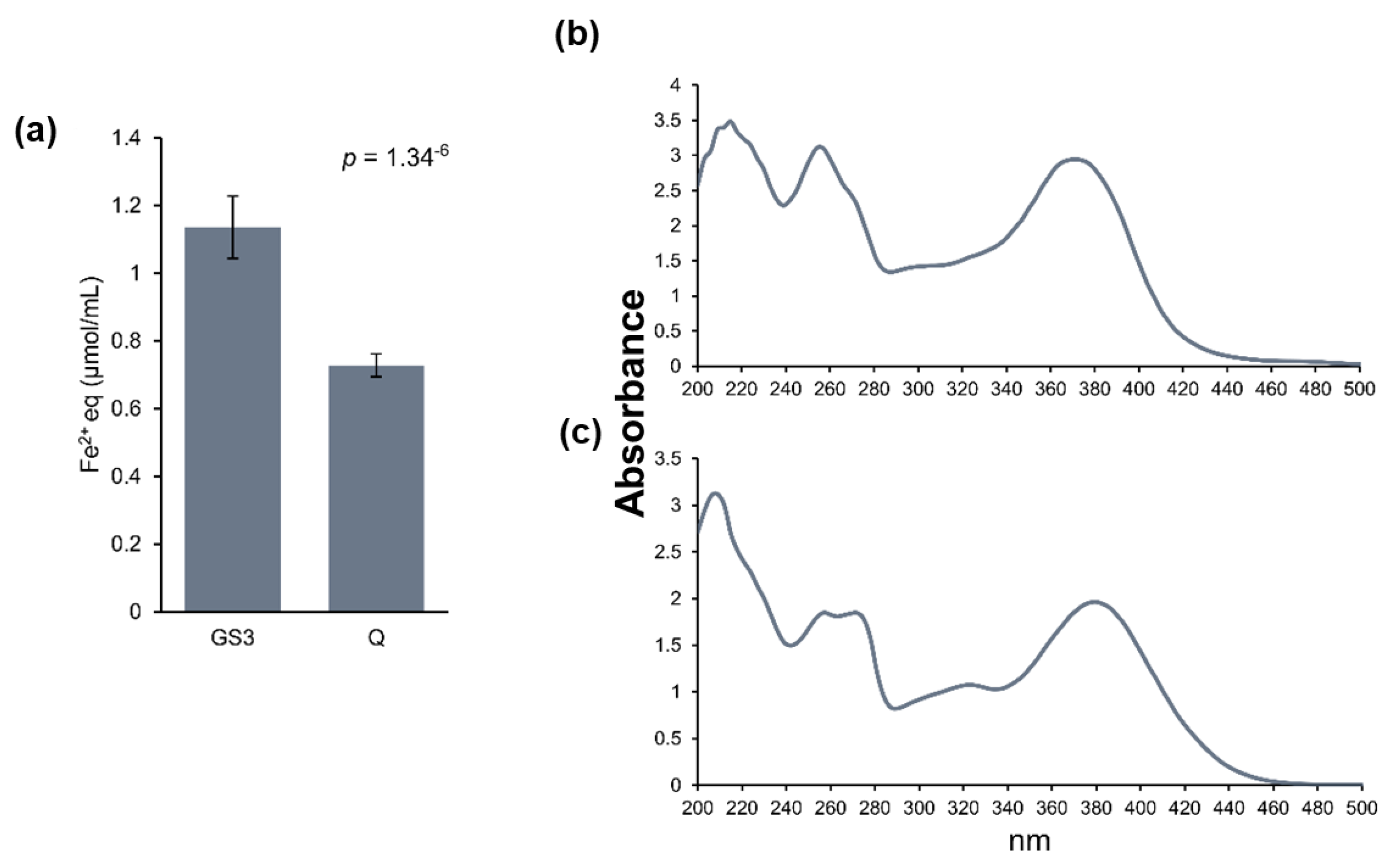
2.3. Relative Flavonoid Accumulation and Antioxidant Power of the Leaf Metabolite Extracts, Phyto-Courier, and Control Solutions
3. Discussion
4. Materials and Methods
4.1. Experimental Set-Up and Infiltration
4.2. Sampling and RNA Extraction
4.3. RNA Quality Check and Quantification
4.4. Primer Design
4.5. RT-qPCR
4.6. Preparation of Samples for Optical Microscopy
4.7. Preparation of Methanolic Extracts and Quercetin Extraction from the GS3 Phyto-Courier and Q Solution
4.8. Spectrophotometric Measurements
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Santini, A.; Ghelardini, L. Plant Pathogen Evolution and Climate Change. CAB Rev. 2015, 10, 1–8. [Google Scholar] [CrossRef]
- Elad, Y.; Pertot, I. Climate Change Impacts on Plant Pathogens and Plant Diseases. J. Crop Improv. 2014, 28, 99–139. [Google Scholar] [CrossRef]
- Heil, M.; Bostock, R.M. Induced Systemic Resistance (ISR) against Pathogens in the Context of Induced Plant Defences. Ann. Bot. 2002, 89, 503–512. [Google Scholar] [CrossRef]
- Swarbrick, P.J.; Schulze-Lefert, P.; Scholes, J.D. Metabolic Consequences of Susceptibility and Resistance (Race-Specific and Broad-Spectrum) in Barley Leaves Challenged with Powdery Mildew. Plant Cell Environ. 2006, 29, 1061–1076. [Google Scholar] [CrossRef] [PubMed]
- Savary, S.; Ficke, A.; Hollier, C. Crop Losses Due to Diseases and Their Implications for Global Food Production Losses and Food Security. Food Secur. 2012, 4, 519–537. [Google Scholar] [CrossRef]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant–Pathogen Warfare under Changing Climate Conditions. Curr. Biol. 2018, 28, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Ben Rejeb, I.; Pastor, V.; Mauch-Mani, B. Plant Responses to Simultaneous Biotic and Abiotic Stress: Molecular Mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef]
- Pulizzi, F. Nano in the Future of Crops. Nat. Nanotechnol. 2019, 14, 507. [Google Scholar] [CrossRef]
- Shahcheraghi, N.; Golchin, H.; Sadri, Z.; Tabari, Y.; Borhanifar, F.; Makani, S. Nano-Biotechnology, an Applicable Approach for Sustainable Future. 3 Biotech 2022, 12, 65. [Google Scholar] [CrossRef]
- Fraceto, L.F.; Grillo, R.; de Medeiros, G.A.; Scognamiglio, V.; Rea, G.; Bartolucci, C. Nanotechnology in Agriculture: Which Innovation Potential Does It Have? Front. Environ. Sci. 2016, 4, 20. [Google Scholar] [CrossRef]
- Machado, T.O.; Beckers, S.J.; Fischer, J.; Müller, B.; Sayer, C.; De Araújo, P.H.H.; Landfester, K.; Wurm, F.R. Bio-Based Lignin Nanocarriers Loaded with Fungicides as a Versatile Platform for Drug Delivery in Plants. Biomacromolecules 2020, 21, 2755–2763. [Google Scholar] [CrossRef]
- Guerriero, G.; Maria Sutera, F.; Torabi-Pour, N.; Renaut, J.; Hausman, J.F.; Berni, R.; Pennington, H.C.; Welsh, M.; Dehsorkhi, A.; Zancan, L.R.; et al. Phyto-Courier, a Silicon Particle-Based Nanobiostimulant: Evidence from Cannabis sativa Exposed to Salinity. ACS Nano 2021, 15, 3061–3069. [Google Scholar] [CrossRef]
- Guerriero, G.; Sutera, F.M.; Hoffmann, J.; Leclercq, C.C.; Planchon, S.; Berni, R.; Hausman, J.-F.; Renaut, J.; Torabi-Pour, N.; Pennington, H.C.; et al. Nanoporous Quercetin-Loaded Silicon-Stabilized Hybrid Lipid Nanoparticles Alleviate Salt Stress in Tomato Plants. ACS Appl. Nano Mater. 2023, 6, 3647–3660. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The Role of Quercetin in Plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Kurepa, J.; Shull, T.E.; Smalle, J.A. Quercetin Feeding Protects Plants against Oxidative Stress. F1000Research 2016, 5, 2430. [Google Scholar] [CrossRef]
- Parvin, K.; Hasanuzzaman, M.; Borhannuddin Bhuyan, M.H.M.; Mohsin, S.M.; Fujita, M. Quercetin Mediated Salt Tolerance in Tomato through the Enhancement of Plant Antioxidant Defense and Glyoxalase Systems. Plants 2019, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Jańczak-Pieniążek, M.; Migut, D.; Piechowiak, T.; Buczek, J.; Balawejder, M. The Effect of Exogenous Application of Quercetin Derivative Solutions on the Course of Physiological and Biochemical Processes in Wheat Seedlings. Int. J. Mol. Sci. 2021, 22, 6882. [Google Scholar] [CrossRef]
- Migut, D.; Jańczak-pieniążek, M.; Piechowiak, T.; Buczek, J.; Balawejder, M. Article Physiological Response of Maize Plants (Zea mays L.) to the Use of the Potassium Quercetin Derivative. Int. J. Mol. Sci. 2021, 22, 7384. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.F.; Lutts, S.; Guerriero, G. Silicon and Plants: Current Knowledge and Technological Perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef]
- Guerriero, G.; Hausman, J.F.; Legay, S. Silicon and the Plant Extracellular Matrix. Front. Plant Sci. 2016, 7, 463. [Google Scholar] [CrossRef]
- Wang, M.; Gao, L.; Dong, S.; Sun, Y.; Shen, Q.; Guo, S. Role of Silicon on Plant–Pathogen Interactions. Front. Plant Sci. 2017, 8, 701. [Google Scholar] [CrossRef]
- Suriyaprabha, R.; Karunakaran, G.; Yuvakkumar, R.; Rajendran, V.; Kannan, N. Foliar Application of Silica Nanoparticles on the Phytochemical Responses of Maize (Zea mays L.) and Its Toxicological Behavior. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2014, 44, 1128–1131. [Google Scholar] [CrossRef]
- Bao-shan, L.; Shao-qi, D.; Chun-hui, L.; Li-jun, F.; Shu-chun, Q.; Min, Y. Effect of TMS (Nanostructured Silicon Dioxide on Growth of Changbai Larch Seedlings. J. For. Res. 2004, 15, 138–140. [Google Scholar] [CrossRef]
- Sun, D.; Hussain, H.I.; Yi, Z.; Rookes, J.E.; Kong, L.; Cahill, D.M. Mesoporous Silica Nanoparticles Enhance Seedling Growth and Photosynthesis in Wheat and Lupin. Chemosphere 2016, 152, 81–91. [Google Scholar] [CrossRef]
- Yuvakkumar, R.; Elango, V.; Rajendran, V.; Kannan, N.S.; Prabu, P. Influence of Nanosilica Powder on the Growth of Maize Crop (Zea mays L.). Int. J. Green Nanotechnol. 2011, 3, 180–190. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Torabian, S. Nano-Silicon Alters Antioxidant Activities of Soybean Seedlings under Salt Toxicity. Protoplasma 2018, 255, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Živčák, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of Silicon Nanoparticles in Agriculture. 3 Biotech 2019, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, M.; Hausman, J.F.; Isenborghs, A.; Guerriero, G.; Lutts, S. Impact of Cadmium and Zinc on Proteins and Cell Wall-Related Gene Expression in Young Stems of Hemp (Cannabis sativa L.) and Influence of Exogenous Silicon. Environ. Exp. Bot. 2021, 183, 104363. [Google Scholar] [CrossRef]
- Liang, Y.; Si, J.; Römheld, V. Silicon Uptake and Transport Is an Active Process in Cucumis sativus. New Phytol. 2005, 167, 797–804. [Google Scholar] [CrossRef]
- El-Shetehy, M.; Moradi, A.; Maceroni, M.; Reinhardt, D.; Petri-Fink, A.; Rothen-Rutishauser, B.; Mauch, F.; Schwab, F. Silica Nanoparticles Enhance Disease Resistance in Arabidopsis Plants. Nat. Nanotechnol. 2021, 16, 344–353. [Google Scholar] [CrossRef]
- Grimberg, Å.; Carlsson, A.S.; Marttila, S.; Bhalerao, R.; Hofvander, P. Transcriptional Transitions in Nicotiana benthamiana Leaves upon Induction of Oil Synthesis by WRINKLED1 Homologs from Diverse Species and Tissues. BMC Plant Biol. 2015, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lukasik, E.; Gawehns, F.; Takken, F.L.W. The Use of Agroinfiltration for Transient Expression of Plant Resistance and Fungal Effector Proteins in Nicotiana Benthamiana Leaves. Methods Mol. Biol. 2012, 835, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ju, M.; Qian, J.; Zhang, M.; Liu, T.; Zhang, K. A Tobacco Syringe Agroinfiltration-Based Method for a Phytohormone Transporter Activity Assay Using Endogenous Substrates. Front. Plant Sci. 2021, 12, 660966. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.M.; Albert, N.W.; Lee, R.H.; Gillard, G.B.; Brown, C.M.; Hellens, R.P.; Macknight, R.C. Infiltration-RNAseq: Transcriptome Profiling of Agrobacterium-Mediated Infiltration of Transcription Factors to Discover Gene Function and Expression Networks in Plants. Plant Methods 2016, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, Biological Functions, and Biotechnological Applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Lois, R. Accumulation of UV-Absorbing Flavonoids Induced by UV-B Radiation in Arabidopsis thaliana L. Planta 1994, 194, 498–503. [Google Scholar] [CrossRef]
- Buchweitz, M.; Kroon, P.A.; Rich, G.T.; Wilde, P.J. Quercetin Solubilisation in Bile Salts: A Comparison with Sodium Dodecyl Sulphate. Food Chem. 2016, 211, 356–364. [Google Scholar] [CrossRef]
- Aranega-Bou, P.; de la O Leyva, M.; Finiti, I.; Garcfa-Agustfn, P.; Gonzalez-Bosch, C. Priming of Plant Resistance by Natural Compounds. Hexanoic Acid as a Model. Front. Plant Sci. 2014, 5, 488. [Google Scholar] [CrossRef]
- Desmedt, W.; Vanholme, B.; Kyndt, T. Plant Defence Prming in the Field: A Review. In Recent Highlights in the Discovery and Optimization of Crop Protection Products; Maienfisch, P., Mangelinckx, S., Eds.; Academic Press: Ghent, Belgium, 2021; pp. 87–124. ISBN 978-0-12-821035-2. [Google Scholar]
- Richmond, K.E.; Sussman, M. Got Silicon? The Non-Essential Beneficial Plant Nutrient. Curr. Opin. Plant Biol. 2003, 6, 268–272. [Google Scholar] [CrossRef]
- Guerriero, G.; Stokes, I.; Valle, N.; Hausman, J.; Exley, C. Visualising Silicon in Plants: Histochemistry, Silica Sculptures and Elemental Imaging. Cells 2020, 9, 1066. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan, M.; Ali, Q.; Ali, S. Seed Priming with Silicon Nanoparticles Improved the Biomass and Yield While Reduced the Oxidative Stress and Cadmium Concentration in Wheat Grains. Environ. Sci. Pollut. Res. 2019, 26, 7579–7588. [Google Scholar] [CrossRef] [PubMed]
- El-Serafy, R.S.; El-Sheshtawy, A.-N.; Atteya, A.K.G.; Al-Hashimi, A.; Abbasi, A.M.; Al-Ashkar, I. Seed Priming with Silicon as a Potential to Increase Salt Stress Tolerance in Lathyrus odoratus. Plants 2021, 10, 2140. [Google Scholar] [CrossRef] [PubMed]
- Hatami, M.; Khanizadeh, P.; Bovand, F.; Aghaee, A. Silicon Nanoparticle-Mediated Seed Priming and Pseudomonas spp. Inoculation Augment Growth, Physiology and Antioxidant Metabolic Status in Melissa officinalis L. Plants. Ind. Crops Prod. 2021, 162, 113238. [Google Scholar] [CrossRef]
- Ahn, I.P.; Kim, S.; Lee, Y.H.; Suh, S.C. Vitamin B1-Induced Priming Is Dependent on Hydrogen Peroxide and the NPR1 Gene in Arabidopsis. Plant Physiol. 2007, 143, 838–848. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Sun, M.; Sun, F.; Deng, S.; Dong, H. Riboflavin-Induced Priming for Pathogen Defense in Arabidopsis thaliana. J. Integr. Plant Biol. 2009, 51, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zou, B.; Wang, X.; Qiu, J.; Ma, H.; Gou, Z.; Song, S.; Dong, H. Quercetin-Induced H2O2 Mediates the Pathogen Resistance against Pseudomonas syringae Pv. Tomato DC3000 in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2010, 396, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Uzma Jalil, S.; Mishra, M.; Ansari, M.I. Current View on Chitinase for Plant Defence. Trends Biosci. 2015, 8, 6733–6743. [Google Scholar]
- Gutsch, A.; Keunen, E.; Guerriero, G.; Renaut, J.; Cuypers, A.; Hausman, J.-F.; Sergeant, K. Long-term Cadmium Exposure Influences the Abundance of Proteins That Impact the Cell Wall Structure in Medicago sativa Stems. Plant Biol. J. 2018, 20, 1023–1035. [Google Scholar] [CrossRef]
- Bowles, D.J. Defense-Related Proteins in Higher Plants. Annu. Rev. Biochem. 1990, 59, 873–907. [Google Scholar] [CrossRef]
- Békésiová, B.; Hraška, Š.; Libantová, J.; Moravčíková, J.; Matušíková, I. Heavy-Metal Stress Induced Accumulation of Chitinase Isoforms in Plants. Mol. Biol. Rep. 2008, 35, 579–588. [Google Scholar] [CrossRef]
- Ward, E.R.; Payne, G.B.; Moyer, M.B.; Williams, S.C.; Dincher, S.S.; Sharkey, K.C.; Beck, J.J.; Taylor, H.T.; Ahl-Goy, P.; Meins, F.; et al. Differential Regulation of β-1,3-Glucanase Messenger RNAs in Response to Pathogen Infection. Plant Physiol. 1991, 96, 390–397. [Google Scholar] [CrossRef]
- Mauch, F.; Staehelin, L.A. Functional Lmplications of the Subcellular Localization of Ethylene-Lnduced Chitinase and β-1,3-Glucanase in Bean Leaves. Plant Cell 1989, 1, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Stanford, A.; Bevan, M.; Northcote, D. Differential Expression within a Family of Novel Wound-Induced Genes in Potato. Mol. Gen. Genet. 1989, 215, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Breen, S.; Williams, S.J.; Outram, M.; Kobe, B.; Solomon, P.S. Emerging Insights into the Functions of Pathogenesis-Related Protein 1. Trends Plant Sci. 2017, 22, 871–879. [Google Scholar] [CrossRef]
- Hiraga, S.; Ito, H.; Sasaki, K.; Yamakawa, H.; Mitsuhara, I.; Toshima, H.; Matsui, H.; Honma, M.; Ohashi, Y. Wound-Induced Expression of a Tobacco Peroxidase Is Not Enhanced by Ethephon and Suppressed by Methyl Jasmonate and Coronatine. Plant Cell Physiol. 2000, 41, 165–170. [Google Scholar] [CrossRef]
- Depège, N.; Bellafiore, S.; Rochaix, J.-D. Role of Chloroplast Protein Kinase Stt7 in LHCII Phosphorylation and State Transition in Chlamydomonas. Science 2003, 299, 1572–1575. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Li, M.; Pan, X. Dynamic Regulation of the Light-Harvesting System through State Transitions in Land Plants and Green Algae. Plants 2023, 12, 1173. [Google Scholar] [CrossRef] [PubMed]
- Mullineaux, C.W.; Emlyn-Jones, D. State Transitions: An Example of Acclimation to Low-Light Stress. J. Exp. Bot. 2005, 56, 389–393. [Google Scholar] [CrossRef]
- Pérez-Bueno, M.L.; Pineda, M.; Barón, M. Phenotyping Plant Responses to Biotic Stress by Chlorophyll Fluorescence Imaging. Front. Plant Sci. 2019, 10, 1135. [Google Scholar] [CrossRef]
- Erickson, J.L.; Ziegler, J.; Guevara, D.; Abel, S.; Klösgen, R.B.; Mathur, J.; Rothstein, S.J.; Schattat, M.H. Agrobacterium-Derived Cytokinin Influences Plastid Morphology and Starch Accumulation in Nicotiana benthamiana during Transient Assays. BMC Plant Biol. 2014, 14, 127. [Google Scholar] [CrossRef]
- Biswas, K.; Adhikari, S.; Tarafdar, A.; Kumar, R.; Saha, S.; Ghosh, P. Reactive Oxygen Species and Antioxidant Defence Systems in Plants: Role and Crosstalk Under Biotic Stress. In Sustainable Agriculture in the Era of Climate Change; Roychowdhury, R., Hasanuzzaman, M., Choudhury, S., Srivastava, A., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 265–292. ISBN 978-3-030-45668-9. [Google Scholar]
- Pietta, P.G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Gutsch, A.; Hendrix, S.; Guerriero, G.; Renaut, J.; Lutts, S.; Alseekh, S.; Fernie, A.R.; Hausman, J.-F.; Vangronsveld, J.; Cuypers, A.; et al. Long-Term Cd Exposure Alters the Metabolite Profile in Stem Tissue of Medicago sativa. Cells 2020, 9, 2707. [Google Scholar] [CrossRef] [PubMed]
- Momić, T.; Savić, J.; Vasić, V. Oxidation of Quercetin by Myeloperoxidase. Adv. Phys. Chem. 2009, 2009, e614362. [Google Scholar] [CrossRef]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The Biological Activities, Chemical Stability, Metabolism and Delivery Systems of Quercetin: A Review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Zhou, A.; Sadik, O.A. Comparative Analysis of Quercetin Oxidation by Electrochemical, Enzymatic, Autoxidation, and Free Radical Generation Techniques: A Mechanistic Study. J. Agric. Food Chem. 2008, 56, 12081–12091. [Google Scholar] [CrossRef]
- Mangeot-Peter, L.; Legay, S.; Hausman, J.F.; Esposito, S.; Guerriero, G. Identification of Reference Genes for RT-QPCR Data Normalization in Cannabis sativa Stem Tissues. Int. J. Mol. Sci. 2016, 17, 1556. [Google Scholar] [CrossRef]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A.M. Primer3Plus, an Enhanced Web Interface to Primer3. Nucleic Acids Res. 2007, 35, 71–74. [Google Scholar] [CrossRef]
- Liu, D.; Shi, L.; Han, C.; Yu, J.; Li, D.; Zhang, Y. Validation of Reference Genes for Gene Expression Studies in Virus-Infected Nicotiana Benthamiana Using Quantitative Real-Time PCR. PLoS ONE 2012, 7, e46451. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. QBase Relative Quantification Framework and Software for Management and Automated Analysis of Real-Time Quantitative PCR Data. Genome Biol. 2008, 8, R19. [Google Scholar] [CrossRef]
- Wong, C.C.; Li, H.B.; Cheng, K.W.; Chen, F. A Systematic Survey of Antioxidant Activity of 30 Chinese Medicinal Plants Using the Ferric Reducing Antioxidant Power Assay. Food Chem. 2006, 97, 705–711. [Google Scholar] [CrossRef]

| Samples | sshLNPs | Trehalose | Quercetin | Hypromellose + Pluronic L61 Vehicle | Total Volume (25 mL PBS Addition) | ||
|---|---|---|---|---|---|---|---|
| Si-NPs | Hydro-PC | Arginine: Glycine | |||||
| B | 25 mL | 50 mL | |||||
| GS3 | 4 mg | 16 mg | 4 mg: 2 mg | 4 mg | 25 mg | 25 mL | 50 mL |
| Q | 25 mg | 25 mL | 50 mL | ||||
| Primer Name | Gene Name | Identifier | Primer Sequence fwd (5′→3′) | Primer Sequence rev (5′→3′) | Size (bp) | Efficiency % |
|---|---|---|---|---|---|---|
| Genes of interest [34] | ||||||
| NB_Chit6 | Chitinase 6 | k58:171433 | GATCGCTGCTTTCTTTGCTC | CGCCTGAAGGACCATTTATC | 80 | 95.69 |
| NB_Chn-A | Endochitinase A | k72:280831 | AACCTTCTTGCCACGATGTC | GTGATGACACCAAATCCAG | 91 | 92.96 |
| NB_GEβGluc | Glucan endo-1,3-beta-glucosidase | k60:395851 | GCTGCTTGTTGGGAGAAAAC | AGCCTGGACCTATTGAAACC | 102 | 95.17 |
| NB_PDRP1 | Pleiotropic drug resistance protein | k80:119278 | AGGTTTCATCGTTCCACGAC | AGGTCTCCGAATTGAGATGC | 111 | 92.55 |
| NB_PR-1a | Pathogenesis-related protein 1A | k58:459599 | TCCAACACGAACCGAGTTAC | TTGAGATGTGGGTCGATGAG | 116 | 81.84 |
| NB_PR-Q | Acidic endochitinase Q | k78:23474 | CCCCAGGAGCAACATTTAAC | AATGACGCAGTGGAAGATCG | 70 | 91.93 |
| NB_RNAP-β | DNA-directed-RNA polymerase subunit beta’ | k58:179615 | TCCTCTTATCCCAATCTGGTG | TTGACGGACACAAACTCTGC | 95 | 88.01 |
| NB_VAS | Lipid transfer-like protein | k64:395173 | TAGTCACGGTGGCGATTATG | GCGGTTTGGTGGAATTAAGG | 105 | 91.8 |
| NB_WIN2 | Wound-induced protein | k58:223872 | CCGTCAAAGGGTAAACATGG | GATGGAAGAGGGAATCAACG | 144 | 97.23 |
| NB_OEE1 | Oxygen-evolving enhancer protein 1 | k80:117138 | CCACATCATTCACGGTCAAG | TGCCATCAGAAGACACTTCG | 135 | 92.6 |
| NB_RuBisCO-BP | RuBisCO large subunit-binding protein subunit beta | k64:394932 | TACTGGCTTTTCCGTTCACC | TTGAGCAAGAAGCACTAGC | 103 | 88.00 |
| NB_SerThrKin | Serine/threonine-protein kinase | k78:183558 | TACCGATACCGTCCAATTCC | TGCACAGTCATGGTCTTG | 129 | 92.94 |
| NB_PSI-N | Photosystem I reaction centre subunit N | k76:226809 | GGCAGCAATGAACTCAAGTG | TGATTGGGAAGCCATAGAGG | 100 | 87.00 |
| Reference genes [70] | ||||||
| Nb_F-Box | F-box protein | Niben.v0.3.Ctg24993647 (At5g15710) | GGCACTCACAAACGTCTATTTC | ACCTGGGAGGCATCCTGCTTAT | 127 | 100.8 |
| Nb_SAND | Sand family protein | Niben.v0.3.Ctg25188435 (At2g28390) | ACCACCAACACCTATGAATGCT | CAGTCTCGCCTCATCTGGGTCA | 83 | 88.27 |
| Nb_L23 | 60S ribosomal protein | TC19271 (At2g39460) | AAGGATGCCGTGAAGAAGATGT | GCATCGTAGTCAGGAGTCAACC | 110 | 91.46 |
| Nb_UK | Uridylate kinase | EH363935 (At5g26667) | CTAGGAGTATATTGGAAGAGCG | AAAGATACATCGCCTTGCTGAA | 107 | 96.72 |
| Nb_GBP | GTP-binding protein | TC20872 (At5g59840) | GGAACTGGATTCGCAACATAGA | GACCCTTGGAAGTTGGCACAGC | 114 | 92.5 |
| Nb_RdR6 | Putative RNA-dependent RNA polymerase SDE1 | AY722008 (At3g49500) | TTCAGGAATGTCTTCGAGCG | AGTGATCTAGCAACCCAATGAG | 134 | 93.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutsch, A.; Berni, R.; Hausman, J.-F.; Sutera, F.M.; Dehsorkhi, A.; Torabi-Pour, N.; Saffie-Siebert, S.; Guerriero, G. A Study on the Use of the Phyto-Courier Technology in Tobacco Leaves Infected by Agrobacterium tumefaciens. Int. J. Mol. Sci. 2023, 24, 14153. https://doi.org/10.3390/ijms241814153
Gutsch A, Berni R, Hausman J-F, Sutera FM, Dehsorkhi A, Torabi-Pour N, Saffie-Siebert S, Guerriero G. A Study on the Use of the Phyto-Courier Technology in Tobacco Leaves Infected by Agrobacterium tumefaciens. International Journal of Molecular Sciences. 2023; 24(18):14153. https://doi.org/10.3390/ijms241814153
Chicago/Turabian StyleGutsch, Annelie, Roberto Berni, Jean-Francois Hausman, Flavia Maria Sutera, Ashkan Dehsorkhi, Nissim Torabi-Pour, Suzanne Saffie-Siebert, and Gea Guerriero. 2023. "A Study on the Use of the Phyto-Courier Technology in Tobacco Leaves Infected by Agrobacterium tumefaciens" International Journal of Molecular Sciences 24, no. 18: 14153. https://doi.org/10.3390/ijms241814153






