Genome-Wide Identification and Evolution of the GRF Gene Family and Functional Characterization of PbGRF18 in Pear
Abstract
:1. Introduction
2. Results
2.1. Identification of Pear GRF Genes
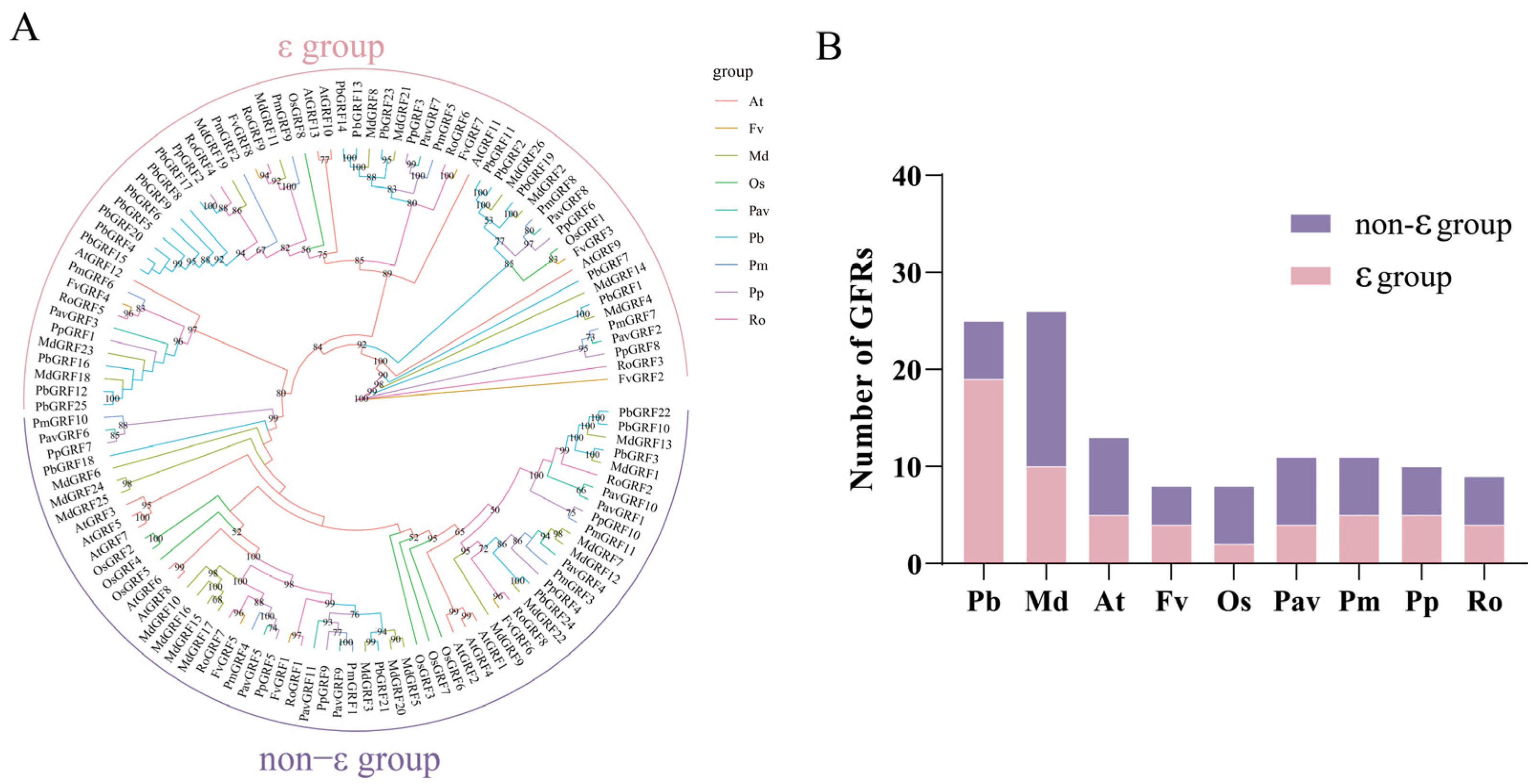
2.2. Phylogenetic Analysis of the GRF Proteins
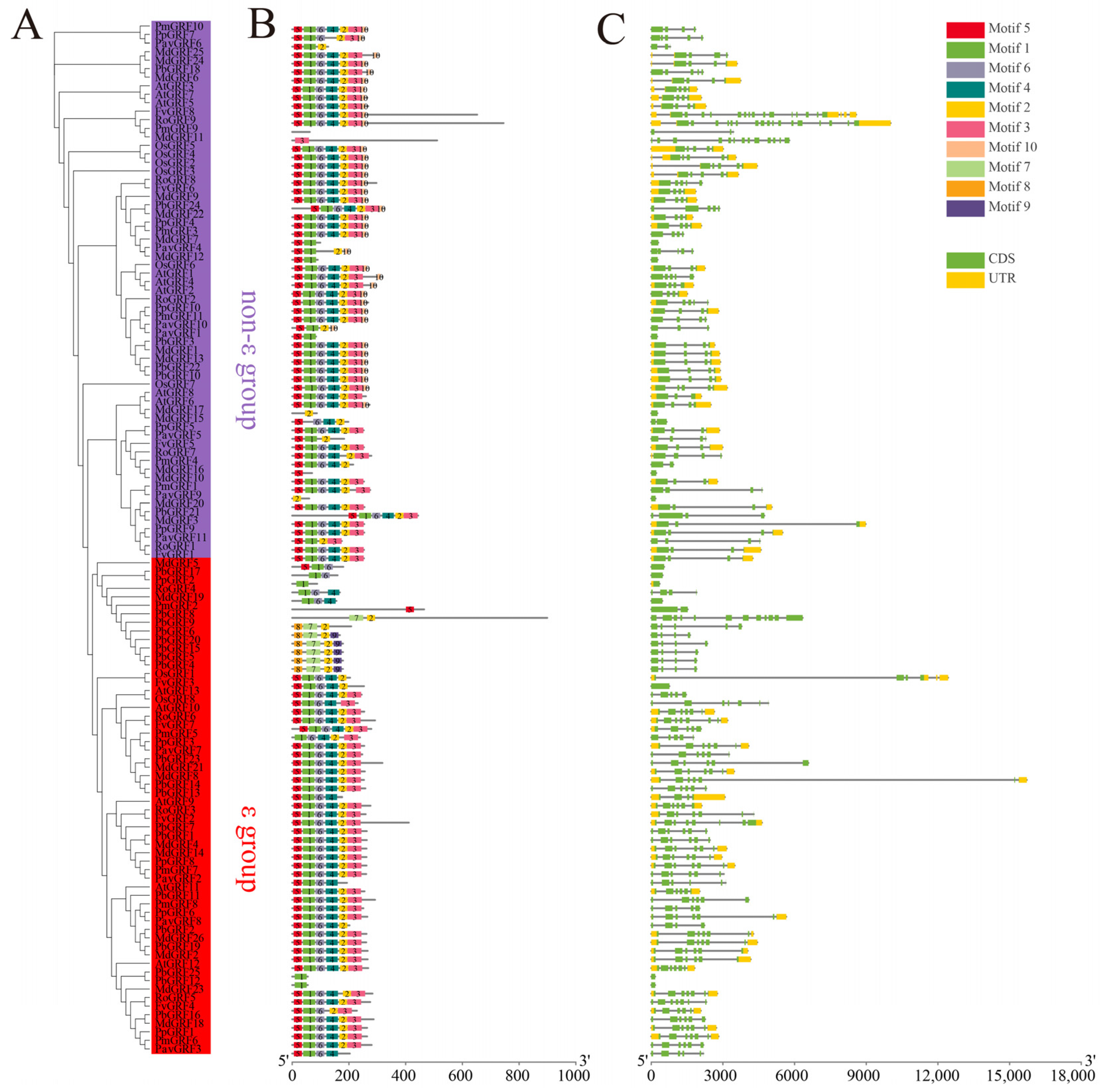
2.3. Structural and Motif Distribution Analyses of the GRF Genes and Proteins
2.4. Synteny Analysis of the GRF Genes
2.5. CREs in the GRF Gene Promoter Regions
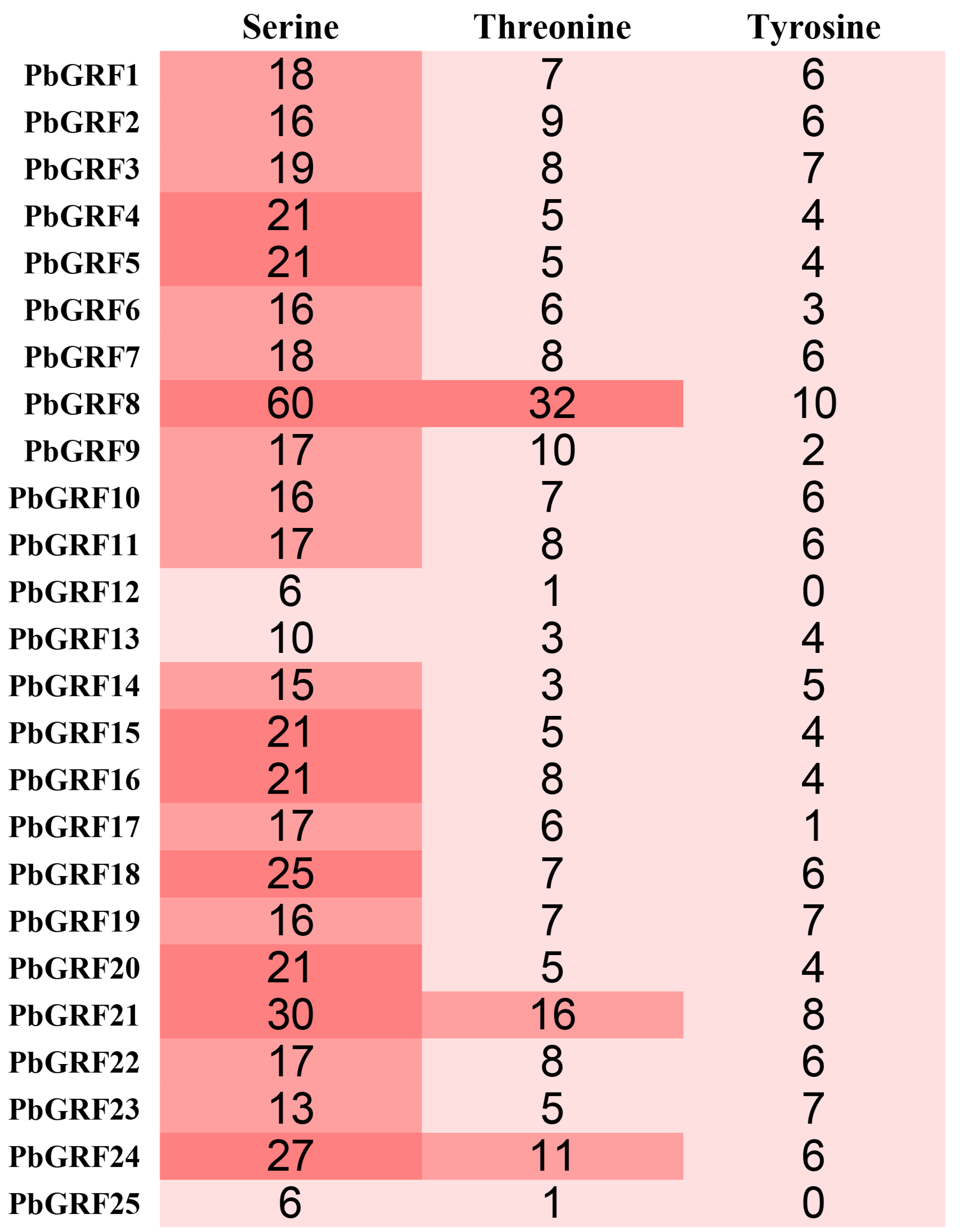
2.6. Phosphorylation Site Analysis
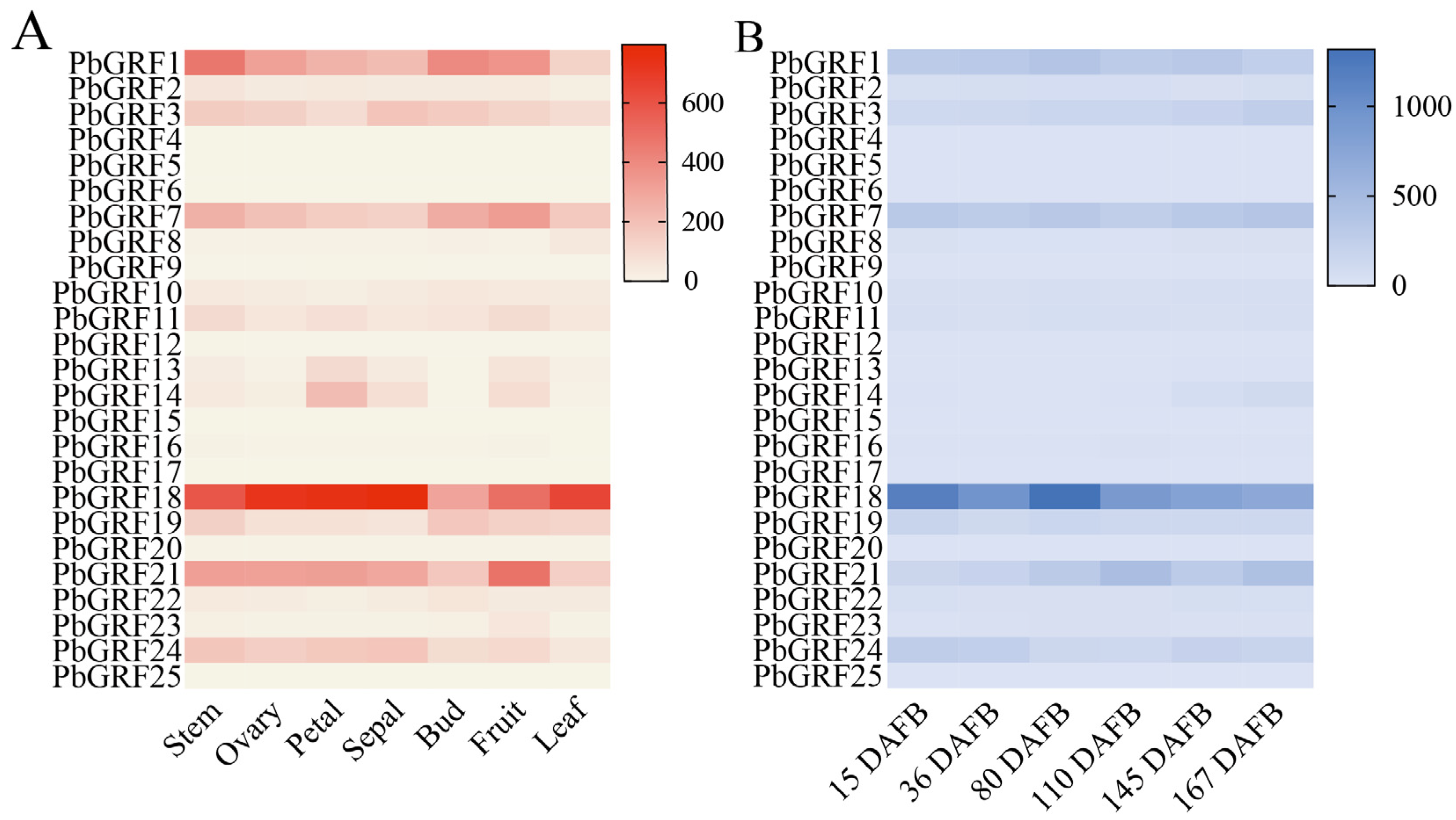
2.7. Expression Characteristics of GRF Genes in Different Tissues of Pear
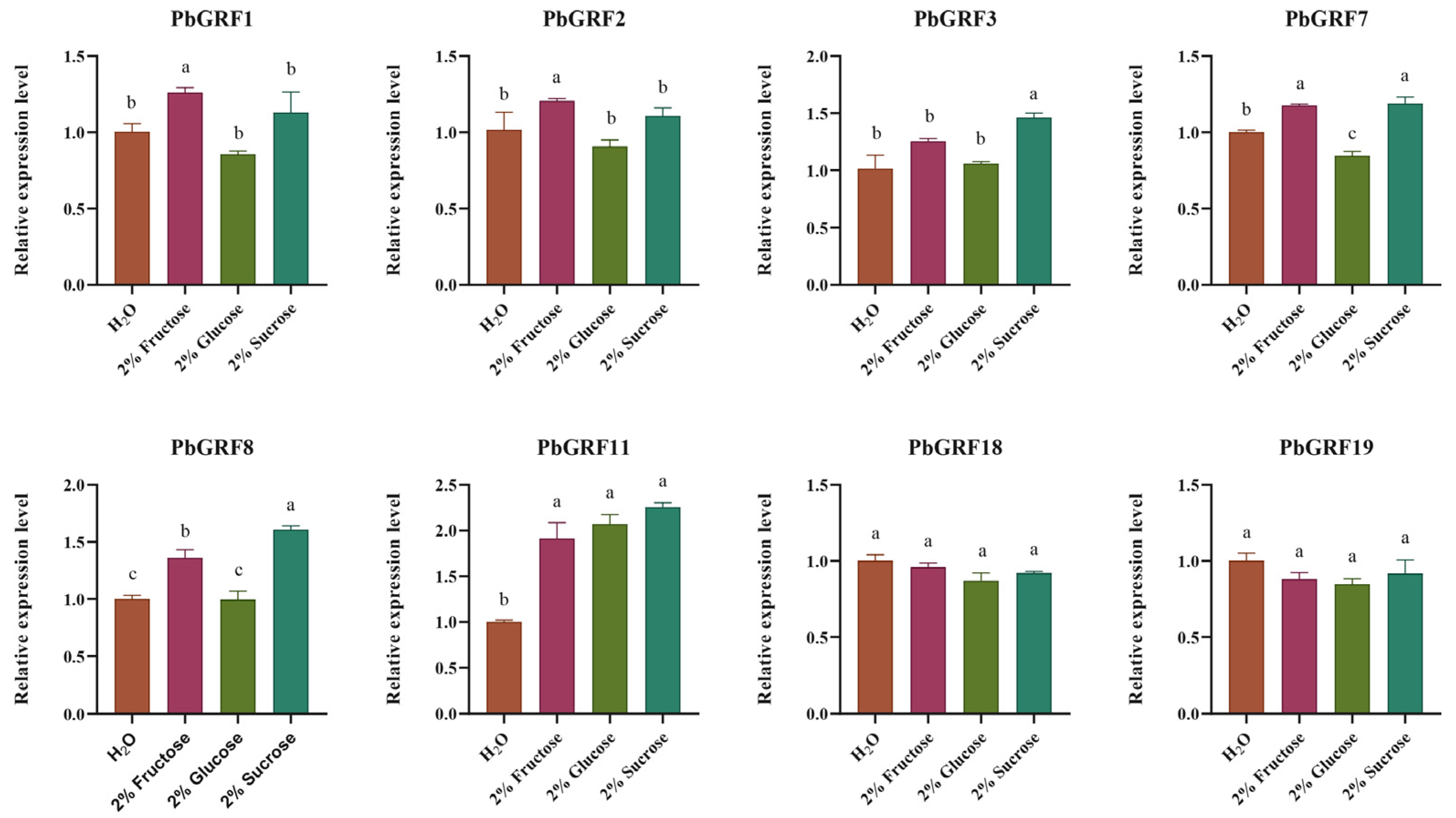
2.8. PbGRF Gene Expression Can Respond to Sugar Content
2.9. PbGRF18 Could Regulate Plant Growth and Fruit Sugar Accumulation
3. Discussion
3.1. Identification and Characterization of GRF Genes in Pear
3.2. Evolution History of the PbGRF Gene Family
3.3. CREs and Phosphorylation Sites Identification in GRF of Pear
3.4. Functional Characterication of PbGRF18
4. Materials and Methods
4.1. Plant Materials
4.2. Identification of GRF Genes in Pear
4.3. The Phylogenetic Tree Construction
4.4. Gene Duplication and Collinearity Analysis
4.5. Analysis of Cis-Regulatory Elements (CREs) in the GRF Genes
4.6. Prediction of Phosphorylation Sites, Protein Motifs, and pI
4.7. Expression Profiles of PbGRF Genes Determined from RNA-seq Datasets
4.8. RNA Extraction for Gene Cloning and qRT-PCR Analysis
4.9. Subcellular Location
4.10. Micro-Tom Transformation and Soluble Sugar Quantification
4.11. Oligonucleotide Primers
4.12. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Potter, D.; Eriksson, T.; Evans, R.C.; Oh, S.; Smedmark, J.E.E.; Morgan, D.R.; Kerr, M.; Robertson, K.R.; Arsenault, M.; Dickinson, T.A.; et al. Phylogeny and classification of Rosaceae. Plant Syst. Evol. 2007, 266, 5–43. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Xu, J.; Korban, S.S.; Fei, Z.; Tao, S.; Ming, R.; Tai, S.; Khan, A.M.; Postman, J.D.; et al. Diversification and independent domestication of Asian and European pears. Genome Biol. 2018, 19, 77. [Google Scholar] [CrossRef]
- Lastdrager, J.; Hanson, J.; Smeekens, S. Sugar signals and the control of plant growth and development. J. Exp. Bot. 2014, 65, 799–807. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, M.; Zhang, H.; Zhang, S.; Qian, M.; Zhang, Z.; Luo, W.; Fan, J.; Liu, Z.; Wang, L. Genome-wide analysis of polygalacturonase gene family from pear genome and identification of the member involved in pear softening. BMC Plant Biol. 2019, 19, 587. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.; Sun, X.; Liu, Z.; Ma, M.; Fan, J.; Luo, W.; Wang, L.; Zhang, S. Identification and characterization of invertase family genes reveal their roles in vacuolar sucrose metabolism during Pyrus bretschneideri Rehd. fruit development. Genomics 2021, 113, 1087–1097. [Google Scholar] [CrossRef]
- Yu, J.; Song, B.; Gu, K.; Cao, B.; Zhao, K.; Wu, J.; Li, J. Genome-Wide identification and expression analysis of CAMTA gene family implies PbrCAMTA2 involved in fruit softening in Pear. Horticulturae 2023, 9, 467. [Google Scholar] [CrossRef]
- Shulaev, V.; Sargent, D.J.; Crowhurst, R.N.; Mockler, T.C.; Folkerts, O.; Delcher, A.L.; Jaiswal, P.; Mockaitis, K.; Liston, A.; Mane, S.P.; et al. The genome of woodland strawberry (Fragaria vesca). Nat. Genet. 2011, 43, 109–116. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Z.; Shi, Z.; Zhang, S.; Ming, R.; Zhu, S.; Khan, M.A.; Tao, S.; Korban, S.S.; Wang, H.; et al. The genome of the pear (Pyrus bretschneideri Rehd.). Genome Res. 2013, 23, 396–408. [Google Scholar] [CrossRef]
- Amborella Genome Project. The Amborella genome and the evolution of flowering plants. Science 2013, 342, 1241089. [Google Scholar] [CrossRef]
- Verde, I.; Abbott, A.G.; Scalabrin, S.; Jung, S.; Shu, S.; Marroni, F.; Zhebentyayeva, T.; Dettori, M.T.; Grimwood, J.; Cattonaro, F.; et al. The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nat. Genet. 2013, 45, 487–494. [Google Scholar] [CrossRef]
- DeLille, J.M.; Sehnke, P.C.; Ferl, R.J. The Arabidopsis 14-3-3 family of signaling regulators. Plant Physiol. 2001, 126, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Rosenquist, M.; Alsterfjord, M.; Larsson, C.; Sommarin, M. Data mining the Arabidopsis genome reveals fifteen 14-3-3 genes. Expression is demonstrated for two out of five novel genes. Plant Physiol. 2001, 127, 142–149. [Google Scholar] [CrossRef] [PubMed]
- de Vetten, N.C.; Ferl, R.J. Two Genes Encoding GF14 (14-3-3) Proteins in zea mays (structure, expression, and potential regulation by the G-Box-Binding complex). Plant Physiol. 1994, 106, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.H.; Ley, S.; Aitken, A. Isoforms of 14-3-3 protein can form homo- and heterodimers in vivo and in vitro: Implications for function as adapter proteins. FEBS Lett. 1995, 368, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Wilker, E.W.; Grant, R.A.; Artim, S.C.; Yaffe, M.B. A structural basis for 14-3-3sigma functional specificity. J. Biol. Chem. 2005, 280, 18891–18898. [Google Scholar] [CrossRef] [PubMed]
- Sehnke, P.C.; DeLille, J.M.; Ferl, R.J. Consummating signal transduction: The role of 14-3-3 proteins in the completion of signal-induced transitions in protein activity. Plant Cell 2002, 14, S339–S354. [Google Scholar] [CrossRef]
- Aitken, A. 14-3-3 proteins: A historic overview. Semin. Cancer Biol. 2006, 16, 162–172. [Google Scholar] [CrossRef]
- Paul, A.L.; Denison, F.C.; Schultz, E.R.; Zupanska, A.K.; Ferl, R.J. 14-3-3 phosphoprotein interaction networks—Does isoform diversity present functional interaction specification? Front. Plant Sci. 2012, 3, 190. [Google Scholar] [CrossRef]
- Camoni, L.; Visconti, S.; Aducci, P.; Marra, M. 14-3-3 proteins in plant hormone signaling: Doing several things at once. Front. Plant Sci. 2018, 9, 297. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, H.; Huang, F.; Long, J.; Song, G.; Lin, W. The 14-3-3 protein GF14f negatively affects grain filling of inferior spikelets of rice (Oryza sativa L.). Plant J. 2019, 99, 344–358. [Google Scholar] [CrossRef]
- Wang, L.; Cui, N.; Zhao, X.; Fan, H.; Li, T. Accumulation of carbohydrate and regulation of 14-3-3 protein on sucrose phosphate synthase (SPS) activity in two tomato species. J. Integr. Agric. 2014, 13, 358–364. [Google Scholar] [CrossRef]
- Żuk, M.; Skała, J.; Biernat, J.; Szopa, J. Repression of six 14-3-3 protein isoforms resulting in the activation of nitrate and carbon fixation key enzymes from transgenic potato plants. Plant Sci. 2003, 165, 731–741. [Google Scholar] [CrossRef]
- Chen, Y.; David Ho, T.H.; Liu, L.; Lee, D.H.; Lee, C.; Chen, Y.; Lin, S.; Lu, C.; Yu, S. Sugar starvation-regulated MYBS2 and 14-3-3 protein interactions enhance plant growth, stress tolerance, and grain weight in rice. Proc. Natl. Acad. Sci. USA 2019, 116, 21925–21935. [Google Scholar] [CrossRef]
- Li, M.; Ren, L.; Xu, B.; Yang, X.; Xia, Q.; He, P.; Xiao, S.; Guo, A.; Hu, W.; Jin, Z. Genome-Wide identification, phylogeny, and expression analyses of the 14-3-3 family reveal their involvement in the development, ripening, and abiotic stress response in banana. Front. Plant Sci. 2016, 7, 1442. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, D.; He, H.; Zhang, N.; Ge, F.; Chen, C. Molecular cloning of a 14-3-3 protein gene from lilium regale wilson and overexpression of this gene in tobacco increased resistance to pathogenic fungi. Sci. Hortic. 2014, 168, 9–16. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, Y. Pear 14-3-3a gene (Pp14-3-3a) is regulated during fruit ripening and senescense, and involved in response to salicylic acid and ethylene signalling. J. Genet. 2014, 93, 747–753. [Google Scholar] [CrossRef]
- Sehnke, P.C.; Chung, H.J.; Wu, K.; Ferl, R.J. Regulation of starch accumulation by granule-associated plant 14-3-3 proteins. Proc. Natl. Acad. Sci. USA 2001, 98, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, G.; Kim, G.T.; Tsukaya, H. The transcription factor AtGRF5 and the transcription coactivator AN3 regulate cell proliferation in leaf primordia of Arabidopsis thaliana. Plant J. 2005, 43, 68–78. [Google Scholar] [CrossRef]
- Xia, L.; He, X.; Hu, W.; Mo, X.; Zhu, J.; Huang, X.; Li, Y.; Zhang, Y.; Yang, X.; Li, K.; et al. Overexpression of the mango MiGF6A and MiGF6B genes promotes early flowering in transgenic Arabidopsis plants. Sci. Hortic. 2023, 318, 112074. [Google Scholar] [CrossRef]
- van Kleeff, P.J.M.; Gao, J.; Mol, S.; Zwart, N.; Zhang, H.; Li, K.W.; de Boer, A.H. The Arabidopsis GORK K(+)-channel is phosphorylated by calcium-dependent protein kinase 21 (CPK21), which in turn is activated by 14-3-3 proteins. Plant Physiol. Biochem. 2018, 125, 219–231. [Google Scholar] [CrossRef]
- Chen, F.; Li, Q.; Sun, L.; He, Z. The Rice 14-3-3 Gene Family and its involvement in responses to biotic and abiotic stress. DNA Res. 2006, 13, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wang, Y.; Chai, F.; Li, S.; Xin, H.; Liang, Z. Genome-wide identification and characterization of the 14-3-3 family in Vitis vinifera L. during berry development and cold- and heat-stress response. BMC Genomics 2018, 19, 579. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Chen, W.; Zhu, X.; Zhou, X.; Jin, Y.; Zhan, C.; Liu, G.; Liu, X.; Ma, D.; Qiao, Y. Genome-Wide identification and characterization of wheat 14-3-3 genes unravels the role of TaGRF6-A in salt stress tolerance by binding MYB transcription factor. Int. J. Mol. Sci. 2021, 22, 1904. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.; Chen, G.; Pan, D.; Chen, J.; She, W. Molecular analysis of 14-3-3 genes in citrus sinensis and their responses to different stresses. Int. J. Mol. Sci. 2021, 22, 568. [Google Scholar] [CrossRef]
- Xia, L.; He, X.; Huang, X.; Yu, H.; Lu, T.; Xie, X.; Zeng, X.; Zhu, J.; Luo, C. Genome-Wide identification and expression analysis of the 14-3-3 gene family in Mango (Mangifera indica L.). Int. J. Mol. Sci. 2022, 23, 1593. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Wang, S.; Guan, Q.; Zhang, W.; Feng, Z. Expansion and diversification of the 14-3-3 gene family in Camellia sinensis. J. Mol. Evol. 2022, 90, 296–306. [Google Scholar] [CrossRef]
- Qiao, X.; Li, Q.; Yin, H.; Qi, K.; Li, L.; Wang, R.; Zhang, S.; Paterson, A.H. Gene duplication and evolution in recurring polyploidization-diploidization cycles in plants. Genome Biol. 2019, 20, 38. [Google Scholar] [CrossRef]
- de Boer, A.H.; van Kleeff, P.J.; Gao, J. Plant 14-3-3 proteins as spiders in a web of phosphorylation. Protoplasma 2013, 250, 425–440. [Google Scholar] [CrossRef]
- Djamei, A.; Pitzschke, A.; Nakagami, H.; Wagner, I.; Hirt, H. Trojan horse strategy in agrobacterium transformation: Abusing MAPK defense signaling. Science 2007, 318, 453–456. [Google Scholar] [CrossRef]
- Muslin, A.J.; Tanner, J.W.; Allen, P.M.; Shaw, A.S. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell 1996, 84, 889–897. [Google Scholar] [CrossRef]
- Yaffe, M.B.; Rittinger, K.; Volinia, S.; Caron, P.R.; Aitken, A.; Leffers, H.; Gamblin, S.J.; Smerdon, S.J.; Cantley, L.C. The structural basis for 14-3-3:phosphopeptide binding specificity. Cell 1997, 91, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Xi, Y.; Lu, P.; Lu, Y.; Wang, Y.; Wang, Y. Genome-wide analysis of the G-box regulating factors protein family reveals its roles in response to Sclerotinia sclerotiorum infection in rapeseed (Brassica napus L.). Front. Plant Sci. 2022, 13, 986635. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; He, Y.; Yang, L.; Lu, C.; Zhu, Y.; Sun, C.; Ma, D.; Yin, J. Genome-wide analysis of growth-regulating factors (GRFs) in Triticum aestivum. PeerJ 2021, 9, e10701. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Xie, F.; Zhang, B. Transcriptome-wide identification and stress properties of the 14-3-3 gene family in cotton (Gossypium hirsutum L.). Funct. Integr. Genom. 2011, 11, 627–636. [Google Scholar] [CrossRef]
- Wang, Y.; Ling, L.; Jiang, Z.; Tan, W.; Liu, Z.; Wu, L.; Zhao, Y.; Xia, S.; Ma, J.; Wang, G.; et al. Genome-wide identification and expression analysis of the 14-3-3 gene family in soybean (Glycine max). PeerJ 2019, 7, e7950. [Google Scholar] [CrossRef]
- Liao, G.L.; Liu, Q.; Li, Y.Q.; Zhong, M.; Huang, C.H.; Jia, D.F.; Xu, X.B. Identification and expression profiling analysis of ascorbate peroxidase gene family in Actinidia chinensis (Hongyang). J. Plant Res. 2020, 133, 715–726. [Google Scholar] [CrossRef]
- Sakharkar, M.K.; Chow, V.T.; Chaturvedi, I.; Mathura, V.S.; Shapshak, P.; Kangueane, P. A report on single exon genes (SEG) in eukaryotes. Front. Biosci. 2004, 9, 3262–3267. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, Y.; Xia, E.; Gao, L. Prevalent role of gene features in determining evolutionary fates of whole-genome duplication duplicated genes in flowering plants. Plant Physiol. 2013, 161, 1844–1861. [Google Scholar] [CrossRef]
- Li, J.; Qin, M.; Qiao, X.; Cheng, Y.; Li, X.; Zhang, H.; Wu, J. A new insight into the evolution and functional divergence of SWEET transporters in Chinese white pear (Pyrus bretschneideri). Plant Cell Physiol. 2017, 58, 839–850. [Google Scholar] [CrossRef]
- Liao, G.; Li, Y.; Wang, H.; Liu, Q.; Zhong, M.; Jia, D.; Huang, C.; Xu, X. Genome-wide identification and expression profiling analysis of sucrose synthase (SUS) and sucrose phosphate synthase (SPS) genes family in Actinidia chinensis and A. eriantha. BMC Plant Biol. 2022, 22, 215. [Google Scholar] [CrossRef]
- Li, J.M.; Zheng, D.M.; Li, L.T.; Qiao, X.; Wei, S.W.; Bai, B.; Zhang, S.L.; Wu, J. Genome-Wide function, evolutionary characterization and expression analysis of sugar transporter family genes in pear (Pyrus bretschneideri Rehd). Plant Cell Physiol. 2015, 56, 1721–1737. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.; Thomson, C.E.; Kaiserli, E.; Christie, J.M. Interaction specificity of Arabidopsis 14-3-3 proteins with phototropin receptor kinases. FEBS Lett. 2009, 583, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Folta, K.M.; Paul, A.L.; Mayfield, J.D.; Ferl, R.J. 14-3-3 isoforms participate in red light signaling and photoperiodic flowering. Plant Signal Behav. 2008, 3, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Ferl, R.J.; Manak, M.S.; Reyes, M.F. The 14-3-3s. Genome Biol. 2002, 3, R3010. [Google Scholar] [CrossRef]
- Denison, F.C.; Paul, A.L.; Zupanska, A.K.; Ferl, R.J. 14-3-3 proteins in plant physiology. Semin. Cell Dev. Biol. 2011, 22, 720–727. [Google Scholar] [CrossRef]
- Gökirmak, T.; Paul, A.L.; Ferl, R.J. Plant phosphopeptide-binding proteins as signaling mediators. Curr. Opin. Plant Biol. 2010, 13, 527–532. [Google Scholar] [CrossRef]
- Li, H.; Wong, W.S.; Zhu, L.; Guo, H.W.; Ecker, J.; Li, N. Phosphoproteomic analysis of ethylene-regulated protein phosphorylation in etiolated seedlings of Arabidopsis mutant ein2 using two-dimensional separations coupled with a hybrid quadrupole time-of-flight mass spectrometer. Proteomics 2009, 9, 1646–1661. [Google Scholar] [CrossRef]
- Xue, L.; Wang, P.; Wang, L.; Renzi, E.; Radivojac, P.; Tang, H.; Arnold, R.; Zhu, J.K.; Tao, W.A. Quantitative measurement of phosphoproteome response to osmotic stress in Arabidopsis based on Library-Assisted eXtracted Ion Chromatogram (LAXIC). Mol. Cell. Proteom. 2013, 12, 2354–2369. [Google Scholar] [CrossRef]
- Camoni, L.; Harper, J.F.; Palmgren, M.G. 14-3-3 proteins activate a plant calcium-dependent protein kinase (CDPK). FEBS Lett. 1998, 430, 381–384. [Google Scholar] [CrossRef]
- Swatek, K.N.; Wilson, R.S.; Ahsan, N.; Tritz, R.L.; Thelen, J.J. Multisite phosphorylation of 14-3-3 proteins by calcium-dependent protein kinases. Biochem. J. 2014, 459, 15–25. [Google Scholar] [CrossRef]
- Li, J.; Zhu, R.; Zhang, M.; Cao, B.; Li, X.; Song, B.; Liu, Z.; Wu, J. Natural variations in the PbCPK28 promoter regulate sugar content through interaction with PbTST4 and PbVHA-A1 in pear. Plant J. 2023, 114, 124–141. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef] [PubMed]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Clamp, M.; Cuff, J.; Searle, S.M.; Barton, G.J. The Jalview Java alignment editor. Bioinformatics 2004, 20, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Li, X.; Xue, C.; Li, J.; Qiao, X.; Li, L.; Yu, L.; Huang, Y.; Wu, J. Genome-Wide identification, evolution and functional divergence of MYB transcription factors in Chinese white pear (Pyrus bretschneideri). Plant Cell Physiol. 2016, 57, 824–847. [Google Scholar] [CrossRef]
- Huang, S.; Kang, Z.; Xu, Z.; Liu, Q. Robust deep k-means: An effective and simple method for data clustering. Pattern Recogn. 2021, 117, 107996. [Google Scholar] [CrossRef]
- Sun, H.J.; Uchii, S.; Watanabe, S.; Ezura, H. A highly efficient transformation protocol for Micro-Tom, a model cultivar for tomato functional genomics. Plant Cell Physiol. 2006, 47, 426–431. [Google Scholar] [CrossRef]




| Gene | Chr | Gene Locus | Length (aa) | MW (kDa) | pI |
|---|---|---|---|---|---|
| PbGRF1 | Chr5 | 25,101,995–25,104,450 | 263 | 29.66 kDa | 4.47 |
| PbGRF2 | scaffold1087.0 | 102,423–106,698 | 262 | 29.52 kDa | 4.43 |
| PbGRF3 | scaffold1332.0 | 65,750–68,405 | 262 | 29.36 kDa | 4.47 |
| PbGRF4 | Chr11 | 9,687,156–9,689,055 | 179 | 19.71 kDa | 5.92 |
| PbGRF5 | Chr12 | 9,955,316–9,957,215 | 179 | 19.71 kDa | 5.92 |
| PbGRF6 | Chr12 | 19,547,164–19,548,792 | 168 | 18.68 kDa | 5.92 |
| PbGRF7 | Chr10 | 3,657,525–3,659,852 | 263 | 29.68 kDa | 4.47 |
| PbGRF8 | Chr10 | 4,192,289–4,198,629 | 899 | 99.97 kDa | 8.84 |
| PbGRF9 | Chr12 | 19,034,476–19,038,260 | 208 | 22.94 kDa | 9.39 |
| PbGRF10 | Chr9 | 17,273,908–17,276,829 | 262 | 29.39 kDa | 4.51 |
| PbGRF11 | Chr17 | 2,782,823–2,786,908 | 292 | 32.59 kDa | 4.48 |
| PbGRF12 | Chr2 | 1,111,149–1,111,313 | 55 | 6.19 kDa | 9.19 |
| PbGRF13 | Chr8 | 1,222,808–1,225,889 | 176 | 20.19 kDa | 6.37 |
| PbGRF14 | Chr8 | 1,520,693–1,523,000 | 258 | 29.37 kDa | 4.8 |
| PbGRF15 | scaffold489.0.1 | 279,520–281,468 | 179 | 19.71 kDa | 5.92 |
| PbGRF16 | Chr13 | 4,575,039–4,577,295 | 287 | 32.56 kDa | 4.68 |
| PbGRF17 | Chr2 | 1,234,860–1,235,339 | 160 | 18.41 kDa | 8.33 |
| PbGRF18 | Chr9 | 19,676,961–19,679,128 | 282 | 31.69 kDa | 4.46 |
| PbGRF19 | Chr15 | 39,603,193–39,607,232 | 266 | 29.87 kDa | 4.44 |
| PbGRF20 | scaffold556.0 | 98,479–100,829 | 179 | 19.71 kDa | 5.92 |
| PbGRF21 | Chr10 | 20,105,222–20,109,957 | 444 | 49.05 kDa | 6.79 |
| PbGRF22 | Chr10 | 21,562,227–21,565,109 | 262 | 29.41 kDa | 4.51 |
| PbGRF23 | Chr15 | 36,285,362–36,291,941 | 318 | 35.45 kDa | 4.47 |
| PbGRF24 | Chr15 | 36,562,201–36,565,061 | 323 | 36.35 kDa | 5.1 |
| PbGRF25 | scaffold956.0 | 67,356–67,520 | 55 | 6.19 kDa | 9.19 |
| Elements | PbGRF | MdGRF | FvGRF | PmGRF | PpGRF | RoGRF | PavGRF | OsGRF | AtGRF |
|---|---|---|---|---|---|---|---|---|---|
| auxin responsive element | 10 | 20 | 10 | 6 | 9 | 9 | 5 | 2 | 11 |
| cis-acting element involved in defense and stress responsiveness | 12 | 12 | 2 | 3 | 4 | 3 | 2 | 4 | 12 |
| cis-acting element involved in gibberellin responsiveness | 5 | 4 | 3 | 0 | 2 | 0 | 2 | 0 | 1 |
| cis-acting element involved in light responsiveness | 6 | 4 | 1 | 0 | 2 | 2 | 1 | 2 | 4 |
| cis-acting element involved in low-temperature responsiveness | 19 | 24 | 3 | 7 | 8 | 9 | 10 | 2 | 9 |
| cis-acting element involved in salicylic acid responsiveness | 14 | 21 | 9 | 6 | 8 | 4 | 5 | 3 | 14 |
| cis-acting element involved in the abscisic acid responsiveness | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 29 |
| cis-acting regulatory element essential for the anaerobic induction | 50 | 46 | 18 | 28 | 35 | 17 | 26 | 14 | 32 |
| cis-acting regulatory element involved in light responsiveness | 69 | 92 | 17 | 28 | 50 | 18 | 38 | 42 | 28 |
| cis-acting regulatory element involved in the MeJA responsiveness | 76 | 88 | 34 | 42 | 36 | 34 | 44 | 44 | 48 |
| cis-acting regulatory element involved in zein metabolism regulation | 15 | 16 | 8 | 5 | 5 | 7 | 7 | 8 | 10 |
| cis-acting regulatory element related to meristem expression | 17 | 11 | 2 | 5 | 5 | 8 | 4 | 5 | 5 |
| gibberellin responsive element | 15 | 20 | 4 | 5 | 6 | 9 | 7 | 5 | 13 |
| light responsive element | 66 | 30 | 22 | 15 | 9 | 11 | 15 | 11 | 26 |
| MYB binding site involved in drought-inducibility | 19 | 22 | 7 | 11 | 7 | 6 | 10 | 11 | 6 |
| MYB binding site involved in light responsiveness | 6 | 11 | 2 | 5 | 4 | 5 | 1 | 2 | 11 |
| part of a conserved DNA module involved in light responsiveness | 65 | 34 | 12 | 20 | 27 | 16 | 14 | 11 | 30 |
| part of a light responsive element | 70 | 89 | 31 | 31 | 24 | 26 | 39 | 19 | 37 |
| part of an auxin responsive element | 1 | 1 | 0 | 2 | 1 | 0 | 1 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, R.; Cao, B.; Sun, M.; Wu, J.; Li, J. Genome-Wide Identification and Evolution of the GRF Gene Family and Functional Characterization of PbGRF18 in Pear. Int. J. Mol. Sci. 2023, 24, 14690. https://doi.org/10.3390/ijms241914690
Zhu R, Cao B, Sun M, Wu J, Li J. Genome-Wide Identification and Evolution of the GRF Gene Family and Functional Characterization of PbGRF18 in Pear. International Journal of Molecular Sciences. 2023; 24(19):14690. https://doi.org/10.3390/ijms241914690
Chicago/Turabian StyleZhu, Rongxiang, Beibei Cao, Manyi Sun, Jun Wu, and Jiaming Li. 2023. "Genome-Wide Identification and Evolution of the GRF Gene Family and Functional Characterization of PbGRF18 in Pear" International Journal of Molecular Sciences 24, no. 19: 14690. https://doi.org/10.3390/ijms241914690





