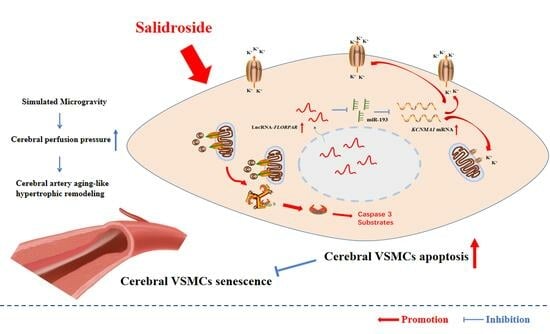
Discovery of Salidroside as a Novel Non-Coding RNA Modulator to Delay Cellular Senescence and Promote BK-Dependent Apoptosis in Cerebrovascular Smooth Muscle Cells of Simulated Microgravity Rats
Abstract
:1. Introduction
2. Results
2.1. General Data
2.2. SAL-Delayed Cellular Senescence in Cerebral Arteries of Simulated Microgravity Rats and Cultured VSMCs
2.3. SAL-Promoted BK-Dependent Apoptosis in Cerebral Arteries of Simulated Microgravity Rats and Cultured VSMCs
2.4. BK-Dependent Apoptosis Was Induced by Simulated Microgravity-Activated LncRNA-FLORPAR in VSMCs
2.5. MiR-193 Negatively Modulated Apoptosis by Targeting BK mRNA
2.6. MiR-193 Was Sponged by LncRNA-FLORPAR
2.7. SAL Activated LncRNA-FLORPAR/miR-193 Pathway and Then Induced BK-Dependent Apoptosis in VSMCs
3. Discussion
3.1. SAL Alleviated Simulated Microgravity-Induced Arterial Hypertrophic Remodeling by Delaying Cellular Senescence and Promoting BK-Dependent Apoptosis in VSMCs
3.2. LncRNA-FLOPPAR-Sponging miR-193 Pathway Induced BK-Dependent Apoptosis in VSMCs of Simulated Microgravity Rats
3.3. SAL Promoted BK-Dependent Apoptosis by Activation of LncRNA-FLORPAR/miR-193 Pathway
3.4. Practical Implications and Limitations
4. Materials and Methods
4.1. Animals
4.2. Isolation of Cerebral Arteries
4.3. Cell Cultures
4.4. Protein Extraction and Western Blotting
4.5. RNA Sequencing
4.6. Analysis of Non-Coding RNA Target
4.7. RNA Extraction and Real-Time Quantitative Reverse Transcription PCR (qRT-PCR)
4.8. Plasmid and Oligonucleotide Transient Transfection
4.9. Dual-Luciferase Report Assay
4.10. RNA Fluorescence In Situ Hybridization (FISH)
4.11. RNA Immunoprecipitation (RIP)
4.12. Hoechst Staining
4.13. Terminal Deoxynucleotidyl Transferase dUTP Nick End-Labeling (TUNEL) Assay
4.14. Flow Cytometry
4.15. Transmission Electron Microscopy (TEM)
4.16. Hematoxylin-Eosin (H&E) Staining
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.F. Region-specific vascular remodeling and its prevention by artificial gravity in weightless environment. Eur. J. Appl. Physiol. 2013, 113, 2873–2895. [Google Scholar]
- Lee, S.M.C.; Feiveson, A.H.; Stein, S.; Stenger, M.B.; Platts, S.H. Orthostatic Intolerance After ISS and Space Shuttle Missions. Aerosp. Med. Hum. Perform. 2015, 86 (Suppl. S12), A54–A67. [Google Scholar] [CrossRef] [PubMed]
- Antonutto, G.; di Prampero, P.E. Cardiovascular deconditioning in microgravity: Some possible countermeasures. Eur. J. Appl. Physiol. 2003, 90, 283–291. [Google Scholar] [CrossRef]
- Zhang, L.F.; Hargens, A.R. Spaceflight-Induced Intracranial Hypertension and Visual Impairment: Pathophysiology and Countermeasures. Physiol. Rev. 2018, 98, 59–87. [Google Scholar] [CrossRef] [PubMed]
- English, K.L.; Bloomberg, J.J.; Mulavara, A.P.; Ploutz-Snyder, L.L. Exercise Countermeasures to Neuromuscular Deconditioning in Spaceflight. Compr. Physiol. 2019, 10, 171–196. [Google Scholar]
- Sibonga, J.; Matsumoto, T.; Jones, J.; Shapiro, J.; Lang, T.; Shackelford, L.; Smith, S.M.; Young, M.; Keyak, J.; Kohri, K.; et al. Resistive exercise in astronauts on prolonged spaceflights provides partial protection against spaceflight-induced bone loss. Bone 2019, 128, 112037. [Google Scholar] [CrossRef]
- Hughson, R.L.; Robertson, A.D.; Arbeille, P.; Shoemaker, J.K.; Rush, J.W.; Fraser, K.S.; Greaves, D.K. Increased postflight carotid artery stiffness and inflight insulin resistance resulting from 6-mo spaceflight in male and female astronauts. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H628–H638. [Google Scholar] [CrossRef]
- Boutouyrie, P.; Chowienczyk, P.; Humphrey, J.D.; Mitchell, G.F. Arterial Stiffness and Cardiovascular Risk in Hypertension. Circ. Res. 2021, 128, 864–886. [Google Scholar] [CrossRef] [PubMed]
- Calcinotto, A.; Kohli, J.; Zagato, E.; Pellegrini, L.; Demaria, M.; Alimonti, A. Cellular Senescence: Aging, Cancer, and Injury. Physiol. Rev. 2019, 99, 1047–1078. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T. Cellular Senescence: A Translational Perspective. EBioMedicine 2017, 21, 21–28. [Google Scholar] [CrossRef]
- Salminen, A.; Ojala, J.; Kaarniranta, K. Apoptosis and aging: Increased resistance to apoptosis enhances the aging process. Cell. Mol. Life Sci. CMLS 2011, 68, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Tchkonia, T.; Zhu, Y.; van Deursen, J.; Campisi, J.; Kirkland, J.L. Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. J. Clin. Investig. 2013, 123, 966–972. [Google Scholar] [CrossRef]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364, eaau8650. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.M.; Stoudemire, J.; Dolan, L.; Downs, M. Leveraging Spaceflight to Advance Cardiovascular Research on Earth. Circ. Res. 2022, 130, 942–957. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.J.; Zhang, L.F.; Ma, J.; Cheng, H.W. Functional alterations in cerebrovascular K+ and Ca2+ channels are comparable between simulated microgravity rat and SHR. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1265–H1276. [Google Scholar] [CrossRef]
- Olivieri, F.; Prattichizzo, F.; Giuliani, A.; Matacchione, G.; Rippo, M.R.; Sabbatinelli, J.; Bonafè, M. miR-21 and miR-146a: The microRNAs of inflammaging and age-related diseases. Ageing Res. Rev. 2021, 70, 101374. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Kang, D.; Lee, E.K.; Lee, J.S. Long Noncoding RNAs and RNA-Binding Proteins in Oxidative Stress, Cellular Senescence, and Age-Related Diseases. Oxidative Med. Cell. Longev. 2017, 2017, 2062384. [Google Scholar] [CrossRef]
- Lozano-Vidal, N.; Bink, D.I.; Boon, R.A. Long noncoding RNA in cardiac aging and disease. J. Mol. Cell Biol. 2019, 11, 860–867. [Google Scholar] [CrossRef]
- Torrens-Spence, M.P.; Pluskal, T.; Li, F.S.; Carballo, V.; Weng, J.K. Complete Pathway Elucidation and Heterologous Reconstitution of Rhodiola Salidroside Biosynthesis. Mol. Plant 2018, 11, 205–217. [Google Scholar] [CrossRef]
- Shi, X.; Dong, N.; Qiu, Q.; Li, S.; Zhang, J. Salidroside Prevents Hypoxia-Induced Human Retinal Microvascular Endothelial Cell Damage Via miR-138/ROBO4 Axis. Investig. Ophthalmol. Vis. Sci. 2021, 62, 25. [Google Scholar] [CrossRef]
- Yu, S.P. Regulation and critical role of potassium homeostasis in apoptosis. Prog. Neurobiol. 2003, 70, 363–386. [Google Scholar] [CrossRef]
- Magder, S. The meaning of blood pressure. Crit. Care 2018, 22, 257. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, L.; Gao, F.; Ma, X.; Zhang, M.; Liu, J.; Zhang, L.; Ma, J. Daily short-period gravitation can prevent functional and structural changes in arteries of simulated microgravity rats. J. Appl. Physiol. 2004, 97, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, M.; Lesniewski, L.; Golding, E.; Bryan, R.; Amin, A.; Wilson, E.; Delp, D.M. Simulated microgravity enhances cerebral artery vasoconstriction and vascular resistance through endothelial nitric oxide mechanism. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1652-61. [Google Scholar] [CrossRef]
- Lin, L.; Gao, F.; Bai, Y.; Bao, J.; Huang, X.; Ma, J.; Zhang, L.F. Contrasting effects of simulated microgravity with and without daily -Gx gravitation on structure and function of cerebral and mesenteric small arteries in rats. J. Appl. Physiol. 2009, 107, 1710–1721. [Google Scholar] [CrossRef]
- Higami, Y.; Shimokawa, I. Apoptosis in the aging process. Cell Tissue Res. 2000, 301, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Savill, J. Apoptosis in disease. Eur. J. Clin. Investig. 1994, 24, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.J.; Ma, Y.G.; Gao, F.; Bai, Y.G.; Cheng, J.H.; Chang, Y.M.; Yu, Z.B.; Ma, J. Activation of BKCa channel is associated with increased apoptosis of cerebrovascular smooth muscle cells in simulated microgravity rats. Am. J. Physiol. Cell Physiol. 2010, 298, C1489–C1500. [Google Scholar] [CrossRef]
- Song, J.-B.; Chen, L.; Zhang, B.; Xie, M.J. Effects of salidroside on apoptosis of vascular smooth muscle cells under high glucose conditions. Chin. J. Aerosp. Med. 2017, 28, 146. [Google Scholar]
- Rong, L.; Li, Z.; Leng, X.; Li, H.; Ma, Y.; Chen, Y.; Song, F. Salidroside induces apoptosis and protective autophagy in human gastric cancer AGS cells through the PI3K/Akt/mTOR pathway. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 122, 109726. [Google Scholar] [CrossRef]
- Ding, S.Y.; Wang, M.T.; Dai, D.F.; Peng, J.L.; Wu, W.L. Salidroside induces apoptosis and triggers endoplasmic reticulum stress in human hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2020, 527, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Q.; Lou, Y.; Xu, J.; Feng, Z.; Chen, Y.; Tang, Q.; Zheng, G.; Zhang, Z.; Wu, Y.; et al. Salidroside attenuates neuroinflammation and improves functional recovery after spinal cord injury through microglia polarization regulation. J. Cell. Mol. Med. 2018, 22, 1148–1166. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, L.; Wang, L.P.; Wan, Q. Therapeutic targets of neuroprotection and neurorestoration in ischemic stroke: Applications for natural compounds from medicinal herbs. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 148, 112719. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zou, L.; Yu, X.; Chen, M.; Guo, R.; Cai, H.; Yao, D.; Xu, X.; Chen, Y.; Ding, C.; et al. Salidroside attenuates chronic hypoxia-induced pulmonary hypertension via adenosine A2a receptor related mitochondria-dependent apoptosis pathway. J. Mol. Cell. Cardiol. 2015, 82, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Dimmeler, S. Long noncoding RNAs in cardiovascular diseases. Circ. Res. 2015, 116, 737–750. [Google Scholar] [CrossRef]
- Leeper, N.; Maegdefessel, L. Non-coding RNAs: Key regulators of smooth muscle cell fate in vascular disease. Cardiovasc. Res. 2018, 114, 611–621. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, H.; Yue, Y.; Li, S.; Zhang, D.; He, R. H19 knockdown suppresses proliferation and induces apoptosis by regulating miR-148b/WNT/β-catenin in ox-LDL -stimulated vascular smooth muscle cells. J. Biomed. Sci. 2018, 25, 11. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, G.; Chen, X.; Lu, W.; Liu, J.; Zhai, S.; Qiao, G. Knockdown of lncRNA PVT1 Inhibits Vascular Smooth Muscle Cell Apoptosis and Extracellular Matrix Disruption in a Murine Abdominal Aortic Aneurysm Model. Mol. Cells 2019, 42, 218–227. [Google Scholar]
- Yang, L.; Liang, H.; Shen, L.; Guan, Z.; Meng, X. LncRNA Tug1 involves in the pulmonary vascular remodeling in mice with hypoxic pulmonary hypertension via the microRNA-374c-mediated Foxc1. Life Sci. 2019, 237, 116769. [Google Scholar] [CrossRef]
- Ming, X.; Yu, X.; Li, J.; Wang, J.; Zheng, J.; Xiong, L. Salidroside Attenuates Airway Inflammation and Remodeling via the miR-323-3p/SOCS5 Axis in Asthmatic Mice. Int. Arch. Allergy Immunol. 2022, 183, 424–434. [Google Scholar] [CrossRef]
- Morey-Holton, E.R.; Globus, R.K. Hindlimb unloading rodent model: Technical aspects. J. Appl. Physiol. 2002, 92, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Morey-Holton, E.; Globus, R.K.; Kaplansky, A.; Durnova, G. The hindlimb unloading rat model: Literature overview, technique update and comparison with space flight data. Adv. Space Biol. Med. 2005, 10, 7–40. [Google Scholar]
- Zhang, B.; Chen, L.; Bai, Y.G.; Song, J.B.; Cheng, J.H.; Ma, H.Z.; Ma, J.; Xie, M.J. miR-137 and its target T-type CaV3.1 channel modulate dedifferentiation and proliferation of cerebrovascular smooth muscle cells in simulated microgravity rats by regulating calcineurin/NFAT pathway. Cell Prolif. 2020, 53, e12774. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, B.; Yang, L.; Bai, Y.G.; Song, J.B.; Ge, Y.L.; Ma, H.Z.; Cheng, J.H.; Ma, J.; Xie, M.J. BMAL1 Disrupted Intrinsic Diurnal Oscillation in Rat Cerebrovascular Contractility of Simulated Microgravity Rats by Altering Circadian Regulation of miR-103/Ca(V)1.2 Signal Pathway. Int. J. Mol. Sci. 2019, 20, 3947. [Google Scholar] [CrossRef] [PubMed]
- Kimes, B.W.; Brandt, B.L. Characterization of two putative smooth muscle cell lines from rat thoracic aorta. Exp. Cell Res. 1976, 98, 349–366. [Google Scholar] [CrossRef]










| Body Weight (g) | Left Soleus Weight (mg) | Soleus/Body Weight (mg/g) | ||
|---|---|---|---|---|
| Initial | Final | |||
| CON | 216.65 ± 4.26 | 405.38 ± 7.42 | 151.29 ± 4.04 | 0.38 ± 0.02 |
| SUS | 220.28 ± 5.34 | 399.21 ± 6.93 | 70.16 ± 2.96 ** | 0.18 ± 0.01 ** |
| CON + SAL | 218.45 ± 4.68 | 404.36 ± 7.74 | 147.43 ± 3.35 | 0.39 ± 0.02 |
| SUS + SAL | 222.42 ± 4.15 | 402.32 ± 7.17 | 72.42 ± 3.29 ** | 0.18 ± 0.01 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, Y.; Zhang, B.; Song, J.; Cao, Q.; Bu, Y.; Li, P.; Bai, Y.; Yang, C.; Xie, M. Discovery of Salidroside as a Novel Non-Coding RNA Modulator to Delay Cellular Senescence and Promote BK-Dependent Apoptosis in Cerebrovascular Smooth Muscle Cells of Simulated Microgravity Rats. Int. J. Mol. Sci. 2023, 24, 14531. https://doi.org/10.3390/ijms241914531
Ge Y, Zhang B, Song J, Cao Q, Bu Y, Li P, Bai Y, Yang C, Xie M. Discovery of Salidroside as a Novel Non-Coding RNA Modulator to Delay Cellular Senescence and Promote BK-Dependent Apoptosis in Cerebrovascular Smooth Muscle Cells of Simulated Microgravity Rats. International Journal of Molecular Sciences. 2023; 24(19):14531. https://doi.org/10.3390/ijms241914531
Chicago/Turabian StyleGe, Yiling, Bin Zhang, Jibo Song, Qinglin Cao, Yingrui Bu, Peijie Li, Yungang Bai, Changbin Yang, and Manjiang Xie. 2023. "Discovery of Salidroside as a Novel Non-Coding RNA Modulator to Delay Cellular Senescence and Promote BK-Dependent Apoptosis in Cerebrovascular Smooth Muscle Cells of Simulated Microgravity Rats" International Journal of Molecular Sciences 24, no. 19: 14531. https://doi.org/10.3390/ijms241914531