Synthesis and In Vitro Evaluation as Potential Anticancer and Antioxidant Agents of Diphenylamine-Pyrrolidin-2-one-Hydrazone Derivatives
Abstract
:1. Introduction
2. Results and Discussion
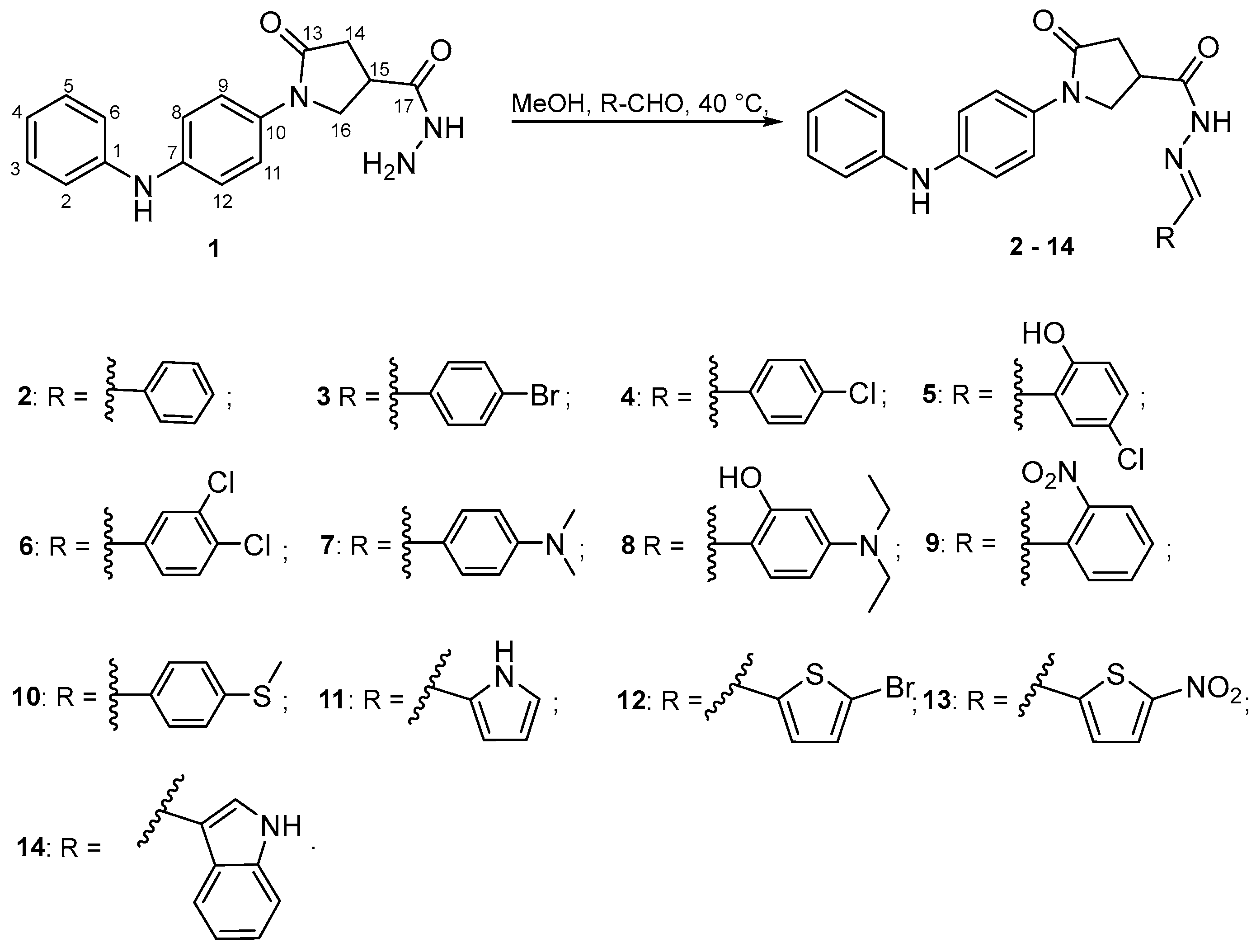
2.1. Chemistry
2.2. Pharmacology
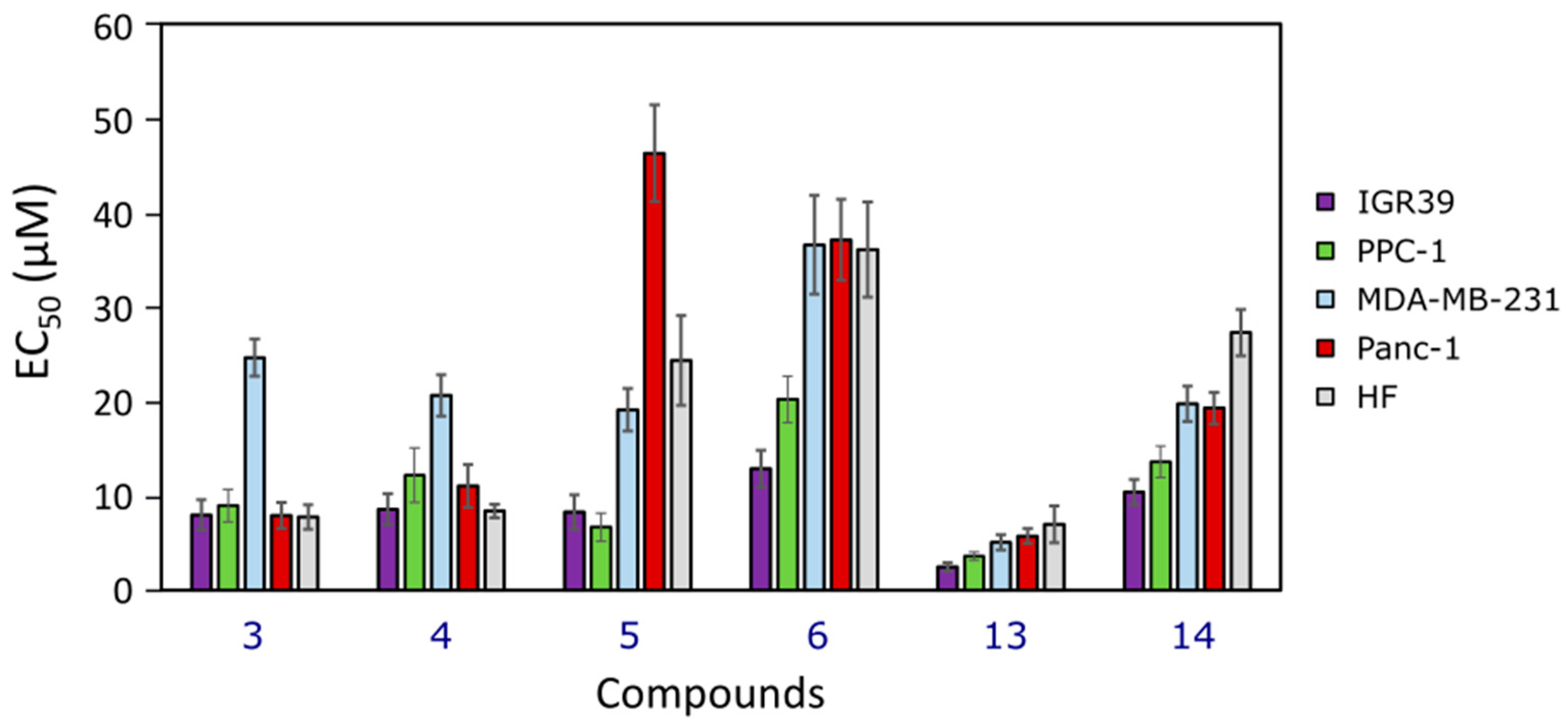
2.2.1. Cytotoxicity
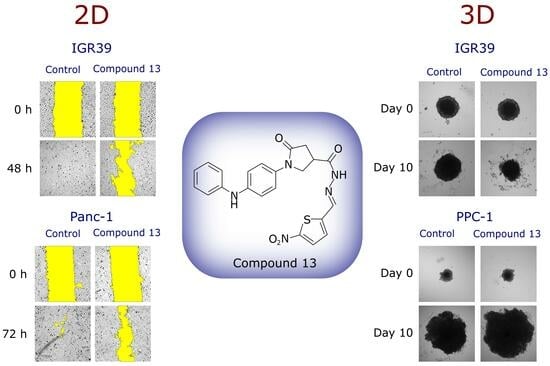
2.2.2. Effect on Cell Migration
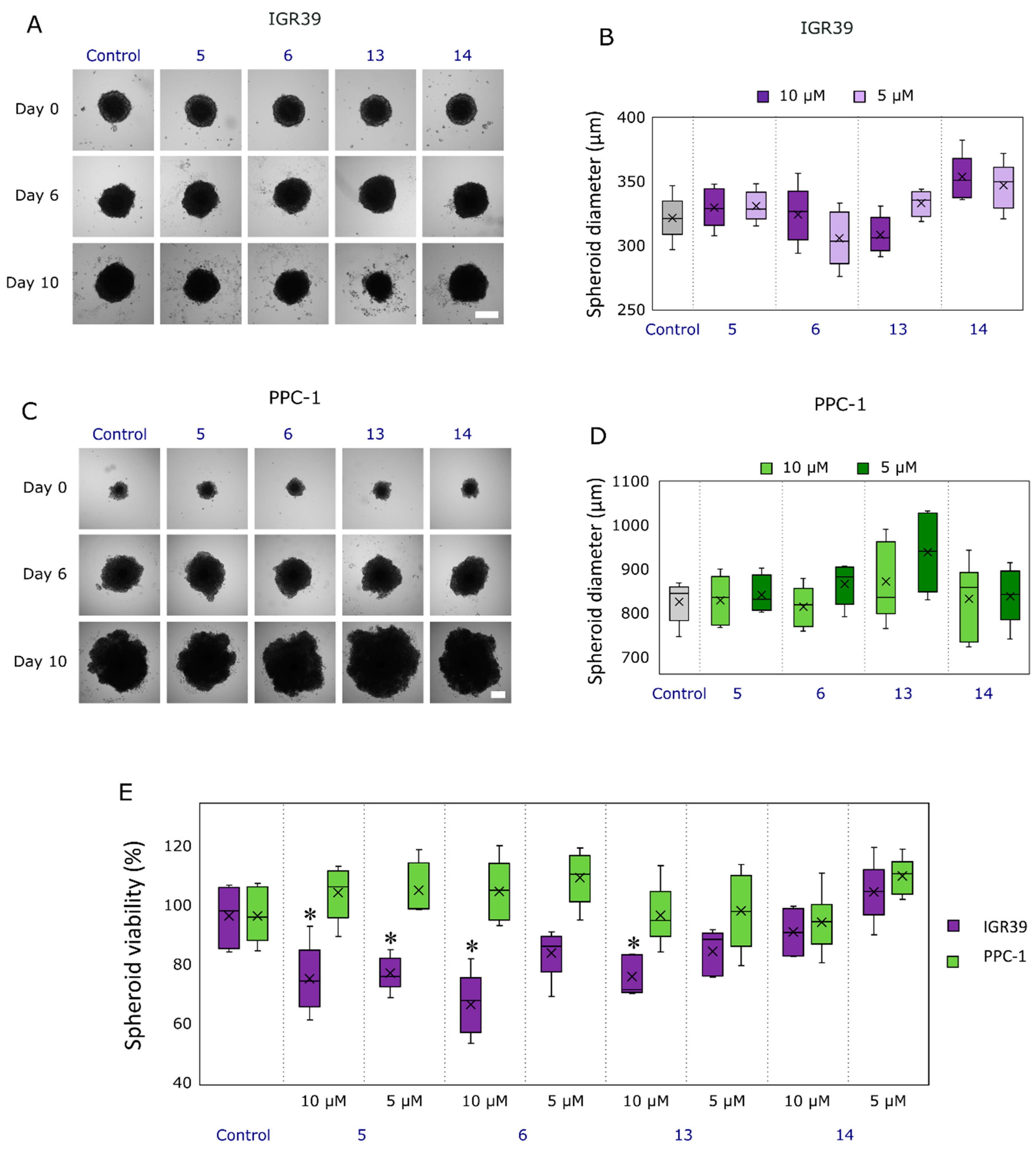
2.2.3. Compound Effect on Tumor Spheroid Growth and Their Viability
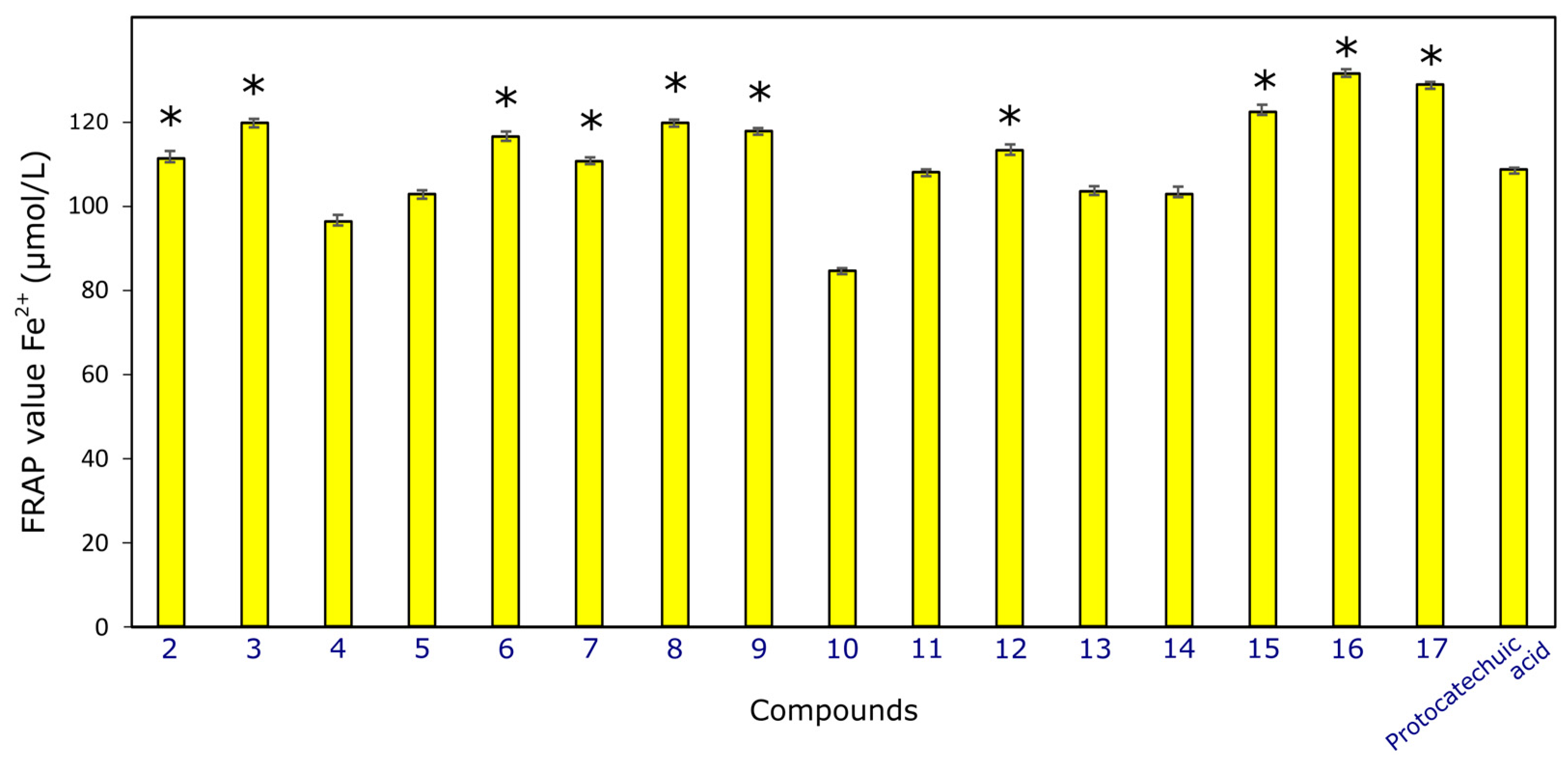
2.2.4. Antioxidant Activity
3. Materials and Methods
3.1. Chemistry
3.1.1. Chemical Reagents and Instruments
3.1.2. 5-Oxo-1-(4-(phenylamino)phenyl)pyrrolidine-3-carbohydrazide (1) Was Synthesized as Described in [32]
3.1.3. General Procedure for the Synthesis of Compounds 2–17
- N′-benzylidene-5-oxo-1-(4-(phenylamino)phenyl)pyrrolidine-3-carbohydrazide (2), Prepared from benzaldehyde. Yield 47% (0.36 g), light blue crystals; m.p. 122–123 °C. IR (KBr) νmax (cm−1): 1604, 1683 (C=O), 3023, 3311 (NH); 1H NMR (400 MHz, DMSO-d6): 2.63–2.81 (m, 2H, H14), 3.89–3.93 (m, 1H, H15), 3.99–4.04 (m, 2H, H16), 6.79 (t, 1H, J = 7.2 Hz, H4), 7.01–7.07 (m, 4H, H2,6,8,12), 7.20 (t, 2H, J = 7.2 Hz, H3,5), 7.43–7.45 (m, 2H, HAr″), 7.49–7.51 (m, 5H, HAr″), 7.86–7.87 (m, 1H, H18), 8.12 (s, 1H, NH), 8.68 (s, 1H, NH); 13C NMR (101 MHz, DMSO-d6): δ 35.37 (C15), 35.51 (C14), 50.75, 52.81 (C16), 116.91, 117.31, 117.65, 120.20, 121.95, 128.96, 129.12, 129.55, 129.81, 130.26, 131.96, 132.06, 134.21, 140.67, 144.04, 162.16 (CAr,Ar′,Ar″+C18), 171.74, 173.97 (C13,17). HRMS (ESI+): m/z calcd for C24H22N4O2 399.1822 [M+H]+, found 399.1708.
- N′-(4-bromobenzylidene)-5-oxo-1-(4-(phenylamino)phenyl)pyrrolidine-3-carbohydrazide (3), Prepared from 4-bromobenzaldehyde. Yield 44% (0.37 g), green crystals; m.p. 198–199 °C. IR (KBr) νmax (cm−1): 1653, 1687 (C=O), 3113, 3397 (NH); 1H NMR (400 MHz, DMSO-d6): 2.65–2.84 (m, 2H, H14), 3.89–3.93 (m, 1H, H15), 3.99–4.10 (m, 2H, H16), 6.79 (t, 1H, J = 7.2 Hz, H4), 7.00–7.07 (m, 4H, H2,6,8,12), 7.20 (t, 2H, J = 7.2 Hz, H3,5), 7.46 (d, 2H, J = 7.2 Hz, H9,11), 7.59–7.65 (m, 4H, HAr″), 7.99 (s, 0.6H, H18), 8.11 (s, 1H, NH), 8.15 (s, 0.4H, H18), 11.55 (s, 0.6H, NH), 11.76 (s, 0.4H, NH); 13C NMR (101 MHz, DMSO-d6): δ 33.20, 35.03 (C15), 35.22, 35.75 (C14), 50.82, 51.18 (C16), 116.65, 116.69, 117.49, 121.67, 121.72, 123.53, 123.85, 129.14, 129.38, 129.59, 131.85, 131.93, 132.20, 133.62, 133.67, 140.35, 140.39, 143.10, 143.86, 146.46, 169.50 (CAr,Ar′,Ar″+C18), 171.77, 172.02, 174.26 (C13,17). HRMS (ESI+): m/z calcd for C24H21BrN4O2 477.0927 [M+H]+, found 477.0926.
- N′-(4-chlorobenzylidene)-5-oxo-1-(4-(phenylamino)phenyl)pyrrolidine-3-carbohydrazide (4), Prepared from 4-chlorobenzaldehyde. Yield 59% (0.4 g), black crystals; m.p. 209–210 °C. IR (KBr) νmax (cm−1): 1655, 1689 (C=O), 3113, 3398 (NH); 1H NMR (400 MHz, DMSO-d6): 1.85–1.92 (m, 2H, H14), 3.04–3.11 (m, 1H, H15), 3.21–3.25 (m, 2H, H16), 5.91 (t, 1H, J = 7.2 Hz, H4), 6.06–6.19 (m, 4H, H2,6,8,12), 6.33 (t, 2H, J = 7.6 Hz, H3,5), 6.60–6.65 (m, 4H, H9,11,Ar″), 6.86 (d, 2H, J = 8.0 Hz, HAr″), 7.15 (s, 0.7H, H18), 7.26 (s, 1H, NH), 7.34 (s, 0.3H, H18), 10.76 (s, 0.7H, NH), 10.83 (s, 0.3H, NH); 13C NMR (101 MHz, DMSO-d6): δ 32.89, 34.74 (C15), 34.92, 35.50 (C14), 50.31, 50.73 (C16), 116.20, 116.24, 117.18, 117.21, 119.38, 121.05, 121.10, 128.55, 128.73, 128.92, 129.18, 131.75, 131.83, 133.10, 134.33, 134.57, 139.82, 139.86, 142.37, 143.68, 145.72, 168.90, 171.16 (CAr,Ar′,Ar″+C18), 171.36, 173.74 (C13,17); HRMS (ESI+): m/z calcd for C24H21ClN4O2 433.1432 [M+H]+, found 433.1428.
- N′-(5-chloro-2-hydroxybenzylidene)-5-oxo-1-(4-(phenylamino)phenyl)pyrrolidine-3-carbohydrazide (5), Prepared from 5-chlorosalicylaldehyde. Yield 51% (0.37 g), light yellow crystals; m.p. 223–224 °C. IR (KBr) νmax (cm−1): 1628, 1674 (C=O), 2855, 3401 (NH); 1H NMR (400 MHz, DMSO-d6): 2.67–2.76 (m, 2H, H14), 3.67–3.68 (m, 1H, H15), 3.94–4.07 (m, 2H, H16), 6.92 (t, 1H, J = 7.2 Hz, H4), 6.98 (d, 2H, J = 7.2 Hz, H2,6), 7.00 (d, 2H J = 7.2 Hz, H8,12), 7.38 (t, 2H J = 7.2 Hz, H3,5), 7.42 (d, 2H J = 7.2 Hz, H9,11), 7.69 (d, 2H, J = 7.6 Hz, HAr″), 7.74 (m, 2H, H18,Ar″), 8.92 (s, 1H, NH), 11.10 (s, 1H, NH), 12.05 (s, 1H, OH); 13C NMR (101 MHz, DMSO-d6): δ 33.31 (C15), 35.14 (C14), 51.08 (C16), 116.25, 117.19, 118.48, 118.56, 119.95, 120.14, 121.08, 121.69, 121.96, 123.15, 123.24, 128.10, 128.68, 129.85, 132,02, 132.71, 133.10, 153.37, 157.30; 157.37, 160.92, 161,25, 162.94, 163.60 (CAr,Ar′,Ar″+C18), 171.71, 174.37 (C13,17); HRMS (ESI+): m/z calcd for C24H21ClN4O3 449.1381 [M+H]+, found 449.1377.
- N′-(3,4-dichlorobenzylidene)-5-oxo-1-(4-(phenylamino)phenyl)pyrrolidine-3-carbohydrazide (6), Prepared from 3,4-dichlorobenzaldehyde. Yield 41% (0.29 g), light blue crystals; m.p. 220–221 °C. IR (KBr) νmax (cm−1): 1603, 1675 (C=O), 2937, 3343 (NH); 1H NMR (400 MHz, DMSO-d6): 2.68–2.77 (m, 2H, H14), 3.86–3.90 (m, 1H, H15), 3.94–4.12 (m, 2H, H16), 6.24 (s, 2H, HAr″), 6.79 (t, 1H, J = 7.2 Hz, H4), 7.01–7.07 (m, 4H, H2,6,8,12), 7.20 (t, 2H, J = 7.2 Hz, H3,5), 7.43–7.48 (m, 3H, H9,11,Ar″), 8.11 (s, 1H, NH), 8.18 (s, 0.7H, H18), 8.33 (s, 0.3H, H18), 11.14 (s, 0.7H, NH), 11.32 (s, 0.3H, NH). 13C NMR (101 MHz, DMSO-d6): δ 34.17, 34.87 (C15), 35.24, 35.88 (C14), 50.95, 51.35 (C16), 91.35, 91.41, 104.08, 116.69, 117.48, 119.96, 121.65, 121.77, 129.60, 131.92, 132.01, 139.52, 140.37, 143.86, 160.22, 162.53 (CAr,Ar′,Ar″+C18), 172.33, 173.46 (C13,17); HRMS (ESI+): m/z calcd for C24H20Cl2N4O2 489.0860 [M+Na]+, found 489.2140.
- N′-(4-(dimethylamino)benzylidene)-5-oxo-1-(4-(phenylamino)phenyl)pyrrolidine-3-carbohydrazide (7), Prepared from 4-(dimethylamino)benzaldehyde. Yield 49% (0.34 g), light red crystals; m.p. 218–219 °C. IR (KBr) νmax (cm−1): 1597, 1674 (C=O), 3077, 3335 (NH); 1H NMR (400 MHz, DMSO-d6): 2.68–2.79 (m, 2H, H14), 2.95, 2.96 (2s, 6H, 2CH3), 3.89–4.12 (m, 3H, H15,16), 6.73 (t, 1H, J = 7.2 Hz, H4), 6.74–6.81 (m, 2H, HAr″), 7.03–7.09 (m, 4H, H2,6,8,12), 7.21 (t, 2H, J = 7.2 Hz, H3,5), 7.47–7.56 (m, 4H, H9,11, Ar″), 7.91 (s, 0.6H, H18), 8.07 (s, 0.4H, H18), 8.14 (s, 1H, NH), 11.28 (s, 0.6H, NH), 11.34 (s, 0.4H, NH); 13C NMR (101 MHz, DMSO-d6): δ 33.74 (C15), 35.60 (C14), 39.65, 39.77 (CH3), 50.47, 50.89 (C16), 111.08, 111.84, 116.19, 117.22, 119.37, 121.02, 121.08, 121.54, 124.53, 128.14, 128.43, 129.18, 131.55, 131.89, 139.78, 143.68, 144.42, 147.82, 151.37, 154.21 (CAr,Ar′,Ar″+C18), 171.50, 173.02 (C13,17); HRMS (ESI+): m/z calcd for C26H27N5O2 442.2244 [M+H]+, found 442.2241.
- N′-(4-(diethylamino)-2-hydroxybenzylidene)-5-oxo-1-(4-(phenylamino)phenyl)pyrrolidine-3-carbohydrazide (8), Prepared from 4-(diethylamino)salicylaldehyde. Yield 42% (0.31 g), light yellow crystals; m.p. 239–240 °C. IR (KBr) νmax (cm−1): 1629, 1676 (C=O), 3004, 3256 (NH), 3423 (OH); 1H NMR (400 MHz, DMSO-d6): 1.02–1.10 (m, 6H, CH3), 2.71–2.81 (m, 2H, H14), 3.35–3.43 (m, 4H, CH2), 4.02–4.08 (m, 3H, H15,16), 6.52 (s, 1H, HAr″), 6.61 (d, 1H, J = 8.8 Hz, HAr″), 6.79 (t, 1H, J = 7.2 Hz, H4), 7.01–7.08 (m, 4H, H2,6,8,12), 7.20 (t, 2H, J = 7.2 Hz, H3,5), 7.39–7.47 (m, 3H, H9,11,Ar″), 8.24 (s, 0.3H, H18), 8.31 (s, 0.7H, H18), 11.29 (s, 0.3H, NH), 11.44 (s, 0.7H, NH), 11.92 (s, 1H, OH); 13C NMR (101 MHz, DMSO-d6): δ 11.93 (CH3), 35.00 (C15), 35.74 (C14), 44.57 (CH2), 51.22 (C16), 40,56 (CH3), 104.96, 107.91, 116.70, 116.74, 117.48, 119.98, 121.70, 121.75, 129.61, 131.85, 131.94, 140.41, 143.86, 149.10, 159.62, 163.75, 169.03 (CAr,Ar′,Ar″+C18), 171.81, 172.06 (C13,17). HRMS (ESI+): m/z calcd for C28H31N5O3 486.2506 [M+H]+, found 486.2510.
- N′-(2-nitrobenzylidene)-5-oxo-1-(4-(phenylamino)phenyl)pyrrolidine-3-carbohydrazide (9), Prepared from 2-nitrobenzaldehyde. Yield 39% (0.32 g), light red crystals; m.p. 192–193 °C. IR (KBr) νmax (cm−1): 1596, 1676 (C=O), 3056, 3342 (NH); 1H NMR (400 MHz, DMSO-d6): 1.87–1.96 (m, 2H, H14), 3.07–3.11 (m, 1H, H15), 3.15–3.25 (m, 2H, H16), 5.92 (t, 1H, J = 7.4 Hz, H4), 6.16–6.22 (m, 4H, H2,6,8,12), 6.34 (t, 2H, J = 7.4 Hz, H3,5), 6.65 (d, 2H, J = 8.8 Hz, HAr″), 6.76–6.93 (m, 2H, HAr″), 7.16–7.25 (m, 2H, H9,11), 7.27 (s, 1H, NH), 7.63–7.80 (m, 2H, HAr″), 7.55 (s, 0.7H, H18), 7.77 (s, 0.3H, H18), 10.98 (s, 0.7H, NH), 11.12 (s, 0.3H, NH); 13C NMR (101 MHz, DMSO-d6): δ 32.89 (C15), 34.72 (C14), 50.23 (C16), 116.21, 117.19, 119.39, 121.10, 124.54, 128.12, 128.36, 128.68, 129.18, 129.44, 130.51, 131.80, 132.16, 133.55, 133.93, 139.15, 139.84, 142.55, 143.66, 148.06, 148.88, 158.70, 169.15 (CAr,Ar′,Ar″+C18), 171.29, 173.96 (C13,17); HRMS (ESI+): m/z calcd for C24H21N5O4 444.1673 [M+H]+, found 444.1672.
- N′-(4-(methylthio)benzylidene)-5-oxo-1-(4-(phenylamino)phenyl)pyrrolidine-3-carbohydrazide (10), Prepared from 4-(methylthio)benzaldehyde. Yield 58% (0.41 g), light red crystals; m.p. 193–194 °C. IR (KBr) νmax (cm−1): 1597, 1660 (C=O), 3241, 3311 (NH); 1H NMR (400 MHz, DMSO-d6): 2.51, 2.55 (2s, 3H, CH3), 2.67–2.81 (m, 2H, H14), 3.92–3.98 (m, 1H, H15), 4.00–4.13 (m, 2H, H16), 6.80 (t, 1H, J = 7.0 Hz, H4), 7.03–7.10 (m, 4H, H2,6,8,12), 7.22 (t, 2H, J = 7.0 Hz, H3,5), 7.31 (d, 2H, J = 7.0 Hz, H9,11), 7.52 (d, 2H, J = 8.0 Hz, HAr″), 7.64 (d, 2H, J = 8.0 Hz, HAr″), 8.00 (s, 0.7H, H18), 8.15 (s, 1H, NH), 8.18 (s, 0.3H, H18), 11.54 (s, 0.7H, NH), 11.61 (s, 0.3H, NH); 13C NMR (101 MHz, DMSO-d6): δ 14.30 (CH3), 32.93 (C15), 34.74 (C14), 50.37 (C16), 116.20, 117.21, 119.38, 121.04, 121.09, 125.68, 127.31, 127.51, 129.19, 129.86, 130.60, 131.85, 139.81, 140.73, 141.05, 143.26, 143.68, 146.63, 168.68 (CAr,Ar′,Ar″+C18), 171.40, 173.53 (C13,17); HRMS (ESI+): m/z calcd for C25H24N4O2S 445.1699 [M+H]+, found 445.1695.
- N′-((1H-pyrrol-2-yl)methylene)-5-oxo-1-(4-(phenylamino)phenyl)pyrrolidine-3-carbohydrazide (11), Prepared from pyrrole-2-carboxaldehyde. Yield 34% (0.29 g), black crystals; m.p. 234–235 °C. IR (KBr) νmax (cm−1): 1613, 1671 (C=O), 2926, 3108, 3400 (NH); 1H NMR (400 MHz, DMSO-d6): 2.63–2.78 (m, 2H, H14), 4.00–4.13 (m, 1H, H15), 4.15–4.17 (m, 2H, H16), 5.85–5.88 (m, 1H, HPyrrole), 6.03–6.12 (m, 1H, HPyrrole), 6.21–6.28 (m, 1H, HAr′), 6.41–6.47 (m, 1H, HAr′), 6.79 (t, 1H, J = 7.0 Hz, H4), 6.92–7.48 (m, 7H, HAr′,Pyrrole), 7.84 (s, 0.6H, H18), 8.01 (s, 1H, NH), 8.11 (s, 0.4H, H18), 11.19 (s, 0.4H, NH), 11.33 (s, 0.6H, NH), 11.44 (s, 1H, NH); 13C NMR (101 MHz, DMSO-d6): δ 32.93 (C15), 34.74 (C14), 50.37 (C16), 116.20, 117.21, 119.38, 121.04, 121.09, 125.17, 125.68, 127.31, 127.51, 129.19, 129.86, 130.60, 131.85, 139.81, 140.73, 143.26, 143.68, 146.63, 168.68 (CAr,Ar′,Pyrrole+C18), 171.40, 173.53 (C13,17); HRMS (ESI+): m/z calcd for C22H21N5O2 388.1774 [M+H]+, found 388.1767.
- N′-((5-bromothiophen-2-yl)methylene)-5-oxo-1-(4-(phenylamino)phenyl)pyrrolidine-3-carbohydrazide (12), Prepared from 5-bromo-2-thiophenecarboxaldehyde. Yield 56% (0.36 g), light green crystals; m.p. 240–241 °C. IR (KBr) νmax (cm−1): 1596, 1670 (C=O), 2926, 3384 (NH); 1H NMR (400 MHz, DMSO-d6): 2.63–2.81 (m, 2H, H14), 3.87–4.06 (m, 3H, H15,16), 6.78 (t, 1H, J = 7.2 Hz, H4), 7.01–7.06 (m, 4H, H2,6,8,12), 7.19 (t, 2H, J = 7.2 Hz, H3,5), 7.24–7.29 (m, 1H, HThiophene); 7.42–7.46 (m, 3H, H9,11,Thiophene), 7.83 (s, 0.5H, NH), 8.09 (s, 0.4H, H18), 8.22 (s, 0.3H, H18), 8.31 (s, 0.3H, H18), 8.70 (s, 0.5H, NH), 9.77 (s, 0.4H, NH), 11.54 (s, 0.3H, NH), 11.70 (s, 0.3H, NH); 13C NMR (101 MHz, DMSO-d6): δ 33.34 (C15), 35.16, 35.30 (C14), 52.59 (C16), 114.65, 115.27, 116.66, 116.69, 117.42, 117.46, 119.95, 121.69, 121.72, 124.00, 129.57, 130.46, 131.19, 132.94, 139.02, 143.84, 145.12, 154.77, 155.84 (CAr,Ar′,Ar″+C18), 171.50, 173.73 (C13,17); HRMS (ESI+): m/z calcd for C22H19BrN4O2S 483.0491 [M+H]+, found 483.0483.
- N′-((5-nitrothiophen-2-yl)methylene)-5-oxo-1-(4-(phenylamino)phenyl)pyrrolidine-3-carbohydrazide (13), Prepared from 5-nitro-2-thiophenecarboxaldehyde. Yield 43% (0.31 g), light blue crystals; m.p. 235–236 °C. IR (KBr) νmax (cm−1): 1648, 1693 (C=O), 3080, 3414 (NH); 1H NMR (400 MHz, DMSO-d6): 2.65–2.82 (m, 2H, H14), 3.89–3.96 (m, 1H, H15), 3.99–4.06 (m, 2H, H16), 6.78 (t, 1H, J = 7.2 Hz, H4), 6.93–7.07 (m, 4H, H2,6,8,12), 7.19 (t, 2H, J = 7.2 Hz, H3,5), 7.42–7.48 (m, 4H, H9,11,Thiophene), 8.04 (t, 1H, J = 7.2 Hz, NH), 8.15 (s, 0.6H, H18), 8.40 (s, 0.4H, H18), 11.87 (s, 0.6H, NH), 12.03 (s, 0.4H, NH); 13C NMR (101 MHz, DMSO-d6): δ 33.34 (C15), 34.99, 35.33 (C14), 50.70, 51.05 (C16), 116.68, 117.45, 119.96, 119.98, 121.71, 129.43, 129.58, 130.05, 130.91, 131.88, 137.37, 140.40, 141.02, 143.84, 146.82, 150.91, 151.29, 169.82 (CAr,Ar′,Thiophene+C18), 171.84, 174.33 (C13,17); HRMS (ESI+): m/z calcd for C22H19N5O4S 450.1234 [M+H]+, found 450.1232.
- N′-((1H-indol-3-yl)methylene)-5-oxo-1-(4-(phenylamino)phenyl)pyrrolidine-3-carbohydrazide (14), Prepared from indole-3-carboxaldehyde. Yield 52% (0.36 g), red crystals; m.p. 238–239 °C. IR (KBr) νmax (cm−1): 1612, 1660 (C=O), 3060, 3310, 3614 (NH); 1H NMR (400 MHz, DMSO-d6): 2.73–2.92 (m, 2H, H14), 3.96–4.04 (m, 1H, H15), 4.10–4.19 (m, 2H, H16), 6.76–6.81 (m, 1H, H4), 7.00–7.49 (m, 12H, H2,3,5,6,8,9,11,12,Indole), 7.74–7.77 (m, 1H, H Indole), 8.12, 8.14 (2s, 0.6H, NH), 8.21, 8.24 (2s, 1H, H18), 8.38 (s, 0.4H, NH), 11.16 (s, 0.6H, NH), 11.36 (s, 0.4H, NH), 11.55 (s, 1H, NH); 13C NMR (101 MHz, DMSO-d6): δ 33.27 (C15), 35.16 (C14), 51.00 (C16), 111.71, 112.25, 112.39, 116.67, 117.51, 119.96, 120.91, 121.20, 121.71, 122.21, 123.13, 124.35, 124.60, 129.60, 130.78, 130.91, 132.00, 137.34, 137.44, 140.36, 141.97, 143.87, 144.86, 168.50 (CAr,Ar′,Indole+C18), 172.01, 173.34 (C13,17); HRMS (ESI+): m/z calcd for C26H23N5O2 438.1931, found 438.1931 [M+H]+.
- 5-Oxo-1-(4-(phenylamino)phenyl)-N′-(1-phenylethylidene)pyrrolidine-3-carbohydrazide (15), Prepared from acetophenone. Yield 47% (0.31 g), light blue crystals; m.p. 225–226 °C. IR (KBr) νmax (cm−1): 1678, 1730 (C=O), 3349, 3376 (NH); 1H NMR (400 MHz, DMSO-d6): 2.90–3.08 (m, 2H, H14), 3.93 (s, 3H, CH3), 4.16–4.30 (m, 3H, H15,16), 7.06 (t, 1H, J = 7.2 Hz, H4), 7.28–7.35 (m, 4H, H2,6,8,12), 7.47 (t, 2H, J = 7.2 Hz, H3,5), 7.57–7.93 (m, 6H, H9,11,Acetophenone), 8.14–8.19 (m, 2H, NH+HAcetophenone), 8.39 (s, 1H, NH); 13C NMR (101 MHz, DMSO-d6): δ 15.08 (CH3), 35.17 (C15), 35.31 (C14), 50.54 (C16), 116.70, 117.44, 119.99, 121.74, 126.82, 128.85, 129.60, 130.19, 131.75, 138.16, 140.46, 143.83, 157.65 (CAr,Ar′,Acetophenone+C18), 171.53, 173.77 (C13,17); HRMS (ESI+): m/z calcd for C25H24N4O2 413.1968 [M+H]+, found 413.1765.
- N′-(1-(4-aminophenyl)ethylidene)-5-oxo-1-(4-(phenylamino)phenyl)pyrrolidine-3-carbohydrazide (16), Prepared from 4-aminoacetophenone. Yield 56% (0.36 g), dark blue crystals; m.p. 230–231 °C. IR (KBr) νmax (cm−1): 1680, 1730 (C=O), 3349, 3376, 3400 (NH); 1H NMR (400 MHz, DMSO-d6): 2.13 (s, 3H, CH3), 2.65–2.81 (m, 2H, H14), 3.89–3.93 (m, 1H, H15), 3.95–4.04 (m, 2H, H16), 5.42 (s, 2H, NH2), 6.55–6.57 (m, 2H, HAcetophenone), 6.79 (t, 1H, J = 7.2 Hz, H4), 7.01–7.07 (m, 4H, H2,6,8,12), 7.20 (t, 2H, J = 7.2 Hz, H3,5), 7.44 (d, 2H, J = 8.8 Hz, H9,11), 7.64–7.66 (m, 2H, HAcetophenone), 8.11 (s, 1H, NH), 10.18 (s, 0.4H, NH), 10.40 (s, 0.6H, NH); 13C NMR (101 MHz, DMSO-d6): δ 26.18 (CH3), 34.39 (C15), 35.17, 35.31 (C14), 50.55 (C16), 112.94, 116.71, 117.44, 117.50, 120.00, 121.66, 121.75, 125.20, 127.66, 129.61, 131.02, 131.75, 140.40, 140.47, 143.83, 153.97 (CAr,Ar′, Acetophenone+C18), 171.55, 173.78 (C13,17); HRMS (ESI+): m/z calcd for C25H25N5O2 428.2087 [M+H]+, found 428.2083.
- N′-(1-(3-aminophenyl)ethylidene)-5-oxo-1-(4-(phenylamino)phenyl)pyrrolidine-3-carbohydrazide (17), Prepared from 3-aminoacetophenone. Yield 64% (0.42 g), black crystals; m.p. 239–240 °C. IR (KBr) νmax (cm−1): 1596, 1665 (C=O), 3094, 3184, 3397 (NH); 1H NMR (400 MHz, DMSO-d6): 2.18, 2.21 (2s, 3H, CH3), 2.66–2.86 (m, 2H, H14), 3.91–3.96 (m, 1H, H15), 4.00–4.12 (m, 2H, H16), 6.60–6.64 (m, 1H, HAcetophenone), 6.79 (t, 1H, J = 7.2 Hz, H4), 6.94 (d, 1H, J = 8.00 Hz, HAcetophenone), 7.01–7.49 (m, 12H, H2,6,8,12,3,5,9,11,Acetophenone+NH2), 8.11 (s, 1H, NH), 10.57 (s, 1H, NH); 13C NMR (101 MHz, DMSO-d6): δ 14.04, 14.76 (CH3), 33.52 (C15), 35.04, 35.16 (C14), 50.98 (C16), 111.89, 112.22, 113.21, 114.70, 115.10, 115.56, 115.80, 116.68, 117.51, 119.97, 121.68, 121.74, 129.33, 129.61, 131.93, 139.03, 140.38, 143.87, 148.73, 154.09, 169.90 (CAr,Ar′,Acetophenone+C18), 172.18, 174.90 (C13,17); HRMS (ESI+): m/z calcd for C25H25N5O2 428.2087 [M+H]+, found 428.2083.
3.2. Pharmacology
3.2.1. Cell Culturing
3.2.2. Cell Viability Assay
3.2.3. ‘Wound Healing’ Assay
3.2.4. Compound Activity in Cell 3D Cultures (Spheroids)
3.2.5. Antioxidant Activity Determined by FRAP Assay
3.2.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Worldwide Cancer Data. Available online: https://www.wcrf.org/cancer-trends/worldwide-cancer-data/ (accessed on 13 January 2023).
- Liebmann, J.; Cook, J.; Lipschultz, C.; Teague, D.; Fisher, J.; Mitchell, J. Cytotoxic Studies of Paclitaxel (Taxol®) in Human Tumour Cell Lines. Br. J. Cancer 1993, 68, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Zahra, K.F.; Lefter, R.; Ali, A.; Abdellah, E.-C.; Trus, C.; Ciobica, A.; Timofte, D. The Involvement of the Oxidative Stress Status in Cancer Pathology: A Double View on the Role of the Antioxidants. Oxid. Med. Cell Longev. 2021, 2021, 9965916. [Google Scholar] [CrossRef] [PubMed]
- Elsayed Azab, A.; A Adwas, A.; Ibrahim Elsayed, A.S.; A Adwas, A.; Ibrahim Elsayed, A.S.; Quwaydir, F.A. Oxidative Stress and Antioxidant Mechanisms in Human Body. J. Appl. Biol. Biotechnol. 2019, 6, 43–47. [Google Scholar] [CrossRef]
- Kong, Q.; Beel, J.A.; Lillehei, K.O. A Threshold Concept for Cancer Therapy. Med. Hypotheses 2000, 55, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Piracetam. DRUGBANK Online. Available online: https://go.drugbank.com/drugs/db09210 (accessed on 13 January 2023).
- Povidone-Iodine. DRUGBANK Online. Available online: https://go.drugbank.com/drugs/db06812 (accessed on 13 January 2023).
- Albratty, M. Quantitative Structure–Activity Relationship Modeling and Docking of Some Synthesized Bioactive Oxopyrolidines against Staphylococcus aureus. J. Saudi Chem. Soc. 2022, 26, 101509. [Google Scholar] [CrossRef]
- Zhu, X.-L.; Tian, X.-Q.; Xu, H.-H.; Wang, H.-M.; Chen, Q.-H.; Zeng, X.-H. Rhopaladins’ Analogue (E)-2-Aroyl-4-(4-Fluorobenzylidene)-5-Oxopyrrolidines Inhibit Proliferation, Promote Apoptosis and down-Regulation of E6/E7 mRNA in Cervical Cancer. Bioorg. Med. Chem. Lett. 2020, 30, 127554. [Google Scholar] [CrossRef]
- Muralidharan, V.P.; Alagumuthu, M.; Iyer, S.K. Iodine Catalyzed Three Component Synthesis of 1-((2-Hydroxy Naphthalen-1-Yl)(Phenyl)(Methyl))Pyrrolidin-2-One Derivatives: Rationale as Potent PI3K Inhibitors and Anticancer Agents. Bioorg. Med. Chem. Lett. 2017, 27, 2510–2514. [Google Scholar] [CrossRef]
- Ali, I.; Lone, M.N.; Alothman, Z.A.; Alwarthan, A. Insights into the Pharmacology of New Heterocycles Embedded with Oxopyrrolidine Rings: DNA Binding, Molecular Docking, and Anticancer Studies. J. Mol. Liq. 2017, 234, 391–402. [Google Scholar] [CrossRef]
- Sasaki, Y.; Tokuhara, H.; Ohba, Y.; Okabe, A.; Nakayama, M.; Nakagawa, H.; Skene, R.; Hoffman, I.; Zou, H.; Yoshida, M. Efficient Synthesis of Tert-Butyl 3-Cyano-3-Cyclopropyl-2-Oxopyrrolidine-4-Carboxylates: Highly Functionalized 2-Pyrrolidinone Enabling Access to Novel Macrocyclic Tyk2 Inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 126963. [Google Scholar] [CrossRef] [PubMed]
- Jasiewicz, B.; Sierakowska, A.; Wandyszewska, N.; Warżajtis, B.; Rychlewska, U.; Wawrzyniak, R.; Mrówczyńska, L. Antioxidant Properties of Thio-Caffeine Derivatives: Identification of the Newly Synthesized 8-[(Pyrrolidin-1-Ylcarbonothioyl)Sulfanyl]Caffeine as Antioxidant and Highly Potent Cytoprotective Agent. Bioorg. Med. Chem. Lett. 2016, 26, 3994–3998. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I.; Saso, L. Advances in Research of Schiff-Base Metal Complexes as Potent Antioxidants. Curr. Med. Chem. 2013, 20, 4609–4632. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, S.; Sheikh, K.A.; Naematullah, M.; Mumtaz Alam, M.; Khan, F.; Garg, M.; Amir, M.; Akhter, M.; Amin, S.; Haque, A.; et al. Synthesis, Biological Evaluation and Docking Studies of Methylene Bearing Cyanopyrimidine Derivatives Possessing a Hydrazone Moiety as Potent Lysine Specific Demethylase-1 (LSD1) Inhibitors: A Promising Anticancer Agents. Bioorg. Chem. 2022, 126, 105885. [Google Scholar] [CrossRef]
- Yamali, C.; Sakagami, H.; Satoh, K.; Bandow, K.; Uesawa, Y.; Bua, S.; Angeli, A.; Supuran, C.T.; Gul, H.I. Investigation of Carbonic Anhydrase Inhibitory Effects and Cytotoxicities of Pyrazole-Based Hybrids Carrying Hydrazone and Zinc-Binding Benzenesulfonamide Pharmacophores. Bioorg. Chem. 2022, 127, 105969. [Google Scholar] [CrossRef] [PubMed]
- Rollas, S.; Küçükgüzel, S. Biological Activities of Hydrazone Derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Verma, G.; Shaquiquzzaman, M.; Marella, A.; Akhtar, M.; Ali, M. A Review Exploring Biological Activities of Hydrazones. J. Pharm. Bioall. Sci. 2014, 6, 69. [Google Scholar] [CrossRef]
- Demurtas, M.; Baldisserotto, A.; Lampronti, I.; Moi, D.; Balboni, G.; Pacifico, S.; Vertuani, S.; Manfredini, S.; Onnis, V. Indole Derivatives as Multifunctional Drugs: Synthesis and Evaluation of Antioxidant, Photoprotective and Antiproliferative Activity of Indole Hydrazones. Bioorg. Chem. 2019, 85, 568–576. [Google Scholar] [CrossRef]
- De Oliveira Carneiro Brum, J.; França, T.C.C.; LaPlante, S.R.; Villar, J.D.F. Synthesis and Biological Activity of Hydrazones and Derivatives: A Review. Mini-Rev. Med. Chem. 2020, 20, 342–368. [Google Scholar] [CrossRef]
- Popiołek, Ł. Updated Information on Antimicrobial Activity of Hydrazide–Hydrazones. Int. J. Mol. Sci. 2021, 22, 9389. [Google Scholar] [CrossRef]
- Dascalu, A.-E.; Ghinet, A.; Lipka, E.; Furman, C.; Rigo, B.; Fayeulle, A.; Billamboz, M. Design, Synthesis and Evaluation of Hydrazine and Acyl Hydrazone Derivatives of 5-Pyrrolidin-2-One as Antifungal Agents. Bioorg. Med. Chem. Lett. 2020, 30, 127220. [Google Scholar] [CrossRef] [PubMed]
- Han, M.İ.; Yeşil Baysal, Ö.D.; Başaran, G.Ş.; Sezer, G.; Telci, D.; Küçükgüzel, Ş.G. Design, Synthesis and Anticancer Activity Studies of Novel 4-Butylaminophenyl Hydrazide-Hydrazones as Apoptotic Inducers. Tetrahedron 2022, 115, 132797. [Google Scholar] [CrossRef]
- Ohta, K.; Chiba, Y.; Kaise, A.; Endo, Y. Structure–Activity Relationship Study of Diphenylamine-Based Estrogen Receptor (ER) Antagonists. Bioorg. Med. Chem. 2015, 23, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mishra, A.K. Pharmacological Applications of Diphenylamine and Its Derivative as Potent Bioactive Compound: A Review. Curr. Bioact. Comp. 2018, 14, 217–233. [Google Scholar] [CrossRef]
- Shimizu, T.; Fujiwara, Y.; Osawa, T.; Sakai, T.; Kubo, K.; Kubo, K.; Nishitoba, T.; Kimura, K.; Senga, T.; Murooka, H.; et al. Orally Active Anti-Proliferation Agents: Novel Diphenylamine Derivatives as FGF-R2 Autophosphorylation Inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Abou-Seri, S.M. Synthesis and Biological Evaluation of Novel 2,4′-Bis Substituted Diphenylamines as Anticancer Agents and Potential Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors. Eur. J. Med. Chem. 2010, 45, 4113–4121. [Google Scholar] [CrossRef]
- Sugihara, T.; Rao, G.; Hebbel, R.P. Diphenylamine: An Unusual Antioxidant. Free Radic. Biol. Med. 1993, 14, 381–387. [Google Scholar] [CrossRef]
- Tumosienė, I.; Kantminienė, K.; Klevinskas, A.; Petrikaitė, V.; Jonuškienė, I.; Mickevičius, V. Antioxidant and Anticancer Activity of Novel Derivatives of 3-[(4-Methoxyphenyl)Amino]Propanehydrazide. Molecules 2020, 25, 2980. [Google Scholar] [CrossRef]
- Tumosienė, I.; Jonuškienė, I.; Kantminienė, K.; Mickevičius, V.; Petrikaitė, V. Novel N-Substituted Amino Acid Hydrazone-Isatin Derivatives: Synthesis, Antioxidant Activity, and Anticancer Activity in 2D and 3D Models In Vitro. IJMS 2021, 22, 7799. [Google Scholar] [CrossRef]
- Šermukšnytė, A.; Kantminienė, K.; Jonuškienė, I.; Tumosienė, I.; Petrikaitė, V. The Effect of 1,2,4-Triazole-3-Thiol Derivatives Bearing Hydrazone Moiety on Cancer Cell Migration and Growth of Melanoma, Breast, and Pancreatic Cancer Spheroids. Pharmaceuticals 2022, 15, 1026. [Google Scholar] [CrossRef]
- Šermukšnytė, A.; Jonuškienė, I.; Kantminienė, K.; Beresnevičius, Z.J.; Tumosienė, I. 2-((4-Phenyl-5-(2-(p-Tolylamino)Ethyl)-4H-1,2,4-Triazol-3-Yl)Thio)-N′-(1-Phenylethylidene)Acetohydrazide. Molbank 2022, 2022, M1380. [Google Scholar] [CrossRef]
- Tisovský, P.; Csicsai, K.; Donovalová, J.; Šandrik, R.; Sokolík, R.; Gáplovský, A. Effect of a =X-NH-Fragment, (X = C, N), on Z/E Isomerization and ON/OFF Functionality of Isatin Arylhydrazones, ((Arylamino)Methylene)Indolin-2-Ones and Their Anions. Molecules 2020, 25, 3082. [Google Scholar] [CrossRef] [PubMed]
- Nedeljković, M.; Damjanović, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer—How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef] [PubMed]
- Quiñonero, F.; Mesas, C.; Doello, K.; Cabeza, L.; Perazzoli, G.; Jimenez-Luna, C.; Rama, A.R.; Melguizo, C.; Prados, J. The Challenge of Drug Resistance in Pancreatic Ductal Adenocarcinoma: A Current Overview. Cancer Biol. Med. 2019, 16, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Tiago, M.; de Oliveira, E.M.; Brohem, C.A.; Pennacchi, P.C.; Paes, R.D.; Haga, R.B.; Campa, A.; de Moraes Barros, S.B.; Smalley, K.S.; Maria-Engler, S.S. Fibroblasts Protect Melanoma Cells from the Cytotoxic Effects of Doxorubicin. Tissue Eng. Part A 2014, 20, 2412–2421. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V.; Pardee, A.B. Exploiting Cancer Cell Cycling for Selective Protection of Normal Cells. Cancer Res. 2001, 61, 4301–4305. [Google Scholar] [PubMed]
- Bedia, C.; Casas, J.; Andrieu-Abadie, N.; Fabriàs, G.; Levade, T. Acid Ceramidase Expression Modulates the Sensitivity of A375 Melanoma Cells to Dacarbazine. J. Biol. Chem. 2011, 286, 28200–28209. [Google Scholar] [CrossRef]
- Caporali, S.; Alvino, E.; Lacal, P.M.; Levati, L.; Giurato, G.; Memoli, D.; Caprini, E.; Antonini Cappellini, G.C.; D’Atri, S. Targeting the PI3K/AKT/mTOR Pathway Overcomes the Stimulating Effect of Dabrafenib on the Invasive Behavior of Melanoma Cells with Acquired Resistance to the BRAF Inhibitor. Int. J. Oncol. 2016, 49, 1164–1174. [Google Scholar] [CrossRef]
- Keenan, J.C.; Ryan, P.K.; Medford, A.J.; Spring, L.M.; Bardia, A. Management of Metastatic Triple-Negative Breast Cancer: Focus on Targeted Therapies. Touchreviews Oncol. Haematol. 2022, 18, 98–102. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Quach, N.D.; Jenkins, A.; Dabke, I.; Somanath, P.R.; Cummings, B.S. Effect of P21-Activated Kinase 1 (PAK-1) Inhibition on Cancer Cell Growth, Migration, and Invasion. Pharmacol. Res. Perspect. 2019, 7, e00518. [Google Scholar] [CrossRef]
- Zhang, L.; Luga, V.; Armitage, S.K.; Musiol, M.; Won, A.; Yip, C.M.; Plotnikov, S.V.; Wrana, J.L. A Lateral Signalling Pathway Coordinates Shape Volatility during Cell Migration. Nat. Commun. 2016, 7, 11714. [Google Scholar] [CrossRef] [PubMed]
- Bytautaite, M.; Petrikaite, V. Comparative Study of Lipophilic Statin Activity in 2D and 3D in Vitro Models of Human Breast Cancer Cell Lines MDA-MB-231 and MCF-7. Onco Targets Ther. 2020, 13, 13201–13209. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D Tumor Spheroid Models for in Vitro Therapeutic Screening: A Systematic Approach to Enhance the Biological Relevance of Data Obtained. Sci. Rep. 2016, 6, 19103. [Google Scholar] [CrossRef] [PubMed]
- Von Felbert, V.; Bauerschlag, D.; Maass, N.; Bräutigam, K.; Meinhold-Heerlein, I.; Woitok, M.; Barth, S.; Hussain, A.F. A Specific Photoimmunotheranostics Agent to Detect and Eliminate Skin Cancer Cells Expressing EGFR. J. Cancer Res. Clin. Oncol. 2016, 142, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, A.; Gravina, G.L.; Rucci, N.; Millimaggi, D.; Festuccia, C.; Muzi, P.; Teti, A.; Vicentini, C.; Bologna, M. Suppression of EGF-R Signaling Reduces the Incidence of Prostate Cancer Metastasis in Nude Mice. Endocr.-Relat. Cancer 2006, 13, 197–210. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Tortora, G.; D’Armiento, F.P.; De Rosa, G.; Staibano, S.; Autorino, R.; D’Armiento, M.; De Laurentiis, M.; De Placido, S.; Catalano, G.; et al. Expression of Epidermal Growth Factor Receptor Correlates with Disease Relapse and Progression to Androgen-Independence in Human Prostate Cancer. Clin. Cancer Res. 2002, 8, 3438–3444. [Google Scholar]
- Carrión-Salip, D.; Panosa, C.; Menendez, J.A.; Puig, T.; Oliveras, G.; Pandiella, A.; De Llorens, R.; Massaguer, A. Androgen-Independent Prostate Cancer Cells Circumvent EGFR Inhibition by Overexpression of Alternative HER Receptors and Ligands. Int. J. Oncol. 2012, 41, 1128–1138. [Google Scholar] [CrossRef]
- Fontoura, R.; Daroit, D.J.; Corrêa, A.P.F.; Moresco, K.S.; Santi, L.; Beys-da-Silva, W.O.; Yates, J.R.; Moreira, J.C.F.; Brandelli, A. Characterization of a Novel Antioxidant Peptide from Feather Keratin Hydrolysates. New Biotechnol. 2019, 49, 71–76. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Mar, J.M.; Da Silva, L.S.; Moreira, W.P.; Biondo, M.M.; Pontes, F.L.D.; Campos, F.R.; Kinupp, V.F.; Campelo, P.H.; Sanches, E.A.; Bezerra, J.D.A. Edible Flowers from Theobroma Speciosum: Aqueous Extract Rich in Antioxidant Compounds. Food Chem. 2021, 356, 129723. [Google Scholar] [CrossRef]
- Global and Regional Protocatechuic Acid (CAS 99-50-3) Market Details Research Report 2021–2026. Available online: http://Globalmarketmonitor.com (accessed on 11 April 2023).
- Lin, H.; Chen, J.; Huang, C.; Wang, C. Apoptotic Effect of 3,4-dihydroxybenzoic Acid on Human Gastric Carcinoma Cells Involving JNK/P38 MAPK Signaling Activation. Int. J. Cancer 2007, 120, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Labib, M.; Görtz, J.; Brüsseler, C.; Kallscheuer, N.; Gätgens, J.; Jupke, A.; Marienhagen, J.; Noack, S. Metabolic and Process Engineering for Microbial Production of Protocatechuate with Corynebacterium Glutamicum. Biotechnol. Bioeng. 2021, 118, 4414–4427. [Google Scholar] [CrossRef] [PubMed]
- Grigalius, I.; Petrikaite, V. Relationship between Antioxidant and Anticancer Activity of Trihydroxyflavones. Molecules 2017, 22, 2169. [Google Scholar] [CrossRef] [PubMed]
- Daunys, S.; Matulis, D.; Petrikaitė, V. Synergistic Activity of Hsp90 Inhibitors and Anticancer Agents in Pancreatic Cancer Cell Cultures. Sci. Rep. 2019, 9, 16177. [Google Scholar] [CrossRef]
- Balandis, B.; Mickevičius, V.; Petrikaitė, V. Exploration of Benzenesulfonamide-Bearing Imidazole Derivatives Activity in Triple-Negative Breast Cancer and Melanoma 2D and 3D Cell Cultures. Pharmaceuticals 2021, 14, 1158. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubrickė, I.; Jonuškienė, I.; Kantminienė, K.; Tumosienė, I.; Petrikaitė, V. Synthesis and In Vitro Evaluation as Potential Anticancer and Antioxidant Agents of Diphenylamine-Pyrrolidin-2-one-Hydrazone Derivatives. Int. J. Mol. Sci. 2023, 24, 16804. https://doi.org/10.3390/ijms242316804
Zubrickė I, Jonuškienė I, Kantminienė K, Tumosienė I, Petrikaitė V. Synthesis and In Vitro Evaluation as Potential Anticancer and Antioxidant Agents of Diphenylamine-Pyrrolidin-2-one-Hydrazone Derivatives. International Journal of Molecular Sciences. 2023; 24(23):16804. https://doi.org/10.3390/ijms242316804
Chicago/Turabian StyleZubrickė, Irma, Ilona Jonuškienė, Kristina Kantminienė, Ingrida Tumosienė, and Vilma Petrikaitė. 2023. "Synthesis and In Vitro Evaluation as Potential Anticancer and Antioxidant Agents of Diphenylamine-Pyrrolidin-2-one-Hydrazone Derivatives" International Journal of Molecular Sciences 24, no. 23: 16804. https://doi.org/10.3390/ijms242316804