Stem Cells Derived from Human Exfoliated Deciduous Teeth Functional Assessment: Exploring the Changes of Free Fatty Acids Composition during Cultivation
Abstract
:1. Introduction
2. Results
2.1. Growth Kinetics of SHEDs
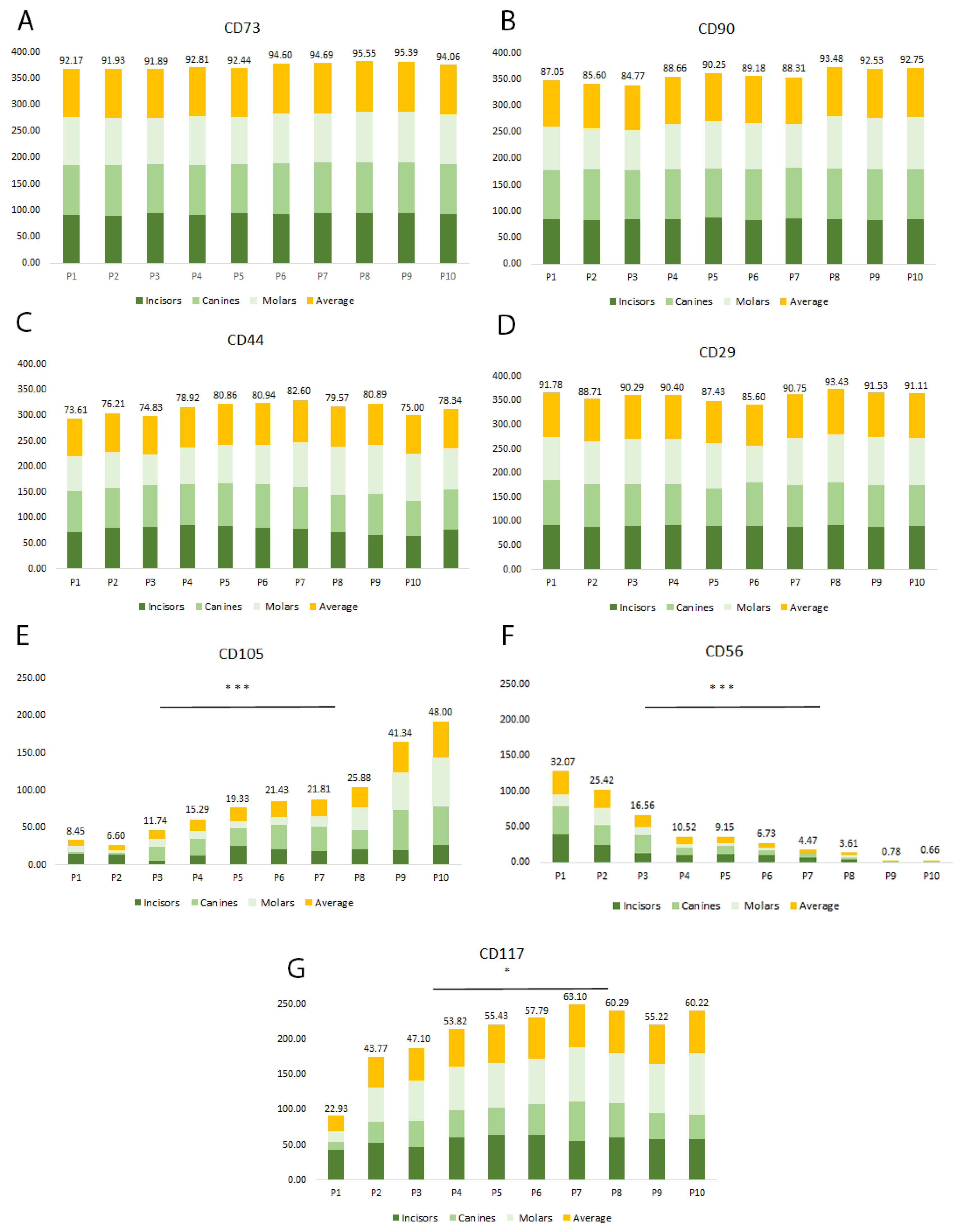
2.2. Multiparametric Immunophenotyping Analysis of SHEDs
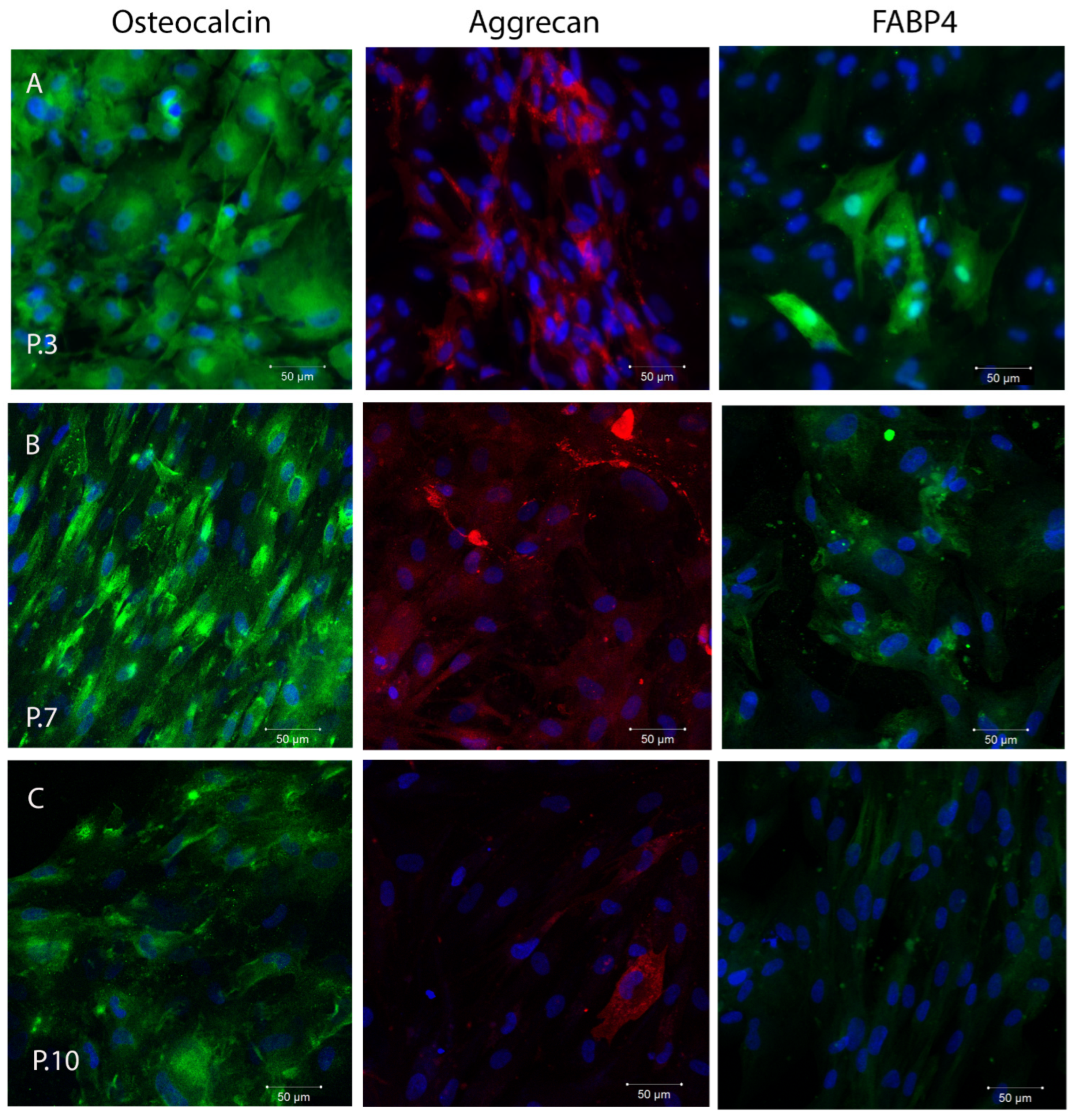
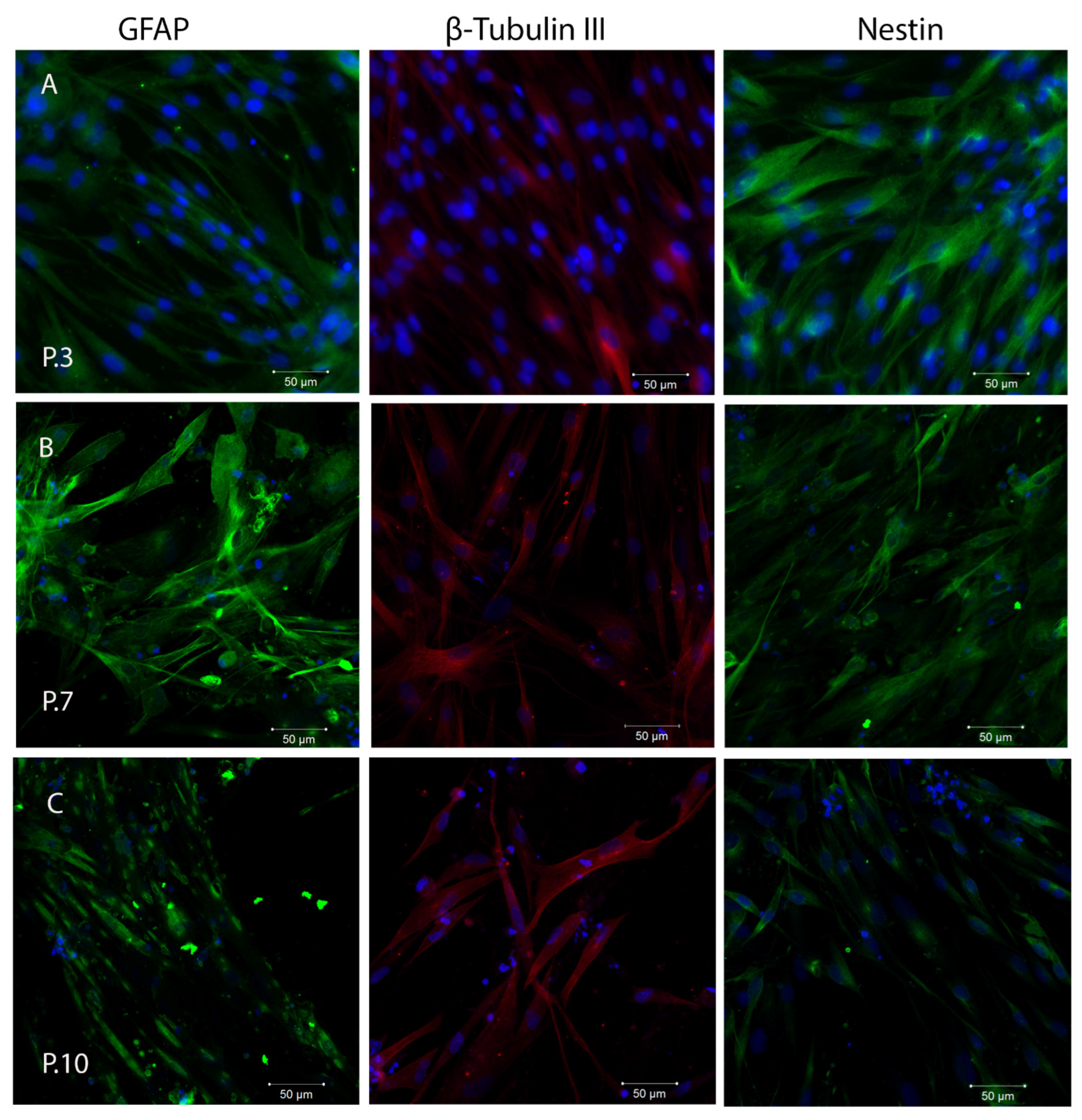
2.3. In Vitro Plasticity Assessment of SHEDs
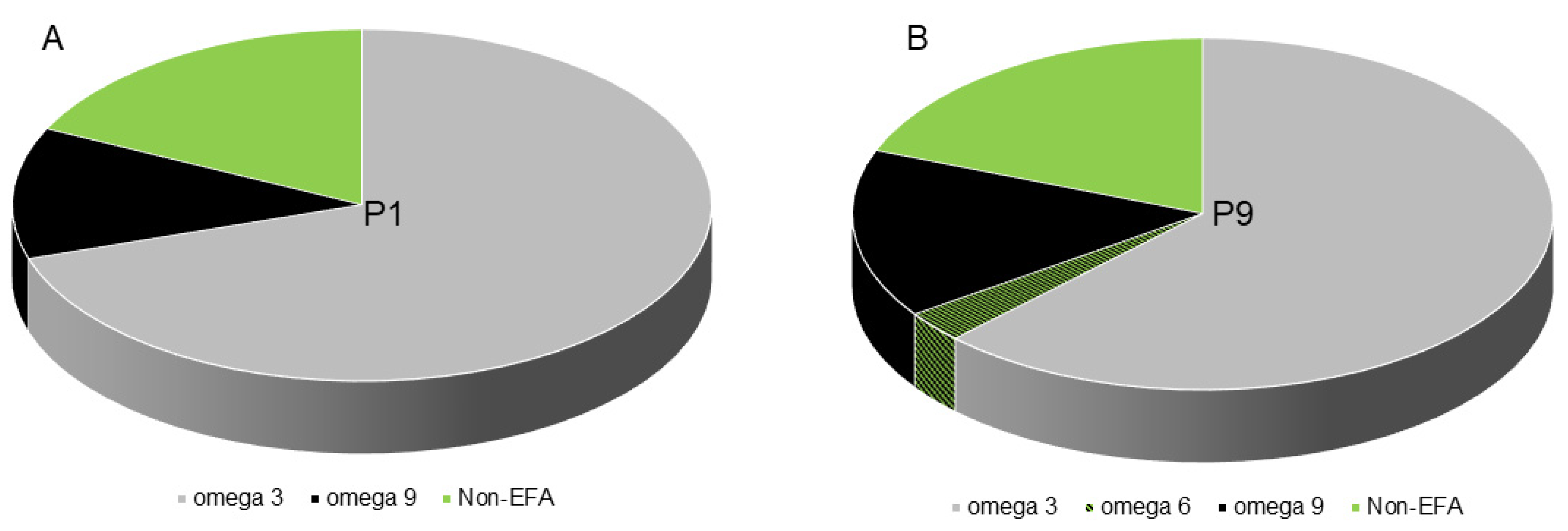
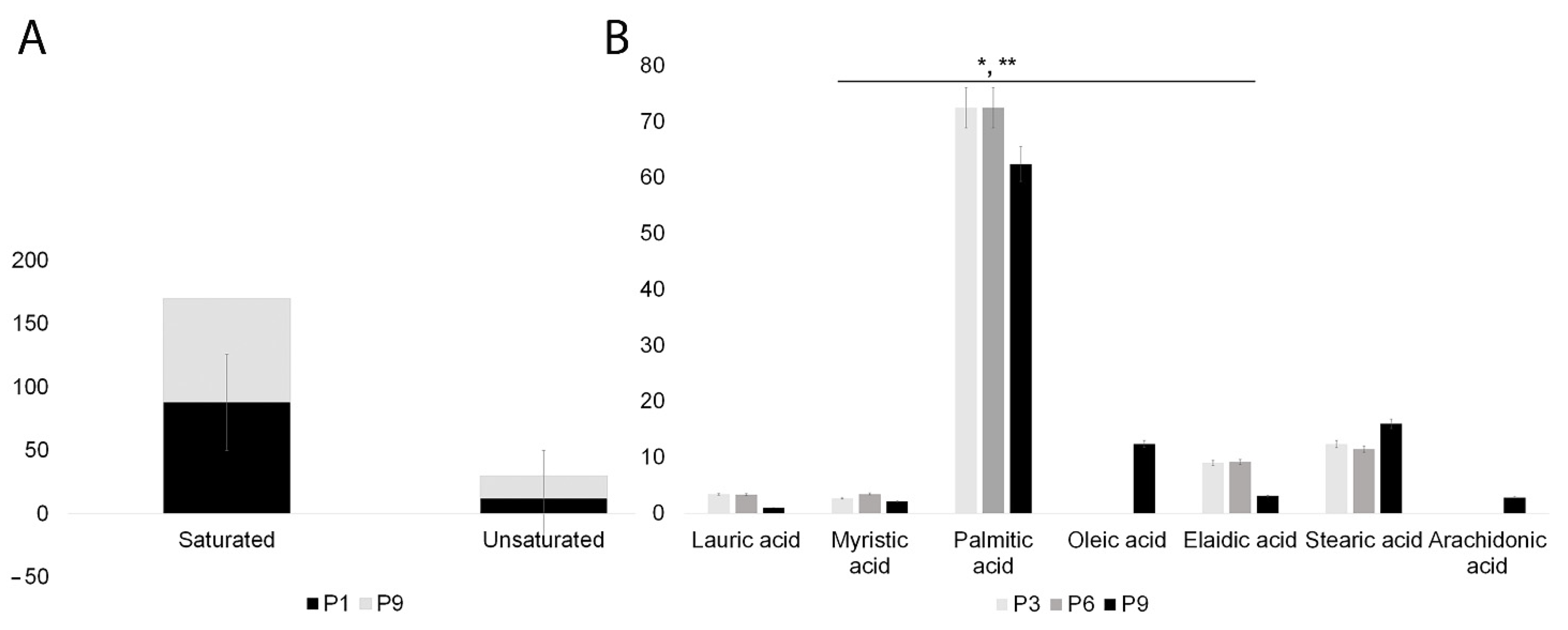
2.4. SHEDs Fatty Acids Content Assessment
2.5. Autophagy Detection
3. Discussion
4. Materials and Methods
4.1. Isolation of SHEDs and Ethical Approval
4.2. Growth Kinetics
4.3. Multiparametric Immunophenotyping of SHEDs
4.4. In Vitro Plasticity of SHEDs
4.5. SHEDs Fatty Acids Content Assessment
4.6. Autophagy Detection
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sheng, G. The developmental basis of mesenchymal stem/stromal cells (MSCs). BMC Dev. Biol. 2015, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Aithal, A.P.; Bairy, L.K.; Seetharam, N.R. Safety and therapeutic potential of human bone marrow-derived mesenchymal stromal cells in regenerative medicine. Stem Cell Investig. 2021, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Costela-Ruiz, V.J.; Melguizo-Rodríguez, L.; Bellotti, C.; Illescas-Montes, R.; Stanco, D.; Arciola, C.R.; Lucarelli, E. Different Sources of Mesenchymal Stem Cells for Tissue Regeneration: A Guide to Identifying the Most Favorable One in Orthopedics and Dentistry Applications. Int. J. Mol. Sci. 2022, 23, 6356. [Google Scholar] [CrossRef] [PubMed]
- Keating, A. Mesenchymal Stromal Cells: New Directions. Cell Stem Cell 2012, 10, 709–716. [Google Scholar] [CrossRef]
- Deszcz, I. Stem Cell-Based Therapy and Cell-Free Therapy as an Alternative Approach for Cardiac Regeneration. Stem Cells Int. 2023, 2023, 2729377. [Google Scholar] [CrossRef]
- Lei, T.; Zhang, X.; Du, H. Characteristics, Classification, and Application of Stem Cells Derived from Human Teeth. Stem Cells Int. 2021, 2021, 8886854. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Turnley, T.; Wishart, A.; Rowe, A.; Kallmeyer, K.; van Vollenstee, F.A.; Thomford, N.E.; Dandara, C.; Chopera, D.; Pepper, M.S.; et al. Fibroblast-Derived Extracellular Matrix Induces Chondrogenic Differentiation in Human Adipose-Derived Mesenchymal Stromal/Stem Cells in Vitro. Int. J. Mol. Sci. 2016, 17, 1259. [Google Scholar] [CrossRef]
- Halfon, S.; Abramov, N.; Grinblat, B.; Ginis, I. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev. 2011, 20, 53–66. [Google Scholar] [CrossRef]
- Martens, W.; Bronckaers, A.; Politis, C.; Jacobs, R.; Lambrichts, I. Dental stem cells and their promising role in neural regeneration: An update. Clin. Oral. Investig. 2013, 17, 1969–1983. [Google Scholar] [CrossRef]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Naderi, F.; Mehdiabadi, M.; Kamarehei, F. The therapeutic effects of stem cells from human exfoliated deciduous teeth on clinical diseases: A narrative review study. Am. J. Stem Cells 2022, 11, 28–36. [Google Scholar]
- O’Connor, K.C. Molecular Profiles of Cell-to-Cell Variation in the Regenerative Potential of Mesenchymal Stromal Cells. Stem Cells Int. 2019, 2019, 5924878. [Google Scholar] [CrossRef]
- Muhammad, S.A.; Nordin, N.; Hussin, P.; Mehat, M.Z.; Abu Kasim, N.H.; Fakurazi, S. Protective effects of stem cells from human exfoliated deciduous teeth derived conditioned medium on osteoarthritic chondrocytes. PLoS ONE 2020, 15, e0238449. [Google Scholar] [CrossRef]
- Luo, P.; Jiang, C.; Ji, P.; Wang, M.; Xu, J. Exosomes of stem cells from human exfoliated deciduous teeth as an anti-inflammatory agent in temporomandibular joint chondrocytes via miR-100-5p/mTOR. Stem Cell Res. Ther. 2019, 10, 216. [Google Scholar] [CrossRef]
- Jindal, L.; Bhat, N.; Vyas, D.; Thakur, K.; Neha, M.S. Stem cells from human exfoliated deciduous teeth (SHED)—Turning useless into miracle: A review article. Acta Sci. Dent. Sci. 2019, 3, 49–54. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, T.; Chen, P.; Wang, L.; Wang, J.; Wang, D.; Guo, W.; Zhou, Y.; Li, Q.; Du, H. Stem cells from human exfoliated deciduous teeth promote hair regeneration in mouse. Cell Transplant. 2021, 30, 9636897211042927. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Tsunekawa, S.; Nakamura, N.; Miura-Yura, E.; Yamada, Y.; Hayashi, Y.; Nakai-Shimoda, H.; Asano, S.; Hayami, T.; Motegi, M.; et al. Secreted Factors from Stem Cells of Human Exfoliated Deciduous Teeth Directly Activate Endothelial Cells to Promote All Processes of Angiogenesis. Cells 2020, 9, 2385. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, X.; Wang, C.; Liu, Y.; Liu, X.; Fan, Y.; Zhang, X. Stem cells from human exfoliated deciduous teeth attenuate trigeminal neuralgia in rats. Stem Cells Int. 2021, 2021, 8819884. [Google Scholar] [CrossRef]
- Chen, Y.R.; Lai, P.L.; Chien, Y.; Lee, P.H.; Lai, Y.H.; Ma, H.I.; Shiau, C.-Y.; Wang, K.-C. Improvement of impaired motor functions by human dental exfoliated deciduous teeth stem cell-derived factors in a rat model of Parkinson’s disease. Int. J. Mol. Sci. 2020, 21, 3807. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiao, X.; Song, J.; Sui, B.; Guo, Z.; Zhao, Y.; Li, J.; Shi, S.; Huang, Q. Therapeutic potential of stem cells from human exfoliated deciduous teeth infusion into patients with type 2 diabetes depends on basal lipid levels and islet function. Stem Cells Transl. Med. 2021, 10, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Kawase-Koga, Y.; Fujii, Y.; Yamakawa, D.; Sato, M.; Chikazu, D. Identification of neurospheres generated from human dental pulp stem cells in xeno-/serum-free conditions. Regen. Ther. 2020, 14, 128–135. [Google Scholar]
- Rosa, V.; Dubey, N.; Islam, I.; Min, K.-S.; Nör, J.E. Pluripotency of Stem Cells from Human Exfoliated Deciduous Teeth for Tissue Engineering. Stem Cells Int. 2016, 2016, 5957806. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- Bojic, S.; Volarevic, V.; Ljujic, B.; Stojkovic, M. Dental stem cells-characteristics and potential. Histol. Histopathol. 2014, 29, 699–706. [Google Scholar]
- Bhandi, S.; Alkahtani, A.; Reda, R.; Mashyakhy, M.; Boreak, N.; Maganur, P.C.; Vishwanathaiah, S.; Mehta, D.; Vyas, N.; Patil, V.; et al. Parathyroid Hormone Secretion and Receptor Expression Determine the Age-Related Degree of Osteogenic Differentiation in Dental Pulp Stem Cells. J. Pers. Med. 2021, 11, 349. [Google Scholar] [CrossRef]
- Hovorakova, M.; Lesot, H.; Peterka, M.; Peterkova, R. Early development of the human dentition revisited. J. Anat. 2018, 233, 135–145. [Google Scholar] [CrossRef]
- Szlasa, W.; Zendran, I.; Zalesińska, A.; Tarek, M.; Kulbacka, J. Lipid composition of the cancer cell membrane. J. Bioenerg. Biomembr. 2020, 52, 321–342. [Google Scholar] [CrossRef]
- Meissen, J.K.; Yuen, B.T.K.; Kind, T.; Riggs, J.W.; Barupal, D.K.; Knoepfler, P.S.; Fiehn, O. Induced Pluripotent Stem Cells Show Metabolomic Differences to Embryonic Stem Cells in Polyunsaturated Phosphatidylcholines and Primary Metabolism. PLoS ONE 2012, 7, e46770. [Google Scholar] [CrossRef]
- Atilla-Gokcumen, G.E.; Muro, E.; Relat-Goberna, J.; Sasse, S.; Bedigian, A.; Coughlin, M.L.; Garcia-Manyes, S.; Eggert, U.S. Dividing Cells Regulate Their Lipid Composition and Localization. Cell 2014, 156, 428–439. [Google Scholar] [CrossRef]
- Symons, J.L.; Cho, K.-J.; Chang, J.T.; Du, G.; Waxham, M.N.; Hancock, J.F.; Levental, I.; Levental, K.R. Lipidomic Atlas of Mammalian Cell Membranes Reveals Hierarchical Variation Induced by Culture Conditions, Subcellular Membranes, and Cell Lineages. Soft Matter 2021, 17, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Uzuner, F.D.; Kaygısız, E.; Darendeliler, N. Defining Dental Age for Chronological Age, Post Mortem Examination and Autopsy—Current Issues From Death to Laboratory Analysis. InTechOpen 2017, 6, 71679–71699. [Google Scholar]
- Proffit, W.; Fields, H.W.; Sarver, D.M. Contemporary Orthodontics, 5th ed.; Elsevier/Mosby: St. Louis, MO, USA, 2012; pp. 66–88. [Google Scholar]
- Fan, W.; Li, J.; Wang, Y.; Pan, J.; Li, S.; Zhu, L.; Guo, C.; Yan, Z. CD105 promotes chondrogenesis of synovium-derived mesenchymal stem cells through Smad2 signaling. Biochem. Biophys. Res. Commun. 2016, 474, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Ishiy, F.A.A.; Fanganiello, R.D.; Kobayashi, G.S.; Kague, E.; Kuriki, P.S.; Passos-Bueno, M.R. CD105 is regulated by hsa-miR-1287 and its expression is inversely correlated with osteopotential in SHED. Bone 2018, 106, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, M.; Vitor, L.; Lopes, N.M.; Neto, N.L.; Dionísio, T.J.; Oliveira, R.C.; Santos, C.F.; Machado, M.A.; Oliveira, T.M. Angiogenic protein synthesis after photobiomodulation therapy on SHED: A preliminary study. Lasers Med. Sci. 2020, 35, 1909–1918. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, Q.; Yang, T.; Qi, Y.; Fu, M.; Yang, X.; Qiao, L.; Ling, Q.; Liu, S.; Zhao, Y. Comparative characterization of SHED and DPSCs during extended cultivation in vitro. Mol. Med. Rep. 2018, 17, 6551–6559. [Google Scholar] [CrossRef]
- Bhandary, M.; Rao, S.; Shetty, A.V.; Kumar, B.M.; Hegde, A.M.; Chhabra, R. Comparison of stem cells from human exfoliated deciduous posterior teeth with varying levels of root resorption. Stem Cell Investig. 2021, 8, 15. [Google Scholar] [CrossRef]
- Naz, S.; Khan, F.R.; Khan, I.; Zohra, R.R.; Salim, A.; Mohammed, N.; Ahmad, T. Comparative analysis of dental pulp stem cells and stem cells from human exfoliated teeth in terms of growth kinetics, immunophenotype, self-renewal and multi lineage differentiation potential for future perspective of calcified tissue regeneration. Pak. J. Med. Sci. 2022, 38, 1228–1237. [Google Scholar] [CrossRef]
- Berezin, V.; Bock, E.; Poulsen, F.M. The neural cell adhesion molecule NCAM. Curr. Opin. Drug Discov. Dev. 2000, 3, 605–609. [Google Scholar]
- Obara, N.; Takeda, M. Expression of neural cell adhesion molecule (NCAM) during the first molar development in the mouse. Anat. Embryol. 1993, 187, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Degistirici, O.; Jaquiery, C.; Schönebeck, B.; Siemonsmeier, J.; Götz, W.; Martin, I.; Thie, M. Defining properties of neural crest-derived progenitor cells from the apex of human developing tooth. Tissue Eng. Part A 2008, 14, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Battula, V.L.; Treml, S.; Bareiss, P.M.; Gieseke, F.; Roelofs, H.; Zwart, P.; Muller, I.; Schewe, B.; Skutella, T.; Fibbe, W.E.; et al. Isolation of functionally distinct mesenchymal stem cell subsets using antibodies against CD56, CD271, and mesenchymal stem cell antigen-1. Haematologica 2009, 94, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Alipour, R.; Sadeghi, F.; Hashemi-Beni, B.; Zarkesh-Esfahani, S.H.; Heydari, F.; Mousavi, S.B.; Adib, M.; Narimani, M.; Esmaeili, N. Phenotypic characterizations and comparison of adult dental stem cells with adipose-derived stem cells. Int. J. Prev. Med. 2010, 1, 164–171. [Google Scholar] [PubMed]
- Gagari, E.; Rand, M.K.; Tayari, L.; Vastardis, H.; Sharma, P.; Hauschka, P.V.; Damoulis, P.D. Expression of stem cell factor and its receptor, c-kit, in human oral mesenchymal cells. Eur. J. Oral. Sci. 2006, 114, 409–415. [Google Scholar] [CrossRef]
- Karaöz, E.; Doğan, B.N.; Aksoy, A.; Gacar, G.; Akyüz, S.; Ayhan, S.; Genç, Z.S.; Yürüker, S.; Duruksu, G.; Demircan, P.Ç.; et al. Isolation and in vitro characterization of dental pulp stem cells from natal teeth. Histochem. Cell Biol. 2010, 133, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Vasandan, A.B.; Shankar, S.R.; Prasad, P.; Sowmya, J.V.; Bhonde, R.R.; Jyothi, P.S. Functional differences in mesenchymal stromal cells from human dental pulp and periodontal ligament. J. Cell Mol. Med. 2014, 18, 344–354. [Google Scholar] [CrossRef]
- Sandra, F.; Sudiono, J.; Binartha, C.T.O.; Chouw, A.; Djamil, M.S. Growth and Osteogenic Differentiation of CD117+Dental Pulp and Periodontal Ligament Cells. Indones. Biomed. J. 2017, 9, 78–83. [Google Scholar] [CrossRef]
- Pan, S.; Dangaria, S.; Gopinathan, G.; Yan, X.; Lu, X.; Kolokythas, A.; Niu, Y.; Luan, X. SCF promotes dental pulp progenitor migration, neovascularization, and collagen remodeling-potential applications as a homing factor in dental pulp regeneration. Stem Cell Rev. 2013, 9, 655–667. [Google Scholar] [CrossRef]
- Suphanantachat, S.; Iwata, T.; Ishihara, J.; Yamato, M.; Okano, T.; Izumi, Y. A role for c-Kit in the maintenance of undifferentiated human mesenchymal stromal cells. Biomaterials 2014, 35, 3618–3626. [Google Scholar] [CrossRef]
- Michalczyk, K.; Ziman, M. Nestin structure and predicted function in cellular cytoskeletal organisation. Histol. Histopathol. 2005, 20, 665–671. [Google Scholar] [PubMed]
- Chai, Y.; Jiang, X.; Ito, Y.; Bringas, P.; Han, J.; Rowitch, D.H.; Soriano, P.; McMahon, A.P.; Sucov, H.M. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 2000, 127, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Kilpinen, L.; Tigistu-Sahle, F.; Oja, S.; Greco, D.; Parmar, A.; Saavalainen, P.; Nikkilä, J.; Korhonen, M.; Lehenkari, P.; Käkelä, R.; et al. Aging bone marrow mesenchymal stromal cells have altered membrane glycerophospholipid composition and functionality. J. Lipid Res. 2013, 54, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Sampath, H.; Ntambi, J.M. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu. Rev. Nutr. 2005, 25, 317–340. [Google Scholar] [CrossRef] [PubMed]
- Tallima, H.; Salah, M.; El-Ridi, R. In vitro and in vivo effects of unsaturated fatty acids on Schistosoma mansoni and S. haematobium lung-stage larvae. J. Parasitol. 2005, 91, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Hyde, C.A.; Missailidis, S. Inhibition of arachidonic acid metabolism and its implication on cell proliferation and tumour-angiogenesis. Int. Immunopharmacol. 2009, 9, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef]
- Wang, D.; Liu, N.; Xie, Y.; Song, B.; Kong, S.; Sun, X. Different culture method changing CD105 expression in amniotic fluid MSCs without affecting differentiation ability or immune function. J. Cell Mol. Med. 2020, 24, 4212–4222. [Google Scholar] [CrossRef]
- Wang, D.; Chen, R.; Zhong, X.; Yong, F.Y.; Lai, W.; Sun, X. Levels of CD105+ cells increase and cell proliferation decreases during S-phase arrest of amniotic fluid cells in long-term culture. Exp. Ther. Med. 2014, 8, 1604–1610. [Google Scholar] [CrossRef]
- Ducret, M.; Fabre, H.; Degoul, O.; Atzeni, G.; McGuckin, C.; Forraz, N.; Mallein-Gerin, F.; Perrier-Groult, E.; Alliot-Licht, B.; Farges, J.C. Immunophenotyping Reveals the Diversity of Human Dental Pulp Mesenchymal Stromal Cells In vivo and Their Evolution upon In vitro Amplification. Front. Physiol. 2016, 7, 512. [Google Scholar] [CrossRef]
- Coller, H.A. The paradox of metabolism in quiescent stem cells. FEBS Lett. 2019, 593, 2817–2839. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, C.; Nantakeeratipat, T.; Murakami, S. Energy Metabolism in Osteogenic Differentiation and Reprogramming: A Possible Future Strategy for Periodontal Regeneration. Front. Dent. Med. 2022, 3, 815140. [Google Scholar] [CrossRef]
- Wong, E.V. Cells. Molecules and mechanism—Fatty Acids; Axolotl Academia Publishing: Louisville, KY, USA, 2009; pp. 29–30. [Google Scholar]
- Chandel, N.S. Lipid Metabolism. Cold Spring Harb. Perspect. Biol. 2021, 13, a040576. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, Y.; Wang, H.; Bai, Y.; Zhao, J.; Zhang, X.; Liang, L.; Chen, Y.; Ye, C.; Li, Y.; et al. Integrated Lipidomics and Transcriptomics Characterization upon Aging-Related Changes of Lipid Species and Pathways in Human Bone Marrow Mesenchymal Stem Cells. J. Proteome Res. 2019, 18, 2065–2077. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Influence of polyunsaturated fatty acids and their metabolites on stem cell biology. Nutrition 2011, 27, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Ageing: Is there a role for arachidonic acid and other bioactive lipids? A review. J. Adv. Res. 2018, 11, 67–79. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, M.; Hwang, S.W. Molecular mechanisms underlying the actions of arachidonic acid-derived prostaglandins on peripheral nociception. J. Neuroinflammation 2020, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.T.; Nara, T.Y. Structure, function, and dietary regulation of delta 6, delta 5, and delta 9 desaturases. Annu. Rev. Nutr. 2004, 24, 345–376. [Google Scholar] [CrossRef]
- Ford, J.H. Saturated fatty acid metabolism is key link between cell division, cancer, and senescence in cellular and whole organism aging. Age 2010, 32, 231–237. [Google Scholar] [CrossRef]
- Yosefy, C.; Viskoper, J.R.; Laszt, A.; Priluk, R.; Guita, E.; Varon, D.; Illan, Z.; Berry, E.M.; Savion, N.; Adan, Y.; et al. The effect of fish oil on hypertension, plasma lipids and hemostasis in hypertensive, obese, dyslipidemic patients with and without diabetes mellitus. Prostaglandins Leukot. Essent. Fat. Acids 1999, 61, 83–87. [Google Scholar] [CrossRef]
- Jové, M.; Mota-Martorell, N.; Pradas, I.; Galo-Licona, J.D.; Martín-Gari, M.; Obis, È.; Sol, J.; Pamplona, R. The Lipidome Fingerprint of Longevity. Molecules 2020, 25, 4343. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Covarrubias, V. Lipidomics in longevity and healthy aging. Biogerontology 2013, 14, 663–672. [Google Scholar] [CrossRef]
- Papsdorf, K.; Brunet, A. Linking lipid metabolism to chromatin regulation in aging. Trends Cell Biol. 2018, 29, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Ha, H.; Lee, B.S.; Kim, B.H.; Song, H.K.; Kim, Y.K. LC3B is an RNA-binding protein to trigger rapid mRNA degradation during autophagy. Nat. Commun. 2022, 13, 1436. [Google Scholar] [CrossRef] [PubMed]
- Revuelta, M.; Matheu, A. Autophagy in stem cell aging. Aging Cell 2017, 16, 912–915. [Google Scholar] [CrossRef]
- Ho, T.T.; Warr, M.R.; Adelman, E.R.; Lansinger, O.M.; Flach, J.; Verovskaya, E.V.; Figueroa, M.E.; Passegué, E. Autophagy maintains the metabolism and function of young and old stem cells. Nature 2017, 543, 205–210. [Google Scholar] [CrossRef]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 2019, 20, 421–435. [Google Scholar] [CrossRef]
- Robey, P.G.; Kuznetsov, S.A.; Ren, J.; Klein, H.G.; Sabatino, M.; Stroncek, D.F. Generation of clinical grade human bone marrow stromal cells for use in bone regeneration. Bone 2015, 70, 87–92. [Google Scholar] [CrossRef]
- Aliborzi, G.; Vahdati, A.; Mehrabani, D.; Hosseini, S.E.; Tamadon, A. Isolation, Characterization and Growth Kinetic Comparison of Bone Marrow and Adipose Tissue Mesenchymal Stem Cells of Guinea Pig. Int. J. Stem Cells 2016, 9, 115–123. [Google Scholar] [CrossRef]
- Jiang, Y.; Henderson, D.; Blackstad, M.; Chen, A.; Miller, R.F.; Verfaillie, C.M. Neuroectodermal differentiation from mouse multipotent adult progenitor cells. Proc. Natl. Acad. Sci. USA 2003, 100 (Suppl. 1), 11854–11860. [Google Scholar] [CrossRef]
- Gruia, A.T.; Barbu-Tudoran, L.; Mic, A.A.; Ordodi, V.L.; Paunescu, V.; Mic, F.A. Arachidonic acid accumulates in the stromal macrophages during thymus involution in diabetes. Histochem. Cell Biol. 2011, 136, 79–92. [Google Scholar] [CrossRef] [PubMed]



| Target | Fluorochrome | Clone | Manufacture | Isotype | Catalog Number |
|---|---|---|---|---|---|
| CD29 | PE | MAR4 | BD | Mouse BALB/c IgG1, k | 555443 |
| CD34 | FITC | 581 | BD | Mouse IgG1, k | 555821 |
| CD44 | FITC | L178 | BD | Mouse BALB/c IgG1, k | 347943 |
| CD45 | PE | HI30 | BD | Mouse IgG1, k | 555483 |
| CD56 | PE | B159 | BD | Mouse IgG1, k | 555516 |
| CD73 | PE | AD2 | BD | Mouse IgG1, k | 550257 |
| CD90 | FITC | 5El0 | BD | Mouse BALB/c IgG1, k | 555595 |
| CD105 | FITC | 266 | BD | Mouse BALB/c IgG1, k | 561443 |
| CD117 | PE | A3C6E2 | Biolegend (San Diego, CA, USA) | Mouse IgG1k | 323408 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivan, A.; Cristea, M.I.; Telea, A.; Oprean, C.; Galuscan, A.; Tatu, C.A.; Paunescu, V. Stem Cells Derived from Human Exfoliated Deciduous Teeth Functional Assessment: Exploring the Changes of Free Fatty Acids Composition during Cultivation. Int. J. Mol. Sci. 2023, 24, 17249. https://doi.org/10.3390/ijms242417249
Ivan A, Cristea MI, Telea A, Oprean C, Galuscan A, Tatu CA, Paunescu V. Stem Cells Derived from Human Exfoliated Deciduous Teeth Functional Assessment: Exploring the Changes of Free Fatty Acids Composition during Cultivation. International Journal of Molecular Sciences. 2023; 24(24):17249. https://doi.org/10.3390/ijms242417249
Chicago/Turabian StyleIvan, Alexandra, Mirabela I. Cristea, Ada Telea, Camelia Oprean, Atena Galuscan, Calin A. Tatu, and Virgil Paunescu. 2023. "Stem Cells Derived from Human Exfoliated Deciduous Teeth Functional Assessment: Exploring the Changes of Free Fatty Acids Composition during Cultivation" International Journal of Molecular Sciences 24, no. 24: 17249. https://doi.org/10.3390/ijms242417249





