Endurance Exercise Attenuates Established Progressive Experimental Autoimmune Encephalomyelitis and Is Associated with an Amelioration of Innate Immune Responses in NOD Mice
Abstract
:1. Introduction
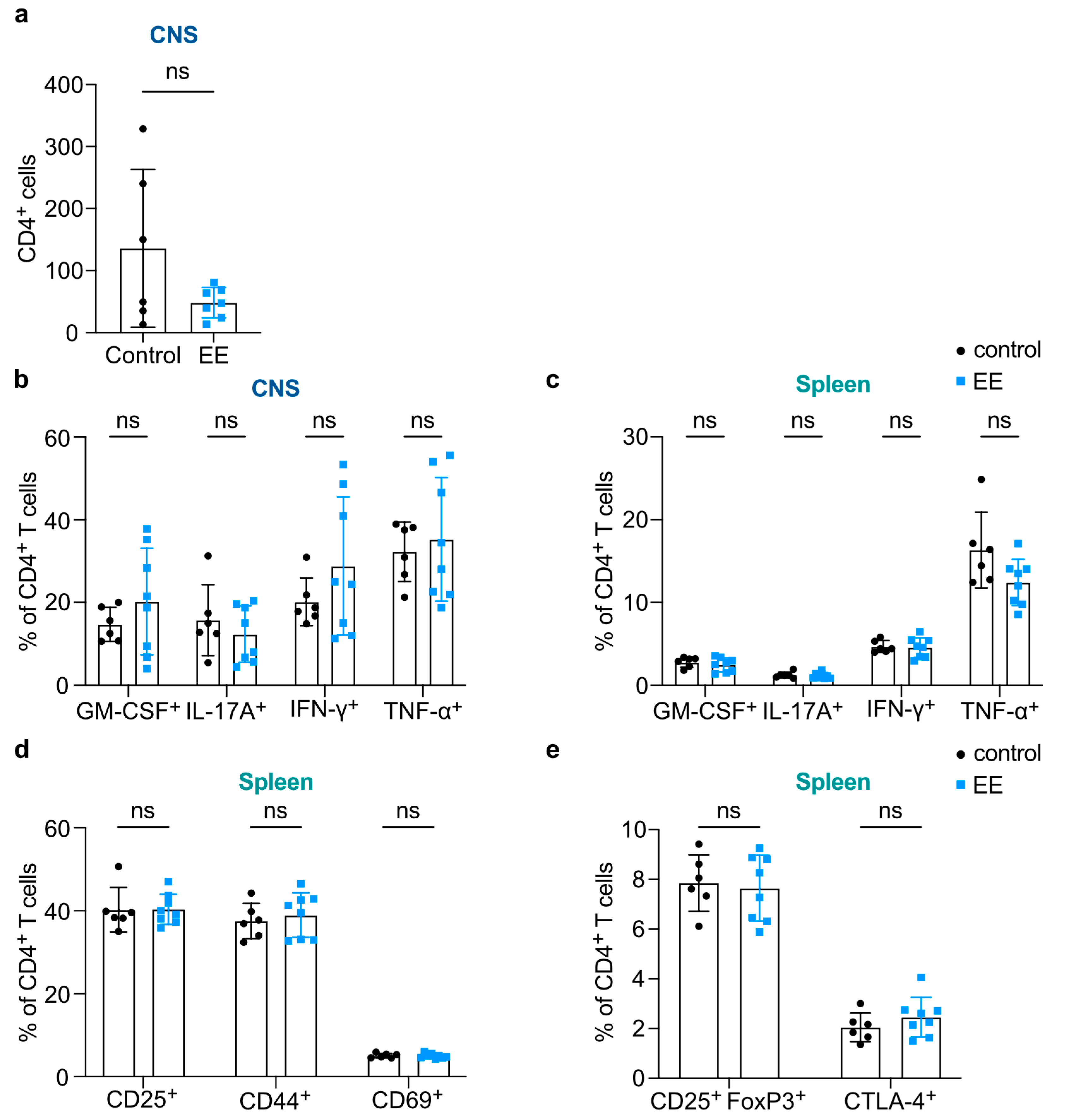
2. Results
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. EAE Induction
4.3. Disease Scoring
4.4. Exercise Intervention
4.5. Isolation of Murine Leukocytes
4.6. Quantitative Detection of Corticosterone
4.7. Flow Cytometry of Murine Leukocytes
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CD | cluster of differentiation |
| CNS | central nervous system |
| CTLA-4 | cytotoxic T lymphocyte antigen 4 |
| dpi | days post induction |
| EAE | experimental autoimmune encephalomyelitis |
| EC1/2 | exercise cycle 1/2 |
| EE | endurance exercise |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| IFN-β/γ | interferon β/γ |
| IL | interleukin |
| MFI | mean fluorescence intensity |
| MHC-II | major histocompatibility complex class II |
| MOG | myelin oligodendrocyte glycoprotein |
| MS | multiple sclerosis |
| NOD | non-obese diabetic |
| NOD1/2 | exercise program 1/2 |
| SE | strength exercise |
| SPMS | secondary progressive multiple sclerosis |
| TNF-α | tumor necrosis factor α |
References
- Ransohoff, R.M.; Hafler, D.A.; Lucchinetti, C.F. Multiple sclerosis—A quiet revolution. Nat. Rev. Neurol. 2015, 11, 134–142. [Google Scholar] [CrossRef]
- Browne, P.; Chandraratna, D.; Angood, C.; Tremlett, H.; Baker, C.; Taylor, B.V.; Thompson, A.J. Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology 2014, 83, 1022–1024. [Google Scholar] [CrossRef]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Gholamzad, M.; Ebtekar, M.; Ardestani, M.S.; Azimi, M.; Mahmodi, Z.; Mousavi, M.J.; Aslani, S. A comprehensive review on the treatment approaches of multiple sclerosis: Currently and in the future. Inflamm. Res. 2019, 68, 25–38. [Google Scholar] [CrossRef]
- Cortese, M.; Riise, T.; Bjørnevik, K.; Myhr, K.M.; The Multiple Sclerosis Conscript Service Database Study Group. Body size and physical exercise, and the risk of multiple sclerosis. Mult. Scler. 2018, 24, 270–278. [Google Scholar] [CrossRef]
- Gold, S.M.; Schulz, K.H.; Hartmann, S.; Mladek, M.; Lang, U.E.; Hellweg, R.; Reer, R.; Braumann, K.M.; Heesen, C. Basal serum levels and reactivity of nerve growth factor and brain-derived neurotrophic factor to standardized acute exercise in multiple sclerosis and controls. J. Neuroimmunol. 2003, 138, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Halabchi, F.; Alizadeh, Z.; Sahraian, M.A.; Abolhasani, M. Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. 2017, 17, 185. [Google Scholar] [CrossRef]
- Dalgas, U.; Stenager, E. Exercise and disease progression in multiple sclerosis: Can exercise slow down the progression of multiple sclerosis? Ther. Adv. Neurol. Disord. 2012, 5, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Flynn, M.G.; McFarlin, B.K.; Markofski, M.M. The anti-inflammatory actions of exercise training. Am. J. Lifestyle Med. 2007, 1, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Coote, S. Progressive resistance therapy is not the best way to rehabilitate deficits due to multiple sclerosis: Yes. Mult. Scler. 2014, 20, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Motl, R.W.; Sandroff, B.M.; Kwakkel, G.; Dalgas, U.; Feinstein, A.; Heesen, C.; Feys, P.; Thompson, A.J. Exercise in patients with multiple sclerosis. Lancet Neurol. 2017, 16, 848–856. [Google Scholar] [CrossRef]
- Souza, P.S.; Gonçalves, E.D.; Pedroso, G.S.; Farias, H.R.; Junqueira, S.C.; Marcon, R.; Tuon, T.; Cola, M.; Silveira, P.C.L.; Santos, A.R.; et al. Physical exercise attenuates experimental autoimmune encephalomyelitis by inhibiting peripheral immune response and blood-brain barrier disruption. Mol. Neurobiol. 2017, 54, 4723–4737. [Google Scholar] [CrossRef]
- Einstein, O.; Fainstein, N.; Touloumi, O.; Lagoudaki, R.; Hanya, E.; Grigoriadis, N.; Katz, A.; Ben-Hur, T. Exercise training attenuates experimental autoimmune encephalomyelitis by peripheral immunomodulation rather than direct neuroprotection. Exp. Neurol. 2018, 299, 56–64. [Google Scholar] [CrossRef]
- Fainstein, N.; Tyk, R.; Touloumi, O.; Lagoudaki, R.; Goldberg, Y.; Agranyoni, O.; Navon-Venezia, S.; Katz, A.; Grigoriadis, N.; Ben-Hur, T.; et al. Exercise intensity-dependent immunomodulatory effects on encephalomyelitis. Ann. Clin. Transl. Neurol. 2019, 6, 1647–1658. [Google Scholar] [CrossRef]
- Xie, Y.; Li, Z.; Wang, Y.; Xue, X.; Ma, W.; Zhang, Y.; Wang, J. Effects of moderate- versus high- intensity swimming training on inflammatory and CD4+ T cell subset profiles in experimental autoimmune encephalomyelitis mice. J. Neuroimmunol. 2019, 328, 60–67. [Google Scholar] [CrossRef]
- Hamdi, L.; Nabat, H.; Goldberg, Y.; Fainstein, N.; Segal, S.; Mediouni, E.; Asis, Y.; Touloumi, O.; Grigoriadis, N.; Katz, A.; et al. Exercise training alters autoimmune cell invasion into the brain in autoimmune encephalomyelitis. Ann. Clin. Transl. Neurol. 2022, 9, 1792–1806. [Google Scholar] [CrossRef]
- Sohrabi, P.; Parnow, A.; Jalili, C. Treadmill aerobic training improve beam-walking test, up-regulate expression of main proteins of myelin and myelination in the hippocampus of cuprizone-fed mice. Neurosci. Lett. 2023, 792, 136936. [Google Scholar] [CrossRef] [PubMed]
- Le Page, C.; Bourdoulous, S.; Béraud, E.; Couraud, P.O.; Rieu, M.; Ferry, A. Effect of physical exercise on adoptive experimental auto-immune encephalomyelitis in rats. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 73, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.M.; Freeman, K.G.; Larson, R.D.; Edwards, G.L.; White, L.J. Chronic exercise confers neuroprotection in experimental autoimmune encephalomyelitis. J. Neurosci. Res. 2015, 93, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, S.; Saitoh, S.; Miyajima, H.; Itokazu, T.; Yamashita, T. Microglia suppress the secondary progression of autoimmune encephalomyelitis. Glia 2019, 67, 1694–1704. [Google Scholar] [CrossRef] [PubMed]
- Colpitts, S.L.; Kasper, E.J.; Keever, A.; Liljenberg, C.; Kirby, T.; Magori, K.; Kasper, L.H.; Ochoa-Repáraz, J. A bidirectional association between the gut microbiota and CNS disease in a biphasic murine model of multiple sclerosis. Gut Microbes 2017, 8, 561–573. [Google Scholar] [CrossRef]
- Dang, P.T.; Bui, Q.; D’Souza, C.S.; Orian, J.M. Modelling MS: Chronic-relapsing EAE in the NOD/Lt mouse strain. In Emerging and Evolving Topics in Multiple Sclerosis Pathogenesis and Treatments; La Flamme, A.C., Orian, J.M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 26, pp. 143–177. [Google Scholar]
- Levy, H.; Assaf, Y.; Frenkel, D. Characterization of brain lesions in a mouse model of progressive multiple sclerosis. Exp. Neurol. 2010, 226, 148–158. [Google Scholar] [CrossRef]
- Baker, D.; Nutma, E.; O’Shea, H.; Cooke, A.; Orian, J.M.; Amor, S. Autoimmune encephalomyelitis in NOD mice is not initially a progressive multiple sclerosis model. Ann. Clin. Transl. Neurol. 2019, 6, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, A.L.; Pinto, A.P.; Kohama, E.B.; Pauli, J.R.; de Moura, L.P.; Cintra, D.E.; Ropelle, E.R.; da Silva, A.S.R. The proinflammatory effects of chronic excessive exercise. Cytokine 2019, 119, 57–61. [Google Scholar] [CrossRef]
- Gong, S.; Miao, Y.L.; Jiao, G.Z.; Sun, M.J.; Li, H.; Lin, J.; Luo, M.J.; Tan, J.H. Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS ONE 2015, 10, e0117503. [Google Scholar] [CrossRef]
- Goldberg, Y.; Fainstein, N.; Zaychik, Y.; Hamdi, L.; Segal, S.; Nabat, H.; Touloumi, O.; Zoidou, S.; Grigoriadis, N.; Hoffman, J.R.; et al. Continuous and interval training attenuate encephalomyelitis by separate immunomodulatory mechanisms. Ann. Clin. Transl. Neurol. 2021, 8, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shen, L.; Liu, Y.; Ma, X.; Long, L.; Ma, X.; Ma, L.; Chen, Z.; Lin, X.; Si, L.; et al. Strength Exercise Confers Protection in Central Nervous System Autoimmunity by Altering the Gut Microbiota. Front. Immunol. 2021, 12, 628629. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, H.; Tang, X.; Yang, Y.; Vieira, V.J.; Niu, Y.; Ma, Y. Effect of exercise training intensity on murine T-regulatory cells and vaccination response. Scand. J. Med. Sci. Sports 2012, 22, 643–652. [Google Scholar] [CrossRef]
- Kuhlmann, T.; Moccia, M.; Coetzee, T.; Cohen, J.A.; Correale, J.; Graves, J.; Marrie, R.A.; Montalban, X.; Yong, V.W.; Thompson, A.J.; et al. Multiple sclerosis progression: Time for a new mechanism-driven framework. Lancet Neurol. 2023, 22, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Antel, J.; Kuhlmann, T. Inflammation in multiple sclerosis: Consequences for remyelination and disease progression. Nat. Rev. Neurol. 2023, 19, 305–320. [Google Scholar] [CrossRef]
- Frodermann, V.; Rohde, D.; Courties, G.; Severe, N.; Schloss, M.J.; Amatullah, H.; McAlpine, C.S.; Cremer, S.; Hoyer, F.F.; Ji, F.; et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat. Med. 2019, 25, 1761–1771. [Google Scholar] [CrossRef]
- Bernardes, D.; Oliveira-Lima, O.C.; Silva, T.V.; Faraco, C.C.; Leite, H.R.; Juliano, M.A.; Santos, D.M.; Bethea, J.R.; Brambilla, R.; Orian, J.M.; et al. Differential brain and spinal cord cytokine and BDNF levels in experimental autoimmune encephalomyelitis are modulated by prior and regular exercise. J. Neuroimmunol. 2013, 264, 24–34. [Google Scholar] [CrossRef]
- Wang, A.; Rojas, O.; Lee, D.; Gommerman, J.L. Regulation of neuroinflammation by B cells and plasma cells. Immunol. Rev. 2021, 299, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.T.; Jabs, C.; Sobel, R.A.; Kuchroo, V.K.; Sharpe, A.H. Studies in B7-deficient mice reveal a critical role for B7 costimulation in both induction and effector phases of experimental autoimmune encephalomyelitis. J. Exp. Med. 1999, 190, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Girvin, A.M.; Dal Canto, M.C.; Rhee, L.; Salomon, B.; Sharpe, A.; Bluestone, J.A.; Miller, S.D. A critical role for B7/CD28 costimulation in experimental autoimmune encephalomyelitis: A comparative study using costimulatory molecule-deficient mice and monoclonal antibody blockade. J. Immunol. 2000, 164, 136–143. [Google Scholar] [CrossRef]
- Genç, K.; Dona, D.L.; Reder, A.T. Increased CD80(+) B cells in active multiple sclerosis and reversal by interferon beta-1b therapy. J. Clin. Investig. 1997, 99, 2664–2671. [Google Scholar] [CrossRef] [PubMed]
- Gerritse, K.; Laman, J.D.; Noelle, R.J.; Aruffo, A.; Ledbetter, J.A.; Boersma, W.J.; Claassen, E. CD40-CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proc. Natl. Acad. Sci. USA 1996, 93, 2499–2504. [Google Scholar] [CrossRef] [PubMed]
- Issazadeh, S.; Navikas, V.; Schaub, M.; Sayegh, M.; Khoury, S. Kinetics of expression of costimulatory molecules and their ligands in murine relapsing experimental autoimmune encephalomyelitis in vivo. J. Immunol. 1998, 161, 1104–1112. [Google Scholar] [CrossRef]
- Becher, B.; Durell, B.G.; Miga, A.V.; Hickey, W.F.; Noelle, R.J. The clinical course of experimental autoimmune encephalomyelitis and inflammation is controlled by the expression of CD40 within the central nervous system. J. Exp. Med. 2001, 193, 967–974. [Google Scholar] [CrossRef]
- Laman, J.D.; ‘t Hart, B.A.; Brok, H.; Meurs, M.; Schellekens, M.M.; Kasran, A.; Boon, L.; Bauer, J.; Boer, M.; Ceuppens, J. Protection of marmoset monkeys against EAE by treatment with a murine antibody blocking CD40 (mu5D12). Eur. J. Immunol. 2002, 32, 2218–2228. [Google Scholar] [CrossRef]
- Fadul, C.E.; Mao-Draayer, Y.; Ryan, K.A.; Noelle, R.J.; Wishart, H.A.; Channon, J.Y.; Kasper, I.R.; Oliver, B.; Mielcarz, D.W.; Kasper, L.H. Safety and Immune Effects of Blocking CD40 Ligand in Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1096. [Google Scholar] [CrossRef]
- Kwidzinski, E.; Mutlu, L.K.; Kovac, A.D.; Bunse, J.; Goldmann, J.; Mahlo, J.; Aktas, O.; Zipp, F.; Kamradt, T.; Nitsch, R.; et al. Self-tolerance in the immune privileged CNS: Lessons from the entorhinal cortex lesion model. In Advances in Research on Neurodegeneration. Journal of Neural Transmission. Supplementa; Springer: Vienna, Austria, 2003; Volume 65, pp. 29–49. [Google Scholar] [CrossRef]
- Bechmann, I.; Peter, S.; Beyer, M.; Gimsa, U.; Nitsch, R. Presence of B-7.2 (CD86) and lack of B7-1 (CD80) on myelin-phagocytosing MHC-II positive rat microglia are associated with nondestructive immunity in vivo. FASEB J. 2001, 15, 1086–1088. [Google Scholar] [CrossRef] [PubMed]
- Krovi, S.H.; Kuchroo, V.K. Activation pathways that drive CD4(+) T cells to break tolerance in autoimmune diseases. Immunol. Rev. 2022, 307, 161–190. [Google Scholar] [CrossRef]
- Klotz, L.; Burgdorf, S.; Dani, I.; Saijo, K.; Flossdorf, J.; Hucke, S.; Alferink, J.; Nowak, N.; Beyer, M.; Mayer, G.; et al. The nuclear receptor PPAR gamma selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J. Exp. Med. 2009, 206, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- Hucke, S.; Floßdorf, J.; Grützke, B.; Dunay, I.R.; Frenzel, K.; Jungverdorben, J.; Linnartz, B.; Mack, M.; Peitz, M.; Brüstle, O.; et al. Licensing of myeloid cells promotes central nervous system autoimmunity and is controlled by peroxisome proliferator-activated receptor γ. Brain 2012, 135, 1586–1605. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Kuzmanov, I.; Hucke, S.; Gross, C.C.; Posevitz, V.; Dreykluft, A.; Schulte-Mecklenbeck, A.; Janoschka, C.; Lindner, M.; Herold, M.; et al. B7-H1 shapes T-cell-mediated brain endothelial cell dysfunction and regional encephalitogenicity in spontaneous CNS autoimmunity. Proc. Natl. Acad. Sci. USA 2016, 113, E6182–E6191. [Google Scholar] [CrossRef]
- Fleck, A.K.; Hucke, S.; Teipel, F.; Eschborn, M.; Janoschka, C.; Liebmann, M.; Wami, H.; Korn, L.; Pickert, G.; Hartwig, M.; et al. Dietary conjugated linoleic acid links reduced intestinal inflammation to amelioration of CNS autoimmunity. Brain 2021, 144, 1152–1166. [Google Scholar] [CrossRef]


| EAE Score | Comment |
|---|---|
| 0 | healthy |
| 1 | first clinical signs of ataxia, retained narrow gait, tip of tail dragging |
| 2 | slight waddle, complete tail dragging |
| 3 | wide gait, moderate ataxia, slight bilateral paralysis of the hindlimbs |
| 4 | uncoordinated gait with moderate bilateral paralysis of hindlimbs, lowered hindquarters |
| 5 | lowered and broad hindquarters, temporary dragging of one hind limb |
| 6 | permanent dragging of one hindlimb or both legs temporarily dragging |
| 7 | complete paralysis of the hindlimbs (paraplegia) |
| 8 | weakness of forelimbs, beginning tetraparesis |
| 9 | no movement, tetraplegia, altered breathing |
| 10 | moribund animal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiffmann, D.; Lampkemeyer, V.; Lindner, M.; Fleck, A.-K.; Koch, K.; Eschborn, M.; Liebmann, M.; Strecker, J.-K.; Minnerup, J.; Wiendl, H.; et al. Endurance Exercise Attenuates Established Progressive Experimental Autoimmune Encephalomyelitis and Is Associated with an Amelioration of Innate Immune Responses in NOD Mice. Int. J. Mol. Sci. 2023, 24, 15798. https://doi.org/10.3390/ijms242115798
Schiffmann D, Lampkemeyer V, Lindner M, Fleck A-K, Koch K, Eschborn M, Liebmann M, Strecker J-K, Minnerup J, Wiendl H, et al. Endurance Exercise Attenuates Established Progressive Experimental Autoimmune Encephalomyelitis and Is Associated with an Amelioration of Innate Immune Responses in NOD Mice. International Journal of Molecular Sciences. 2023; 24(21):15798. https://doi.org/10.3390/ijms242115798
Chicago/Turabian StyleSchiffmann, Daniel, Victoria Lampkemeyer, Maren Lindner, Ann-Katrin Fleck, Kathrin Koch, Melanie Eschborn, Marie Liebmann, Jan-Kolja Strecker, Jens Minnerup, Heinz Wiendl, and et al. 2023. "Endurance Exercise Attenuates Established Progressive Experimental Autoimmune Encephalomyelitis and Is Associated with an Amelioration of Innate Immune Responses in NOD Mice" International Journal of Molecular Sciences 24, no. 21: 15798. https://doi.org/10.3390/ijms242115798





