A Novel Mouse Model of Intrahepatic Cholangiocarcinoma Induced by Azoxymethane
Abstract
:1. Introduction
2. Results
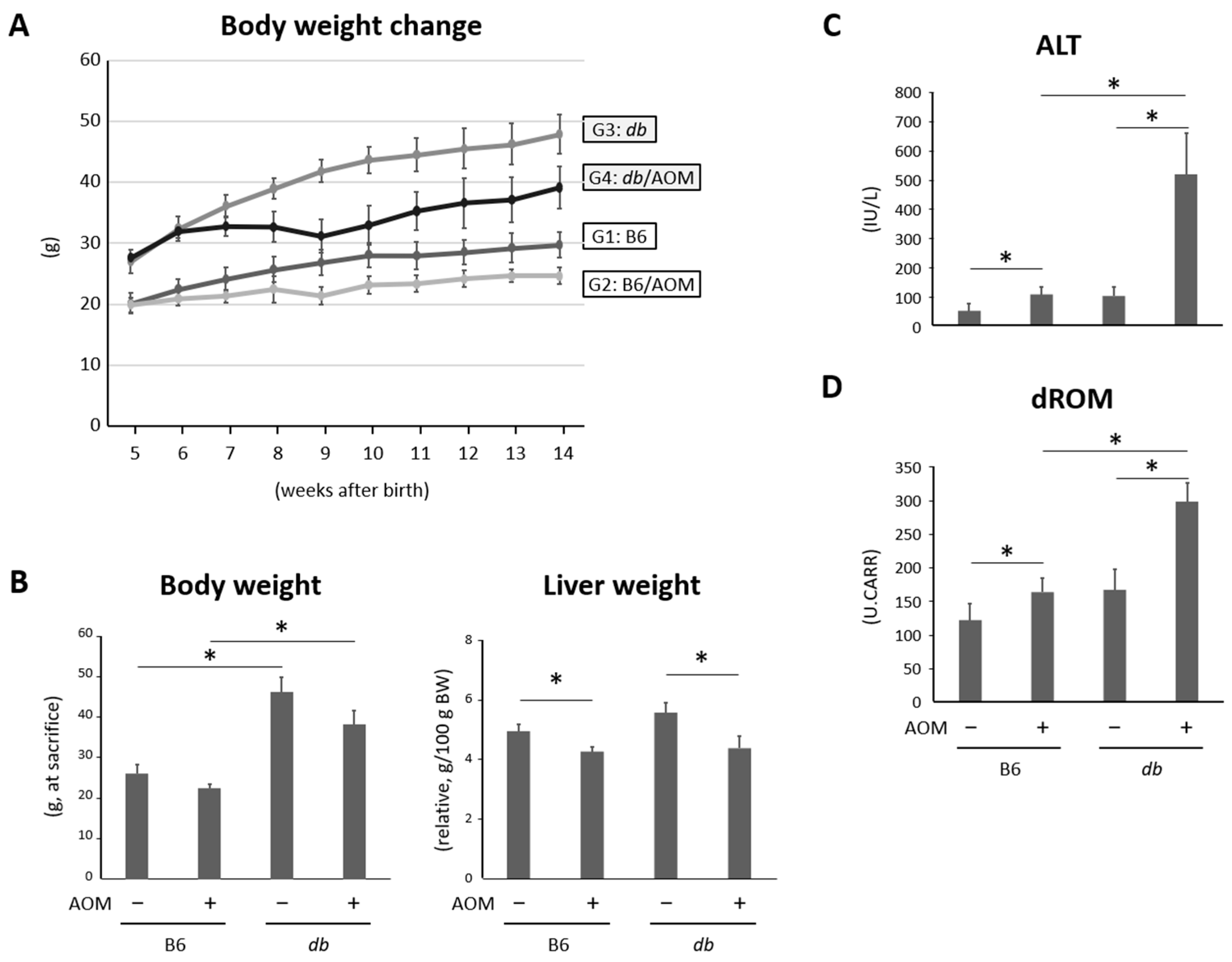
2.1. General Observations
2.2. Liver Damage and Oxidative Stress Were Observed in Mice Given Carcinogens and Were Exacerbated in Obese Mice
2.3. Obese Mice Given Carcinogens Showed Extended Bile Canalicular Epithelial Hyperplasia with Atypia and Cancerous Lesions of the Bile Duct in the Liver
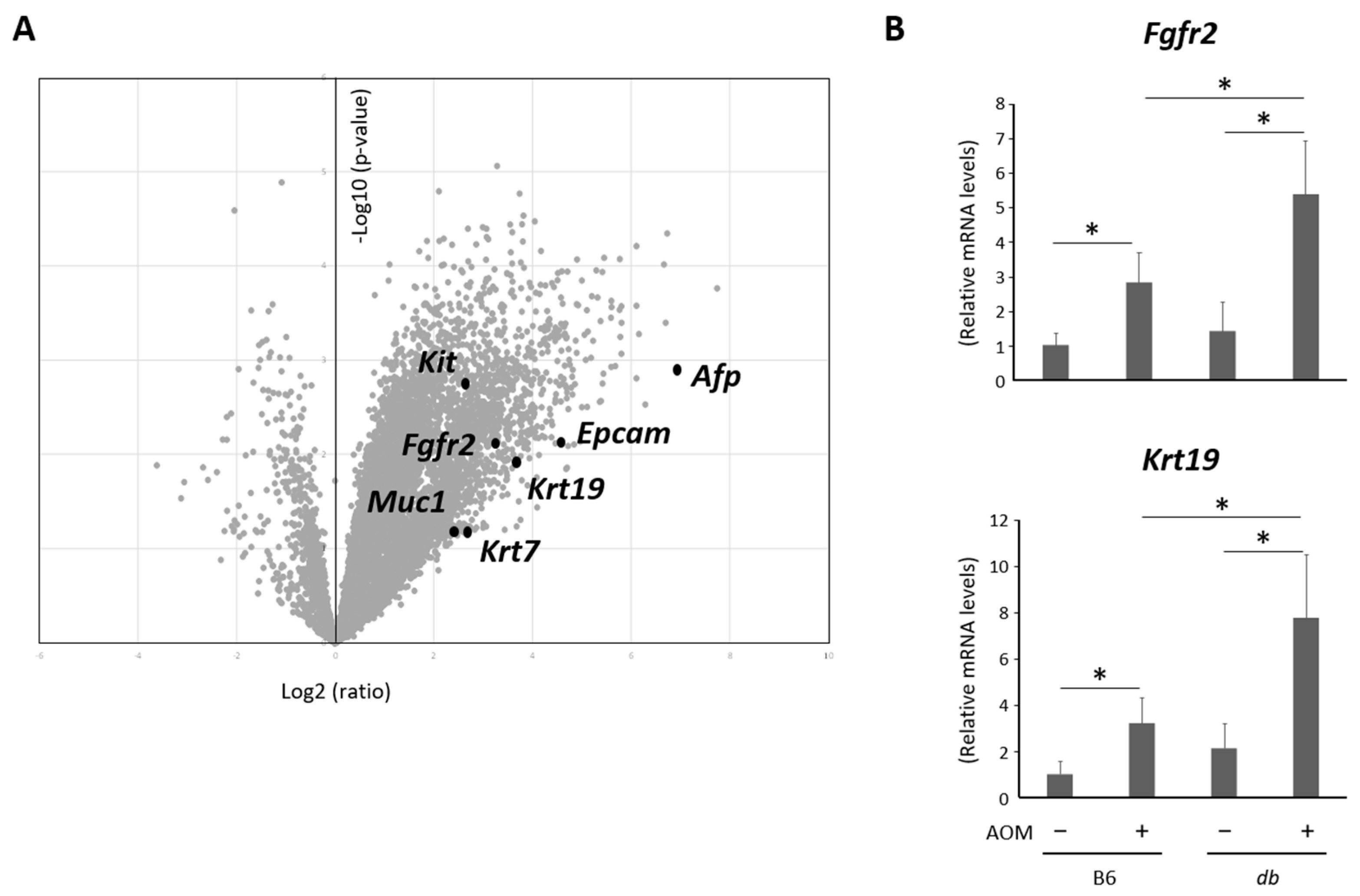
2.4. Hepatic Expression Levels of Genes as Markers to Investigate the Type of Cancerous Lesions Were Analyzed by Microarrays and a qRT-PCR
2.5. Expression Levels of Genes Related with Inflammation Were Elevated in the Liver of Obese and AOM-Treated Mice
3. Discussion
4. Materials and Methods
4.1. Animals, Chemicals, and Diets
4.2. Experimental Procedure
4.3. Blood Biochemistry
4.4. Histological Analysis and Immunohistochemistry
4.5. RNA Extraction and Quantitative Real-Time Reverse Transcription-PCR Analysis
4.6. Microarray Analysis
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welzel, T.M.; McGlynn, K.A.; Hsing, A.W.; O’Brien, T.R.; Pfeiffer, R.M. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J. Natl. Cancer Inst. 2006, 98, 873–875. [Google Scholar] [CrossRef] [PubMed]
- Everhart, J.E.; Ruhl, C.E. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology 2009, 136, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.-H.; Chen, X.; Zhang, X.-H.; Zhang, E.-C.; Sun, C.-X. Clinicopathological characteristics and prognostic factors for intrahepatic cholangiocarcinoma: A population-based study. Sci. Rep. 2021, 11, 3990. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kurzrock, R.; Adashek, J.J. Evolution of the Targeted Therapy Landscape for Cholangiocarcinoma: Is Cholangiocarcinoma the “NSCLC” of GI Oncology? Cancers 2023, 15, 1578. [Google Scholar] [CrossRef]
- Ruff, S.M.; Roychowdhury, S.; Pawlik, T.M. The future of fibroblast growth factor receptor inhibitors and mechanisms of resistance for cholangiocarcinoma. Expert. Opin. Pharmacother. 2023, 24, 779–788. [Google Scholar] [CrossRef]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; Kelley, R.K.; Cassier, P.A.; et al. Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. N. Engl. J. Med. 2023, 388, 228–239. [Google Scholar] [CrossRef]
- Guest, R.V.; Boulter, L.; Kendall, T.J.; Minnis-Lyons, S.E.; Walker, R.; Wigmore, S.J.; Sansom, O.J.; Forbes, S.J. Cell lineage tracing reveals a biliary origin of intrahepatic cholangiocarcinoma. Cancer Res. 2014, 74, 1005–1010. [Google Scholar] [CrossRef]
- Terada, M.; Horisawa, K.; Miura, S.; Takashima, Y.; Ohkawa, Y.; Sekiya, S.; Matsuda-Ito, K.; Suzuki, A. Kupffer cells induce Notch-mediated hepatocyte conversion in a common mouse model of intrahepatic cholangiocarcinoma. Sci. Rep. 2016, 6, 34691. [Google Scholar] [CrossRef]
- Tomita, H.; Tanaka, K.; Hirata, A.; Okada, H.; Imai, H.; Shirakami, Y.; Ohnishi, K.; Sugie, S.; Aoki, H.; Hatano, Y.; et al. Inhibition of FGF10-ERK signal activation suppresses intraductal papillary neoplasm of the bile duct and its associated carcinomas. Cell Rep. 2021, 34, 108772. [Google Scholar] [CrossRef]
- Loeuillard, E.; Fischbach, S.R.; Gores, G.J.; Rizvi, S. Animal models of cholangiocarcinoma. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 982–992. [Google Scholar] [CrossRef] [PubMed]
- De Minicis, S.; Kisseleva, T.; Francis, H.; Baroni, G.S.; Benedetti, A.; Brenner, D.; Alvaro, D.; Alpini, G.; Marzioni, M. Liver carcinogenesis: Rodent models of hepatocarcinoma and cholangiocarcinoma. Dig. Liver Dis. 2013, 45, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, K.; Shirakami, Y.; Maruta, A.; Obara, K.; Iritani, S.; Nakamura, N.; Kochi, T.; Kubota, M.; Sakai, H.; Tanaka, T.; et al. Preventive effects of pentoxifylline on the development of colonic premalignant lesions in obese and diabetic mice. Int. J. Mol. Sci. 2017, 18, 413. [Google Scholar] [CrossRef] [PubMed]
- Kato, J.; Shirakami, Y.; Mizutani, T.; Kubota, M.; Sakai, H.; Ibuka, T.; Shimizu, M. Alpha-Glucosidase Inhibitor Voglibose Suppresses Azoxymethane-Induced Colonic Preneoplastic Lesions in Diabetic and Obese Mice. Int. J. Mol. Sci. 2020, 21, 2226. [Google Scholar] [CrossRef] [PubMed]
- Cadamuro, M.; Strazzabosco, M. Inflammatory pathways and cholangiocarcinoma risk mechanisms and prevention. Adv. Cancer Res. 2022, 156, 39–73. [Google Scholar] [CrossRef]
- Farshidfar, F.; Zheng, S.; Gingras, M.-C.; Newton, Y.; Shih, J.; Robertson, A.G.; Hinoue, T.; Hoadley, K.A.; Gibb, E.A.; Roszik, J.; et al. Integrative Genomic Analysis of Cholangiocarcinoma Identifies Distinct IDH-Mutant Molecular Profiles. Cell Rep. 2017, 18, 2780–2794. [Google Scholar] [CrossRef]
- Xu, J.; Tan, Y.; Shao, X.; Zhang, C.; He, Y.; Wang, J.; Xi, Y. Evaluation of NCAM and c-Kit as hepatic progenitor cell markers for intrahepatic cholangiocarcinomas. Pathol. Res. Pract. 2018, 214, 2011–2017. [Google Scholar] [CrossRef]
- Inagaki, Y.; Xu, H.; Nakata, M.; Seyama, Y.; Hasegawa, K.; Sugawara, Y.; Tang, W.; Norihiro, K. Clinicopathology of sialomucin: MUC1, particularly KL-6 mucin, in gastrointestinal, hepatic and pancreatic cancers. Biosci. Trends. 2009, 3, 220–232. [Google Scholar]
- Tang, W.; Guo, Q.; Qu, X.; Inagaki, Y.; Seyama, Y.; Midorikawa, Y.; Nakata, M.; Makuuchi, M. KL-6 mucin is a useful immunohistochemical marker for cholangiocarcinoma. Oncol. Rep. 2007, 17, 737–741. [Google Scholar] [CrossRef]
- Xu, J.; Sasaki, M.; Harada, K.; Sato, Y.; Ikeda, H.; Kim, J.-H.; Yu, E.; Nakanuma, Y. Intrahepatic cholangiocarcinoma arising in chronic advanced liver disease and the cholangiocarcinomatous component of hepatocellular cholangiocarcinoma share common phenotypes and cholangiocarcinogenesis. Histopathology 2011, 59, 1090–1099. [Google Scholar] [CrossRef]
- Arai, Y.; Totoki, Y.; Hosoda, F.; Shirota, T.; Hama, N.; Nakamura, H.; Ojima, H.; Furuta, K.; Shimada, K.; Okusaka, T.; et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology 2014, 59, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, V.; Neyaz, A.; Chogule, A.; Baiev, I.; Reyes, S.; Barr Fritcher, E.G.; Lennerz, J.K.; Sukov, W.; Kipp, B.; Ting, D.T.; et al. FGFR mRNA Expression in Cholangiocarcinoma and Its Correlation with FGFR2 Fusion Status and Immune Signatures. Clin. Cancer Res. 2022, 28, 5431–5439. [Google Scholar] [CrossRef] [PubMed]
- Imray, C.H.; Newbold, K.M.; Davis, A.; Lavelle-Jones, M.; Neoptolemos, J.P. Induction of cholangiocarcinoma in the Golden Syrian hamster using methylazoxymethyl acetate. Eur. J. Surg. Oncol. 1992, 18, 373–378. [Google Scholar] [PubMed]
- Storandt, M.H.; Jin, Z.; Mahipal, A. Pemigatinib in cholangiocarcinoma with a FGFR2 rearrangement or fusion. Expert. Rev. Anticancer. Ther. 2022, 22, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- O’Dell, M.R.; Huang, J.L.; Whitney-Miller, C.L.; Deshpande, V.; Rothberg, P.; Grose, V.; Rossi, R.M.; Zhu, A.X.; Land, H.; Bardeesy, N.; et al. Kras(G12D) and p53 mutation cause primary intrahepatic cholangiocarcinoma. Cancer Res. 2012, 72, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Ikenoue, T.; Terakado, Y.; Nakagawa, H.; Hikiba, Y.; Fujii, T.; Matsubara, D.; Noguchi, R.; Zhu, C.; Yamamoto, K.; Kudo, Y.; et al. A novel mouse model of intrahepatic cholangiocarcinoma induced by liver-specific Kras activation and Pten deletion. Sci. Rep. 2016, 6, 23899. [Google Scholar] [CrossRef]
- Kiguchi, K.; Carbajal, S.; Chan, K.; Beltrán, L.; Ruffino, L.; Shen, J.; Matsumoto, T.; Yoshimi, N.; DiGiovanni, J. Constitutive expression of ErbB-2 in gallbladder epithelium results in development of adenocarcinoma. Cancer Res. 2001, 61, 6971–6976. [Google Scholar]
- Xu, X.; Kobayashi, S.; Qiao, W.; Li, C.; Xiao, C.; Radaeva, S.; Stiles, B.; Wang, R.-H.; Ohara, N.; Yoshino, T.; et al. Induction of intrahepatic cholangiocellular carcinoma by liver-specific disruption of Smad4 and Pten in mice. J. Clin. Investig. 2006, 116, 1843–1852. [Google Scholar] [CrossRef]
- Farazi, P.A.; Zeisberg, M.; Glickman, J.; Zhang, Y.; Kalluri, R.; DePinho, R.A. Chronic bile duct injury associated with fibrotic matrix microenvironment provokes cholangiocarcinoma in p53-deficient mice. Cancer Res. 2006, 66, 6622–6627. [Google Scholar] [CrossRef]
- Zender, S.; Nickeleit, I.; Wuestefeld, T.; Sörensen, I.; Dauch, D.; Bozko, P.; El-Khatib, M.; Geffers, R.; Bektas, H.; Manns, M.P.; et al. A critical role for notch signaling in the formation of cholangiocellular carcinomas. Cancer Cell. 2013, 23, 784–795. [Google Scholar] [CrossRef]
- Shiode, Y.; Kodama, T.; Shigeno, S.; Murai, K.; Tanaka, S.; Newberg, J.Y.; Jumpei, K.; Shogo, K.; Ryoko, Y.; Hayato, H.; et al. TNF receptor-related factor 3 inactivation promotes the development of intrahepatic cholangiocarcinoma through NF-κB-inducing kinase-mediated hepatocyte transdifferentiation. Hepatology 2023, 77, 395–410. [Google Scholar] [CrossRef] [PubMed]
- Thamavit, W.; Bhamarapravati, N.; Sahaphong, S.; Vajrasthira, S.; Angsubhakorn, S. Effects of dimethylnitrosamine on induction of cholangiocarcinoma in Opisthorchis viverrini-infected Syrian golden hamsters. Cancer Res. 1978, 38, 4634–4639. [Google Scholar] [PubMed]
- Umemura, T.; Kai, S.; Hasegawa, R.; Kanki, K.; Kitamura, Y.; Nishikawa, A.; Hirose, M. Prevention of dual promoting effects of pentachlorophenol, an environmental pollutant, on diethylnitrosamine-induced hepato- and cholangiocarcinogenesis in mice by green tea infusion. Carcinogenesis 2003, 24, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Shirakami, Y.; Gottesman, M.E.; Blaner, W.S. Diethylnitrosamine-induced hepatocarcinogenesis is suppressed in lecithin: Retinol acyltransferase-deficient mice primarily through retinoid actions immediately after carcinogen administration. Carcinogenesis 2012, 33, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, T.W.H.; Peng, J.; Tang, X.; Ko, K.S.; Xia, M.; Aller, M.A. A mouse model of cholestasis-associated cholangiocarcinoma and transcription factors involved in progression. Gastroenterology 2011, 141, 378–388.e4. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-N.; Maitra, A.; Lee, K.-F.; Jan, Y.-Y.; Chen, M.-F. Thioacetamide-induced intestinal-type cholangiocarcinoma in rat: An animal model recapitulating the multi-stage progression of human cholangiocarcinoma. Carcinogenesis 2004, 25, 631–636. [Google Scholar] [CrossRef]
- Hu, Y.; Le Leu, R.K.; Young, G.P. Detection of K-ras mutations in azoxymethane-induced aberrant crypt foci in mice using LNA-mediated real-time PCR clamping and mutant-specific probes. Mutat. Res. 2009, 677, 27–32. [Google Scholar] [CrossRef]
- Hsu, M.; Sasaki, M.; Igarashi, S.; Sato, Y.; Nakanuma, Y. KRAS and GNAS mutations and p53 overexpression in biliary intraepithelial neoplasia and intrahepatic cholangiocarcinomas. Cancer 2013, 119, 1669–1674. [Google Scholar] [CrossRef]
- Szklener, K.; Rudzińska, A.; Juchaniuk, P.; Kabała, Z.; Mańdziuk, S. Ozone in Chemotherapy-Induced Peripheral Neuropathy-Current State of Art, Possibilities, and Perspectives. Int. J. Mol. Sci. 2023, 24, 5279. [Google Scholar] [CrossRef]
- Hajovsky, H.; Hu, G.; Koen, Y.; Sarma, D.; Cui, W.; Moore, D.S.; Staudinger, J.L.; Hanzlik, R.P. Metabolism and toxicity of thioacetamide and thioacetamide S-oxide in rat hepatocytes. Chem. Res. Toxicol. 2012, 25, 1955–1963. [Google Scholar] [CrossRef]
- Fava, G.; Alpini, G.; Rychlicki, C.; Saccomanno, S.; DeMorrow, S.; Trozzi, L.; Candelaresi, C.; Venter, J.; Di Sario, A.; Marzioni, M.; et al. Leptin enhances cholangiocarcinoma cell growth. Cancer Res. 2008, 68, 6752–6761. [Google Scholar] [CrossRef] [PubMed]
- Piattini, F.; Le Foll, C.; Kisielow, J.; Rosenwald, E.; Nielsen, P.; Lutz, T.; Schneider, C.; Kopf, M. A spontaneous leptin receptor point mutation causes obesity and differentially affects leptin signaling in hypothalamic nuclei resulting in metabolic dysfunctions distinct from db/db mice. Mol. Metab. 2019, 25, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Hickman, D.L. Minimal Exposure Times for Irreversible Euthanasia with Carbon Dioxide in Mice and Rats. J. Am. Assoc. Lab. Anim. Sci. 2022, 61, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Shirakami, Y.; Shimizu, M.; Kubota, M.; Ohno, T.; Kochi, T.; Nakamura, N.; Takafumi, S.; Takuji, T.; Hisataka, M.; Mitsuru, S. Pentoxifylline prevents nonalcoholic steatohepatitis-related liver pre-neoplasms by inhibiting hepatic inflammation and lipogenesis. Eur. J. Cancer Prev. 2016, 25, 206–215. [Google Scholar] [CrossRef]
- Shirakami, Y.; Kato, J.; Maeda, T.; Ideta, T.; Imai, K.; Sakai, H.; Shiraki, M.; Shimizu, M. Skeletal muscle atrophy is exacerbated by steatotic and fibrotic liver-derived TNF-α in senescence-accelerated mice. J. Gastroenterol. Hepatol. 2023, 38, 800–808. [Google Scholar] [CrossRef]
- Li, W. Volcano plots in analyzing differential expressions with mRNA microarrays. J. Bioinform. Comput. Biol. 2012, 10, 1231003. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirakami, Y.; Kato, J.; Ohnishi, M.; Taguchi, D.; Maeda, T.; Ideta, T.; Kubota, M.; Sakai, H.; Tomita, H.; Tanaka, T.; et al. A Novel Mouse Model of Intrahepatic Cholangiocarcinoma Induced by Azoxymethane. Int. J. Mol. Sci. 2023, 24, 14581. https://doi.org/10.3390/ijms241914581
Shirakami Y, Kato J, Ohnishi M, Taguchi D, Maeda T, Ideta T, Kubota M, Sakai H, Tomita H, Tanaka T, et al. A Novel Mouse Model of Intrahepatic Cholangiocarcinoma Induced by Azoxymethane. International Journal of Molecular Sciences. 2023; 24(19):14581. https://doi.org/10.3390/ijms241914581
Chicago/Turabian StyleShirakami, Yohei, Junichi Kato, Masaya Ohnishi, Daisuke Taguchi, Toshihide Maeda, Takayasu Ideta, Masaya Kubota, Hiroyasu Sakai, Hiroyuki Tomita, Takuji Tanaka, and et al. 2023. "A Novel Mouse Model of Intrahepatic Cholangiocarcinoma Induced by Azoxymethane" International Journal of Molecular Sciences 24, no. 19: 14581. https://doi.org/10.3390/ijms241914581




