New Flexible Analogues of 8-Aza-7-deazapurine Nucleosides as Potential Antibacterial Agents
Abstract
:1. Introduction
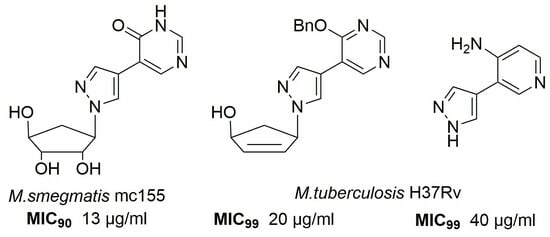
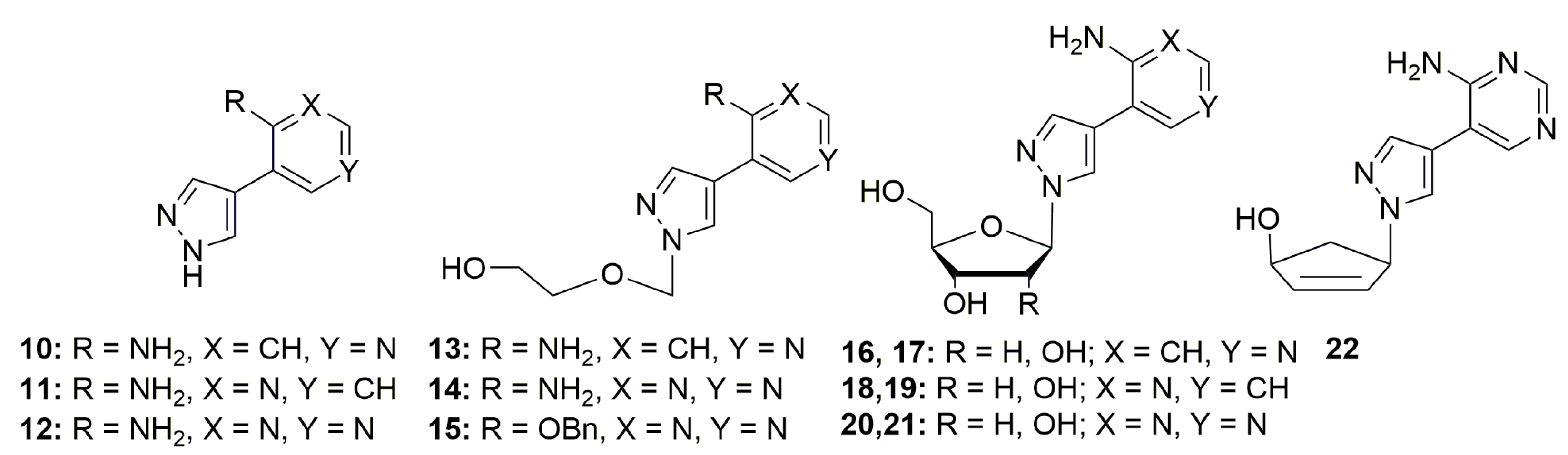
2. Results
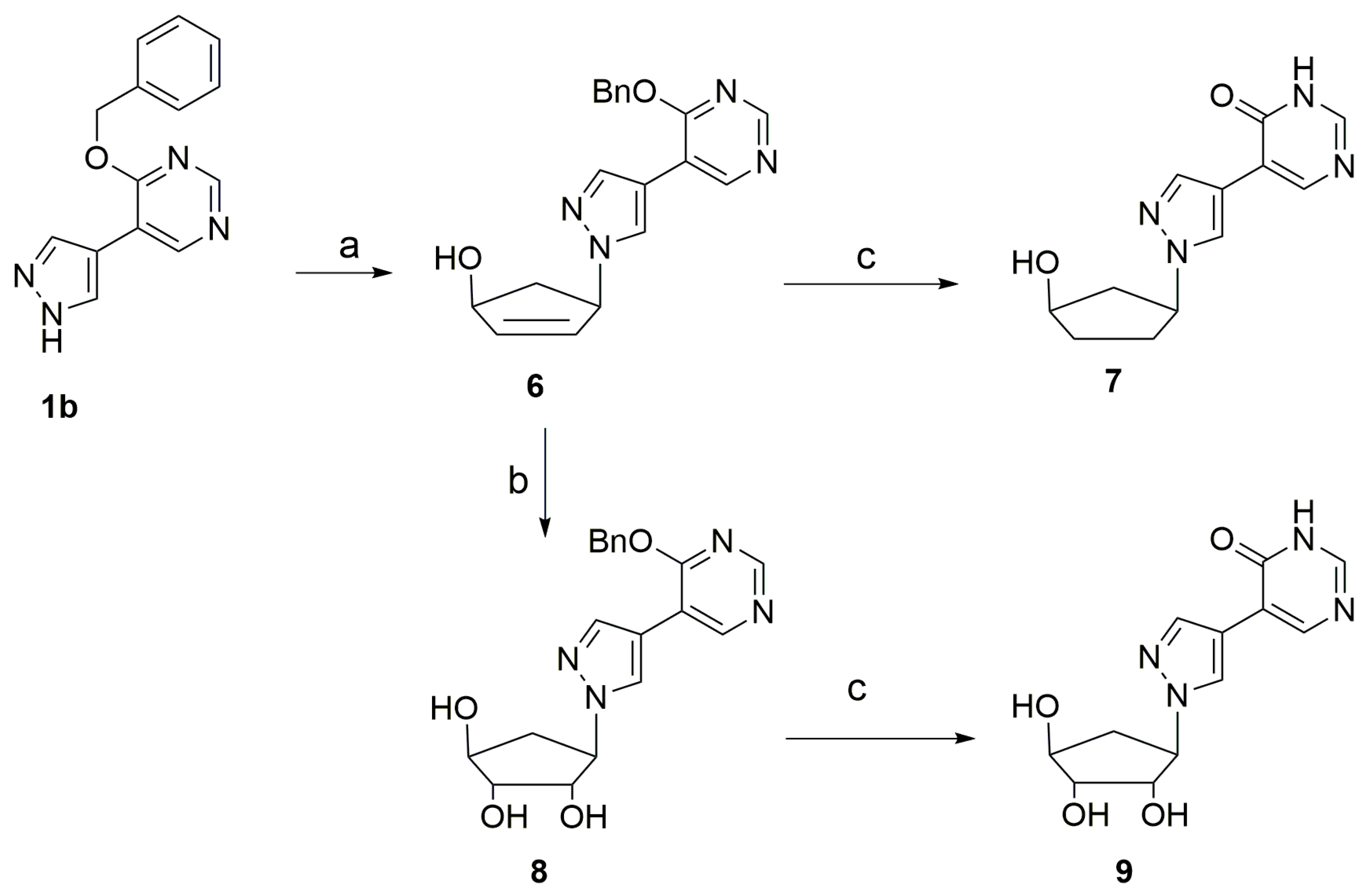
2.1. Synthesis of the Target Compounds
2.2. Antimicrobial Studies
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.2. General Procedure for the Enzymatic Synthesis of Fleximer Nucleosides
4.3. The Palladium-on-Carbon (Pd/C)-Catalyzed Hydrogenative Deprotection of the N-Benzyl-Protecting Group
4.4. Chemical Synthesis of Fleximer Nucleosides
4.5. Antimicrobial Activity
4.6. Antituberculosis Tests
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cook, M.A.; Wright, G.D. The past, present, and future of antibiotics. Sci. Transl. Med. 2022, 14, eabo7793. [Google Scholar] [CrossRef]
- Lewis, K. The Science of Antibiotic Discovery. Cell 2020, 181, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Khawbung, J.L.; Nath, D.; Chakraborty, S. Drug resistant Tuberculosis: A review. Comp. Immunol. Microbiol. Infect. Dis. 2021, 74, 101574. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/ru/news-room/fact-sheets/detail/tuberculosis (accessed on 17 October 2023).
- Mencarini, J.; Spinicci, M.; Zammarchi, L.; Bartoloni, A. Tuberculosis in the European Region. Curr. Trop. Med. Rep. 2023, 10, 88–93. [Google Scholar] [CrossRef]
- Li, J.; Lai, S.; Gao, G.F.; Shi, W. The emergence, genomic diversity and global spread of SARS-CoV-2. Nature 2021, 600, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Abushaheen, M.A.; Fatani, A.J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D.D.; Jhugroo, C.; Vellappally, S.; et al. Antimicrobial resistance, mechanisms and its clinical significance. Dis. Mon. 2020, 66, 100971. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tong, Z.; Shi, J.; Li, R.; Upton, M.; Wang, Z. Drug repurposing for next-generation combination therapies against multidrug-resistant bacteria. Theranostics 2021, 11, 4910–4928. [Google Scholar] [CrossRef]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef]
- Geraghty, R.J.; Aliota, M.T.; Bonnac, L.F. Broad-Spectrum Antiviral Strategies and Nucleoside Analogues. Viruses 2021, 13, 667. [Google Scholar] [CrossRef]
- Seley-Radtke, K.L.; Yates, M.K. The evolution of nucleoside analogue antivirals: A review for chemists and non-chemists. Part 1: Early structural modifications to the nucleoside scaffold. Antivir. Res. 2018, 154, 66–86. [Google Scholar] [CrossRef]
- Yates, M.K.; Seley-Radtke, K.L. The evolution of antiviral nucleoside analogues: A review for chemists and non-chemists. Part II: Complex modifications to the nucleoside scaffold. Antivir. Res. 2019, 162, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Galmarini, C.M.; Mackey, J.R.; Dumontet, C. Nucleoside analogues and nucleobases in cancer treatment. Lancet Oncol. 2002, 3, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.M.; Lamont, I.L. Nucleoside Analogues as Antibacterial Agents. Front. Microbiol. 2019, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Antiretroviral drugs. Curr. Opin. Pharmacol. 2010, 10, 507–515. [Google Scholar] [CrossRef]
- Johar, M.; Manning, T.; Tse, C.; Desroches, N.; Agrawal, B.; Kunimoto, D.Y.; Kumar, R. Growth inhibition of Mycobacterium bovis, Mycobacterium tuberculosis and Mycobacterium avium in vitro: Effect of 1-beta-D-2′-arabinofuranosyl and 1-(2′-deoxy-2′-fluoro-beta-D-2′-ribofuranosyl) pyrimidine nucleoside analogs. J. Med. Chem. 2007, 50, 3696–3705. [Google Scholar] [CrossRef]
- Rai, D.; Johar, M.; Srivastav, N.C.; Manning, T.; Agrawal, B.; Kunimoto, D.Y.; Kumar, R. Inhibition of Mycobacterium tuberculosis, Mycobacterium bovis, and Mycobacterium avium by novel dideoxy nucleosides. J. Med. Chem. 2007, 50, 4766–4774. [Google Scholar] [CrossRef]
- Shakya, N.; Srivastav, N.C.; Desroches, N.; Agrawal, B.; Kunimoto, D.Y.; Kumar, R. 3′-bromo analogues of pyrimidine nucleosides as a new class of potent inhibitors of Mycobacterium tuberculosis. J. Med. Chem. 2010, 53, 4130–4140. [Google Scholar] [CrossRef]
- Srivastav, N.C.; Rai, D.; Tse, C.; Agrawal, B.; Kunimoto, D.Y.; Kumar, R. Inhibition of mycobacterial replication by pyrimidines possessing various C-5 functionalities and related 2′-deoxynucleoside analogues using in vitro and in vivo models. J. Med. Chem. 2010, 53, 6180–6187. [Google Scholar] [CrossRef]
- Matyugina, E.; Khandazhinskaya, A.; Chernousova, L.; Andreevskaya, S.; Smirnova, T.; Chizhov, A.; Karpenko, I.; Kochetkov, S.; Alexandrova, L. The synthesis and antituberculosis activity of 5′-nor carbocyclic uracil derivatives. Bioorg. Med. Chem. 2012, 20, 6680–6686. [Google Scholar] [CrossRef]
- Matyugina, E.; Novikov, M.; Babkov, D.; Ozerov, A.; Chernousova, L.; Andreevskaya, S.; Smirnova, T.; Karpenko, I.; Chizhov, A.; Murthu, P.; et al. 5-Arylaminouracil Derivatives: New Inhibitors of Mycobacterium tuberculosis. Chem. Biol. Drug Des. 2015, 86, 1387–1396. [Google Scholar] [CrossRef]
- Shakya, N.; Srivastav, N.C.; Bhavanam, S.; Tse, C.; Desroches, N.; Agrawal, B.; Kunimoto, D.Y.; Kumar, R. Discovery of novel 5-(ethyl or hydroxymethyl) analogs of 2′-‘up’ fluoro (or hydroxyl) pyrimidine nucleosides as a new class of Mycobacterium tuberculosis, Mycobacterium bovis and Mycobacterium avium inhibitors. Bioorg. Med. Chem. 2012, 20, 4088–4097. [Google Scholar] [CrossRef]
- Srivastav, N.C.; Shakya, N.; Bhavanam, S.; Agrawal, A.; Tse, C.; Desroches, N.; Kunimoto, D.Y.; Kumar, R. Antimycobacterial activities of 5-alkyl (or halo)-3′-substituted pyrimidine nucleoside analogs. Bioorg. Med. Chem. Lett. 2012, 22, 1091–1094. [Google Scholar] [CrossRef]
- Garg, S.; Shakya, N.; Srivastav, N.C.; Agrawal, B.; Kunimoto, D.Y.; Kumar, R. Investigation of C-5 alkynyl (alkynyloxy or hydroxymethyl) and/or N-3 propynyl substituted pyrimidine nucleoside analogs as a new class of antimicrobial agents. Bioorg. Med. Chem. 2016, 24, 5521–5533. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Tiwari, D.; Wilson, D.J.; Seiler, C.L.; Schnappinger, D.; Aldrich, C.C. Bisubstrate Inhibitors of Biotin Protein Ligase in Mycobacterium tuberculosis Resistant to Cyclonucleoside Formation. ACS Med. Chem. Lett. 2013, 4, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Baran, M.; Grimes, K.D.; Sibbald, P.A.; Fu, P.; Boshoff, H.I.M.; Wilson, D.J.; Aldrich, C.C. Development of small-molecule inhibitors of fatty acyl-AMP and fatty acyl-CoA ligases in Mycobacterium tuberculosis. Eur. J. Med. Chem. 2020, 201, 112408. [Google Scholar] [CrossRef] [PubMed]
- Khoje, A.D.; Kulendrn, A.; Charnock, C.; Wan, B.; Franzblau, S.; Gundersen, L.L. Synthesis of non-purine analogs of 6-aryl-9-benzylpurines, and their antimycobacterial activities. Compounds modified in the imidazole ring. Bioorg. Med. Chem. 2010, 18, 7274–7282. [Google Scholar] [CrossRef] [PubMed]
- Read, M.L.; Braendvang, M.; Miranda, P.O.; Gundersen, L.L. Synthesis and biological evaluation of pyrimidine analogs of antimycobacterial purines. Bioorg. Med. Chem. 2010, 18, 3885–3897. [Google Scholar] [CrossRef]
- Khoje, A.D.; Charnock, C.; Wan, B.; Franzblau, S.; Gundersen, L.L. Synthesis and antimycobacterial activities of non-purine analogs of 6-aryl-9-benzylpurines: Imidazopyridines, pyrrolopyridines, benzimidazoles, and indoles. Bioorg. Med. Chem. 2011, 19, 3483–3491. [Google Scholar] [CrossRef]
- Seley-Radtke, K. Flexibility-Not just for yoga anymore! Antivir. Chem. Chemother. 2018, 26, 2040206618756788. [Google Scholar] [CrossRef]
- Chudinov, M.V. Nucleoside Analogs with Fleximer Nucleobase. Chem. Heterocycl. Compd. 2020, 56, 636–643. [Google Scholar] [CrossRef]
- Khandazhinskaya, A.; Eletskaya, B.; Fateev, I.; Kharitonova, M.; Konstantinova, I.; Barai, V.; Azhayev, A.; Hyvonen, M.T.; Keinanen, T.A.; Kochetkov, S.; et al. Novel fleximer pyrazole-containing adenosine analogues: Chemical, enzymatic and highly efficient biotechnological synthesis. Org. Biomol. Chem. 2021, 19, 7379–7389. [Google Scholar] [CrossRef] [PubMed]
- Vorbruggen, H.; Ruh-Polenz, C. Organic Reactions; Paquette, L.A., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2000; Volume 55, pp. 1–394. [Google Scholar]
- Korach, M.; Nielsen, D.R.; Rideout, W.H. Organic Syntheses, Coll.; Dauben, W.G., Ashcraft, C., Eds.; Wiley: Hoboken, NJ, USA, 1973; Volume 5, p. 414. [Google Scholar]
- Trost, B.M.; Kuo, G.H.; Benneche, T. A Transition-Metal-Controlled Synthesis of (+/−)-Aristeromycin and (+/−)-2′,3′-Diepi-Aristeromycin—An Unusual Directive Effect in Hydroxylations. J. Am. Chem. Soc. 1988, 110, 621–622. [Google Scholar] [CrossRef]
- Khandazhinskaya, A.; Fateev, I.; Eletskaya, B.; Maslova, A.; Konstantinova, I.; Seley-Radtke, K.; Kochetkov, S.; Matyugina, E. Design and Synthesis of New Modified Flexible Purine Bases as Potential Inhibitors of Human PNP. Molecules 2023, 28, 928. [Google Scholar] [CrossRef]
- Khandazhinskaya, A.; Fateev, I.; Konstantinova, I.; Esipov, R.; Polyakov, K.; Seley-Radtke, K.; Kochetkov, S.; Matyugina, E. Synthesis of New 5′-Norcarbocyclic Aza/Deaza Purine Fleximers—Noncompetitive Inhibitors of E. coli Purine Nucleoside Phosphorylase. Front. Chem. 2022, 10, 867587. [Google Scholar] [CrossRef]
- Khandazhinskaya, A.L.; Alexandrova, L.A.; Matyugina, E.S.; Solyev, P.N.; Efremenkova, O.V.; Buckheit, K.W.; Wilkinson, M.; Buckheit, R.W., Jr.; Chernousova, L.N.; Smirnova, T.G.; et al. Novel 5′-Norcarbocyclic Pyrimidine Derivatives as Antibacterial Agents. Molecules 2018, 23, 3069. [Google Scholar] [CrossRef] [PubMed]
- Schneller, S.W. Carbocyclic nucleosides (carbanucleosides) as new therapeutic leads. Curr. Top. Med. Chem. 2002, 2, 1087–1092. [Google Scholar] [CrossRef]
- Kausar, S.; Said Khan, F.; Ishaq Mujeeb Ur Rehman, M.; Akram, M.; Riaz, M.; Rasool, G.; Hamid Khan, A.; Saleem, I.; Shamim, S.; Malik, A. A review: Mechanism of action of antiviral drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211002621. [Google Scholar] [CrossRef]
- Matyugina, E.; Petushkov, I.; Surzhikov, S.; Kezin, V.; Maslova, A.; Ivanova, O.; Smirnova, O.; Kirillov, I.; Fedyakina, I.; Kulbachinskiy, A.; et al. Nucleoside Analogs That Inhibit SARS-CoV-2 Replication by Blocking Interaction of Virus Polymerase with RNA. Int. J. Mol. Sci. 2023, 24, 3361. [Google Scholar] [CrossRef]
- Matyugina, E.S.; Valuev-Elliston, V.T.; Babkov, D.A.; Novikov, M.S.; Ivanov, A.V.; Kochetkov, S.N.; Balzarini, J.; Seley-Radtke, K.L.; Khandazinskaya, A.L. 5′-Nor carbocyclic nucleosides: Surprising nonnucleoside inhibitors of HIV-1 reverse transcriptase. MedChemComm 2013, 4, 741–748. [Google Scholar] [CrossRef]
- Matyugina, E.S.; Valuev-Elliston, V.T.; Geisman, A.N.; Novikov, M.S.; Chizhov, A.O.; Kochetkov, S.N.; Seley-Radtke, K.L.; Khandazhinskaya, A.L. Structure-activity evaluation of new uracil-based non-nucleoside inhibitors of HIV reverse tran-scriptase. MedChemComm 2013, 4, 1443–1451. [Google Scholar] [CrossRef]
- Siddiqi, S.H.; Rusch-Gerdes, S. MGIT Procedure Manual for BACTEC MGIT 960 TB System; Foundation for Innovative New Diagnostics: Geneva, Switzerland, 2006; Available online: https://www.finddx.org/wp-content/uploads/2023/02/20061101_rep_mgit_manual_FV_EN.pdf (accessed on 17 October 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khandazhinskaya, A.; Eletskaya, B.; Mironov, A.; Konstantinova, I.; Efremenkova, O.; Andreevskaya, S.; Smirnova, T.; Chernousova, L.; Kondrashova, E.; Chizhov, A.; et al. New Flexible Analogues of 8-Aza-7-deazapurine Nucleosides as Potential Antibacterial Agents. Int. J. Mol. Sci. 2023, 24, 15421. https://doi.org/10.3390/ijms242015421
Khandazhinskaya A, Eletskaya B, Mironov A, Konstantinova I, Efremenkova O, Andreevskaya S, Smirnova T, Chernousova L, Kondrashova E, Chizhov A, et al. New Flexible Analogues of 8-Aza-7-deazapurine Nucleosides as Potential Antibacterial Agents. International Journal of Molecular Sciences. 2023; 24(20):15421. https://doi.org/10.3390/ijms242015421
Chicago/Turabian StyleKhandazhinskaya, Anastasia, Barbara Eletskaya, Anton Mironov, Irina Konstantinova, Olga Efremenkova, Sofya Andreevskaya, Tatiana Smirnova, Larisa Chernousova, Evgenia Kondrashova, Alexander Chizhov, and et al. 2023. "New Flexible Analogues of 8-Aza-7-deazapurine Nucleosides as Potential Antibacterial Agents" International Journal of Molecular Sciences 24, no. 20: 15421. https://doi.org/10.3390/ijms242015421