BMSC–HNC Interaction: Exploring Effects on Bone Integrity and Head and Neck Cancer Progression
Abstract
:1. Introduction
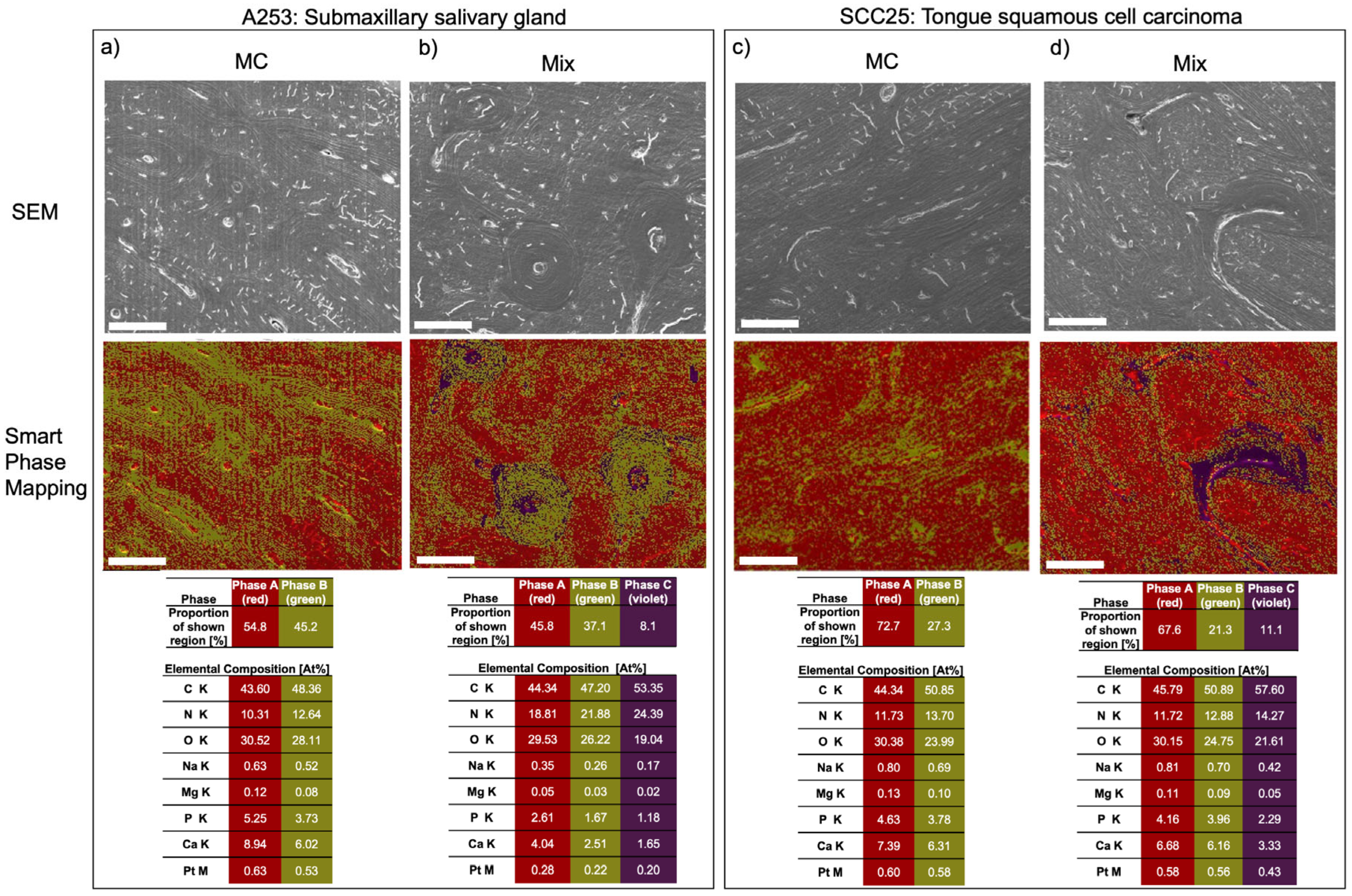
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture Conditions
4.2. Cultivation on Bone Slices
4.3. Generation of Tumor Spheroids
4.4. Calcein-AM Staining
4.5. High Vacuum Scanning Electron Microscopic (HV-SEM) Examination
4.6. Superficial Energy Dispersive X-ray Spectroscopy (EDX)
4.7. Scoring of Samples
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stewart, B.W.; Kleihues, P. World Cancer Report: Cancer Research for Cancer Prevention; IARC: Lyon, France, 2020; ISBN 978-92-832-0447-3. [Google Scholar]
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and Neck Cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Spoerl, S.; Gerken, M.; Fischer, R.; Mamilos, A.; Spoerl, S.; Wolf, S.; Pohl, F.; Klingelhöffer, C.; Ettl, T.; Reichert, T.E.; et al. Lymphatic and Vascular Invasion in Oral Squamous Cell Carcinoma: Implications for Recurrence and Survival in a Population-Based Cohort Study. Oral Oncol. 2020, 111, 105009. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Chen, X.; Cao, Y.; Lu, L.; Zhang, F.; Bornstein, S.; Li, Y.; Owens, P.; Malkoski, S.; Said, S.; et al. Overexpression of PIK3CA in Murine Head and Neck Epithelium Drives Tumor Invasion and Metastasis through PDK1 and Enhanced TGFβ Signaling. Oncogene 2016, 35, 4641–4652. [Google Scholar] [CrossRef] [PubMed]
- Brana, I.; Siu, L.L. Locally Advanced Head and Neck Squamous Cell Cancer: Treatment Choice Based on Risk Factors and Optimizing Drug Prescription. Ann. Oncol. 2012, 23 (Suppl. S10), x178–x185. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Kuo, S.-W.; Fang, K.-H.; Hao, S.-P. Prognostic Impact of Marginal Mandibulectomy in the Presence of Superficial Bone Invasion and the Nononcologic Outcome. Head Neck 2011, 33, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.; Tzadik, A.; Leonard, G. Mandibular Involvement by Oral Squamous Cell Carcinoma. Laryngoscope 1986, 96, 96–101. [Google Scholar] [CrossRef]
- Chen, S.-F.; Nieh, S.; Jao, S.-W.; Wu, M.-Z.; Liu, C.-L.; Chang, Y.-C.; Lin, Y.-S. The Paracrine Effect of Cancer-Associated Fibroblast-Induced Interleukin-33 Regulates the Invasiveness of Head and Neck Squamous Cell Carcinoma: CAF-Induced IL-33 Regulates HNSCC Invasiveness. J. Pathol. 2013, 231, 180–189. [Google Scholar] [CrossRef]
- Scherzed, A.; Hackenberg, S.; Froelich, K.; Kessler, M.; Koehler, C.; Hagen, R.; Radeloff, A.; Friehs, G.; Kleinsasser, N. BMSC Enhance the Survival of Paclitaxel Treated Squamous Cell Carcinoma Cells in Vitro. Cancer Biol. Ther. 2011, 11, 349–357. [Google Scholar] [CrossRef]
- Wessely, A.; Waltera, A.; Reichert, T.E.; Stöckl, S.; Grässel, S.; Bauer, R.J. Induction of ALP and MMP9 Activity Facilitates Invasive Behavior in Heterogeneous Human BMSC and HNSCC 3D Spheroids. FASEB J. 2019, 33, 11884–11893. [Google Scholar] [CrossRef]
- Kong, Y.Y.; Yoshida, H.; Sarosi, I.; Tan, H.L.; Timms, E.; Capparelli, C.; Morony, S.; Oliveira-dos-Santos, A.J.; Van, G.; Itie, A.; et al. OPGL Is a Key Regulator of Osteoclastogenesis, Lymphocyte Development and Lymph-Node Organogenesis. Nature 1999, 397, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Pan, L.; Luo, Z.; Zhao, H.; Cai, S. Interrelationship of Circulating Matrix Metalloproteinase-9, TNF-α, and OPG/RANK/RANKL Systems in COPD Patients with Osteoporosis. COPD 2013, 10, 650–656. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.K.; Singh, M.; Bharti, A.C.; Asotra, K.; Sundaram, S.; Mehrotra, R. Genetic Polymorphisms of Matrix Metalloproteinases and Their Inhibitors in Potentially Malignant and Malignant Lesions of the Head and Neck. J. Biomed. Sci. 2010, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.P.; Shah, P.M.; Rawal, U.M.; Desai, A.A.; Shah, S.V.; Rawal, R.M.; Patel, P.S. Activation of MMP-2 and MMP-9 in Patients with Oral Squamous Cell Carcinoma. J. Surg. Oncol. 2005, 90, 81–88. [Google Scholar] [CrossRef]
- Koontongkaew, S. The Tumor Microenvironment Contribution to Development, Growth, Invasion and Metastasis of Head and Neck Squamous Cell Carcinomas. J. Cancer 2013, 4, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, L.; Stenstrom, B.; Chen, D.; Piperdi, B.; Levey, S.; Lyle, S.; Wang, T.C.; Houghton, J. Human Barrett’s Adenocarcinoma of the Esophagus, Associated Myofibroblasts, and Endothelium Can Arise from Bone Marrow-Derived Cells after Allogeneic Stem Cell Transplant. Stem. Cells Dev. 2011, 20, 11–17. [Google Scholar] [CrossRef]
- Gonzalez, M.E.; Martin, E.E.; Anwar, T.; Arellano-Garcia, C.; Medhora, N.; Lama, A.; Chen, Y.-C.; Tanager, K.S.; Yoon, E.; Kidwell, K.M.; et al. Mesenchymal Stem Cell-Induced DDR2 Mediates Stromal-Breast Cancer Interactions and Metastasis Growth. Cell Rep. 2017, 18, 1215–1228. [Google Scholar] [CrossRef]
- Hill, B.S.; Pelagalli, A.; Passaro, N.; Zannetti, A. Tumor-Educated Mesenchymal Stem Cells Promote pro-Metastatic Phenotype. Oncotarget 2017, 8, 73296–73311. [Google Scholar] [CrossRef]
- Kidd, S.; Spaeth, E.; Dembinski, J.L.; Dietrich, M.; Watson, K.; Klopp, A.; Battula, L.; Weil, M.; Andreeff, M.; Marini, F.C. Direct Evidence of Mesenchymal Stem Cell Tropism for Tumor and Wounding Microenvironments Using In Vivo Bioluminescence Imaging. Stem. Cells 2009, 27, 2614–2623. [Google Scholar] [CrossRef]
- Beckermann, B.M.; Kallifatidis, G.; Groth, A.; Frommhold, D.; Apel, A.; Mattern, J.; Salnikov, A.V.; Moldenhauer, G.; Wagner, W.; Diehlmann, A.; et al. VEGF Expression by Mesenchymal Stem Cells Contributes to Angiogenesis in Pancreatic Carcinoma. Br. J. Cancer 2008, 99, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Colnot, D.R.; Nieuwenhuis, E.J.C.; Kuik, D.J.; Leemans, C.R.; Dijkstra, J.; Snow, G.B.; Van Dongen, G.A.M.S.; Brakenhoff, R.H. Clinical Significance of Micrometastatic Cells Detected by E48 (Ly-6D) Reverse Transcription-Polymerase Chain Reaction in Bone Marrow of Head and Neck Cancer Patients. Clin. Cancer Res. 2004, 10, 7827–7833. [Google Scholar] [CrossRef] [PubMed]
- Pichi, B.; Marchesi, P.; Manciocco, V.; Ruscito, P.; Pellini, R.; Cristalli, G.; Terenzi, V.; Spriano, G. Carcinoma of the Buccal Mucosa Metastasizing to the Talus. J. Craniofac. Surg. 2009, 20, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Gibo, T.; Yamada, S.; Kawamoto, M.; Uehara, T.; Kurita, H. Immunohistochemical Investigation of Predictive Biomarkers for Mandibular Bone Invasion in Oral Squamous Cell Carcinoma. Pathol. Oncol. Res. 2020, 26, 2381–2389. [Google Scholar] [CrossRef]
- Eichberger, J.; Weber, F.; Spanier, G.; Gerken, M.; Schreml, S.; Schulz, D.; Fiedler, M.; Ludwig, N.; Bauer, R.J.; Reichert, T.E.; et al. Loss of MMP-27 Predicts Mandibular Bone Invasion in Oral Squamous Cell Carcinoma. Cancers 2022, 14, 4044. [Google Scholar] [CrossRef]
- Amiri, V.; Mohammadi, M.H.; Rafiee, M.; Mirzaeian, A.; Farsani, M.A. Panobinostat, a Pan-HDAC Inhibitor, Substantially Decreases the Quiescent Population of Leukemic Cells Either in Monoculture or in Co-Culture with Bone Marrow Stromal Cells. Int. J. Cancer Manag. 2022, 15, e120599. [Google Scholar] [CrossRef]
- Zhou, L.; Guo, H.; Jia, F.; Chen, X.; Zhang, X.; Dong, S.; Zhao, L. Studies on the Changes of UPA System in a Co-Culture Model of Bone Marrow Stromal Cells–Leukemia Cells. Biosci. Rep. 2020, 40, BSR20194044. [Google Scholar] [CrossRef]
- Liao, Y.; Fang, Y.; Zhu, H.; Huang, Y.; Zou, G.; Dai, B.; Rausch, M.A.; Shi, B. Concentrated Growth Factors Promote HBMSCs Osteogenic Differentiation in a Co-Culture System with HUVECs. Front. Bioeng. Biotechnol. 2022, 10, 837295. [Google Scholar] [CrossRef]
- Waltera, A.; Schulz, D.; Schaefer, N.; Stoeckl, S.; Pion, E.; Haerteis, S.; Reichert, T.E.; Ettl, T.; Bauer, R.J. Opposing MMP-9 Expression in Mesenchymal Stromal Cells and Head and Neck Tumor Cells after Direct 2D and 3D Co-Culture. Int. J. Mol. Sci. 2023, 24, 1293. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Majumder, A.; Ray, S.; Banerji, A. Epidermal Growth Factor Receptor-Mediated Regulation of Matrix Metalloproteinase-2 and Matrix Metalloproteinase-9 in MCF-7 Breast Cancer Cells. Mol. Cell Biochem. 2019, 452, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-N.; Zhou, X.; Gu, Y.; Yan, J. Prognostic Value of MMP-9 in Ovarian Cancer: A Meta-Analysis. Asian Pac. J. Cancer Prev. 2013, 14, 4107–4113. [Google Scholar] [CrossRef] [PubMed]
- Mundy, G.R. Metastasis to Bone: Causes, Consequences and Therapeutic Opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef]
- Yang, T.; Tang, S.; Peng, S.; Ding, G. The Effects of Mesenchymal Stem Cells on Oral Cancer and Possible Therapy Regime. Front. Genet. 2022, 13, 949770. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Feng, X.; Wang, B.; Wang, X.; Wang, C.; Yu, M.; Cao, G.; Wang, H. Bone Marrow Mesenchymal Stem Cells Promote Head and Neck Cancer Progression through Periostin-Mediated Phosphoinositide 3-Kinase/Akt/Mammalian Target of Rapamycin. Cancer Sci. 2018, 109, 688–698. [Google Scholar] [CrossRef]
- Klopp, A.H.; Gupta, A.; Spaeth, E.; Andreeff, M.; Marini, F. Concise Review: Dissecting a Discrepancy in the Literature: Do Mesenchymal Stem Cells Support or Suppress Tumor Growth? Stem. Cells 2011, 29, 11–19. [Google Scholar] [CrossRef]
- Basmaeil, Y.; Bahattab, E.; Al Subayyil, A.; Kulayb, H.; Alrodayyan, M.; Abumaree, M.; Khatlani, T. Decidua Parietalis Mesenchymal Stem/Stromal Cells and Their Secretome Diminish the Oncogenic Properties of MDA231 Cells In Vitro. Cells 2021, 10, 3493. [Google Scholar] [CrossRef]
- Chen, L.; Tredget, E.E.; Wu, P.Y.G.; Wu, Y. Paracrine Factors of Mesenchymal Stem Cells Recruit Macrophages and Endothelial Lineage Cells and Enhance Wound Healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef]
- Eck, S.M.; Côté, A.L.; Winkelman, W.D.; Brinckerhoff, C.E. CXCR4 and Matrix Metalloproteinase-1 Are Elevated in Breast Carcinoma–Associated Fibroblasts and in Normal Mammary Fibroblasts Exposed to Factors Secreted by Breast Cancer Cells. Mol. Cancer Res. 2009, 7, 1033–1044. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, K.; Zhang, K.; Xu, F.; Yin, Y.; Zhu, L.; Zhou, F. Galectin-1 Knockdown in Carcinoma-Associated Fibroblasts Inhibits Migration and Invasion of Human MDA-MB-231 Breast Cancer Cells by Modulating MMP-9 Expression. Acta Biochim. Biophys. Sin. 2016, 48, 462–467. [Google Scholar] [CrossRef]

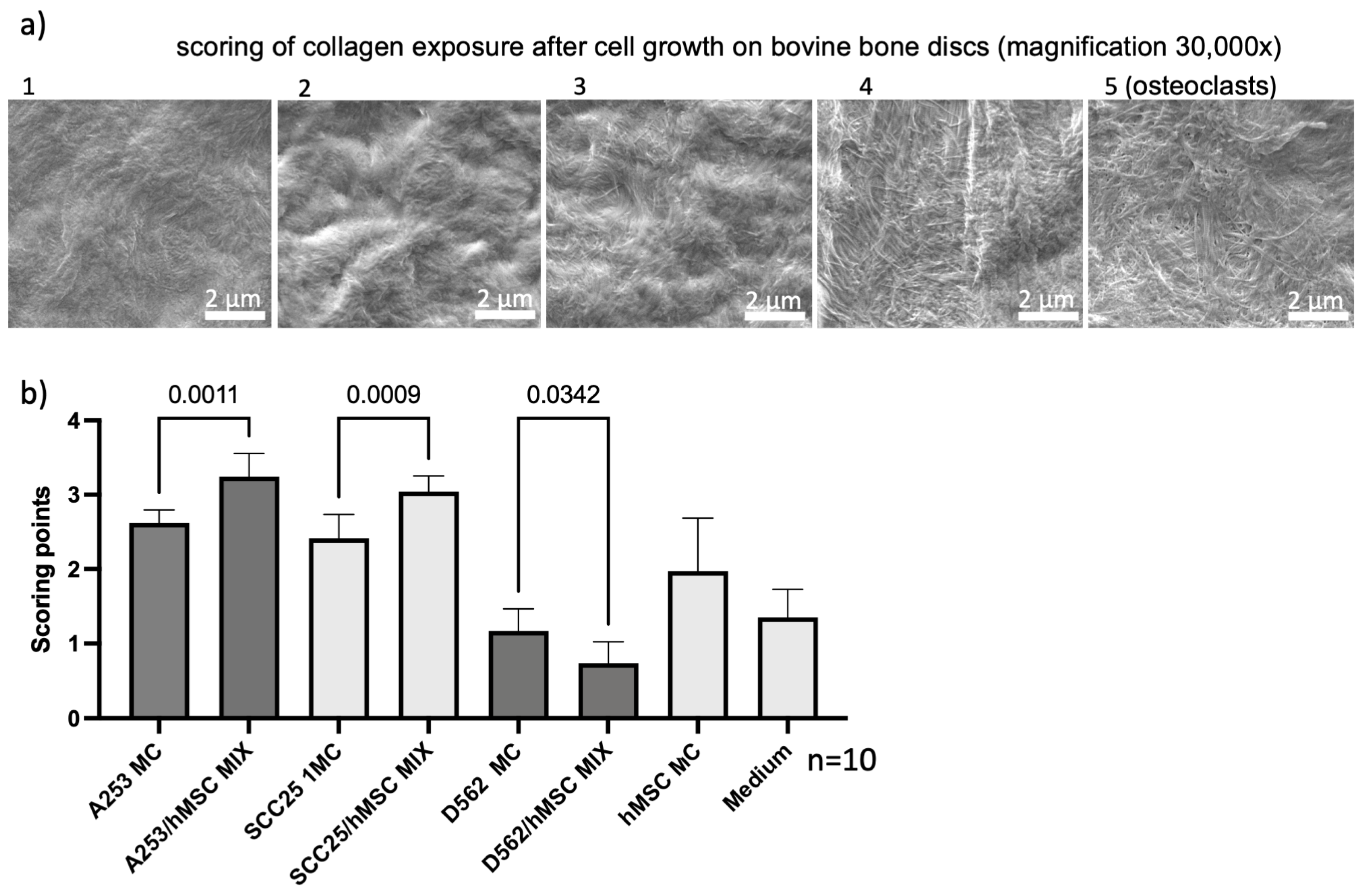

| Grade | Collagen Exposure |
|---|---|
| 1 | No collagen exposure |
| 2 | <30% Collagen exposure |
| 3 | 30–50% Collagen exposure |
| 4 | 50–90% Collagen exposure |
| 5 | >90% Collagen exposure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eichberger, J.; Froschhammer, D.; Schulz, D.; Scholz, K.J.; Federlin, M.; Ebensberger, H.; Reichert, T.E.; Ettl, T.; Bauer, R.J. BMSC–HNC Interaction: Exploring Effects on Bone Integrity and Head and Neck Cancer Progression. Int. J. Mol. Sci. 2023, 24, 14417. https://doi.org/10.3390/ijms241914417
Eichberger J, Froschhammer D, Schulz D, Scholz KJ, Federlin M, Ebensberger H, Reichert TE, Ettl T, Bauer RJ. BMSC–HNC Interaction: Exploring Effects on Bone Integrity and Head and Neck Cancer Progression. International Journal of Molecular Sciences. 2023; 24(19):14417. https://doi.org/10.3390/ijms241914417
Chicago/Turabian StyleEichberger, Jonas, Daniel Froschhammer, Daniela Schulz, Konstantin J. Scholz, Marianne Federlin, Helga Ebensberger, Torsten E. Reichert, Tobias Ettl, and Richard J. Bauer. 2023. "BMSC–HNC Interaction: Exploring Effects on Bone Integrity and Head and Neck Cancer Progression" International Journal of Molecular Sciences 24, no. 19: 14417. https://doi.org/10.3390/ijms241914417






