Isolation of Pro-Osteogenic Compounds from Euptelea polyandra That Reciprocally Regulate Osteoblast and Osteoclast Differentiation
Abstract
:1. Introduction
2. Results
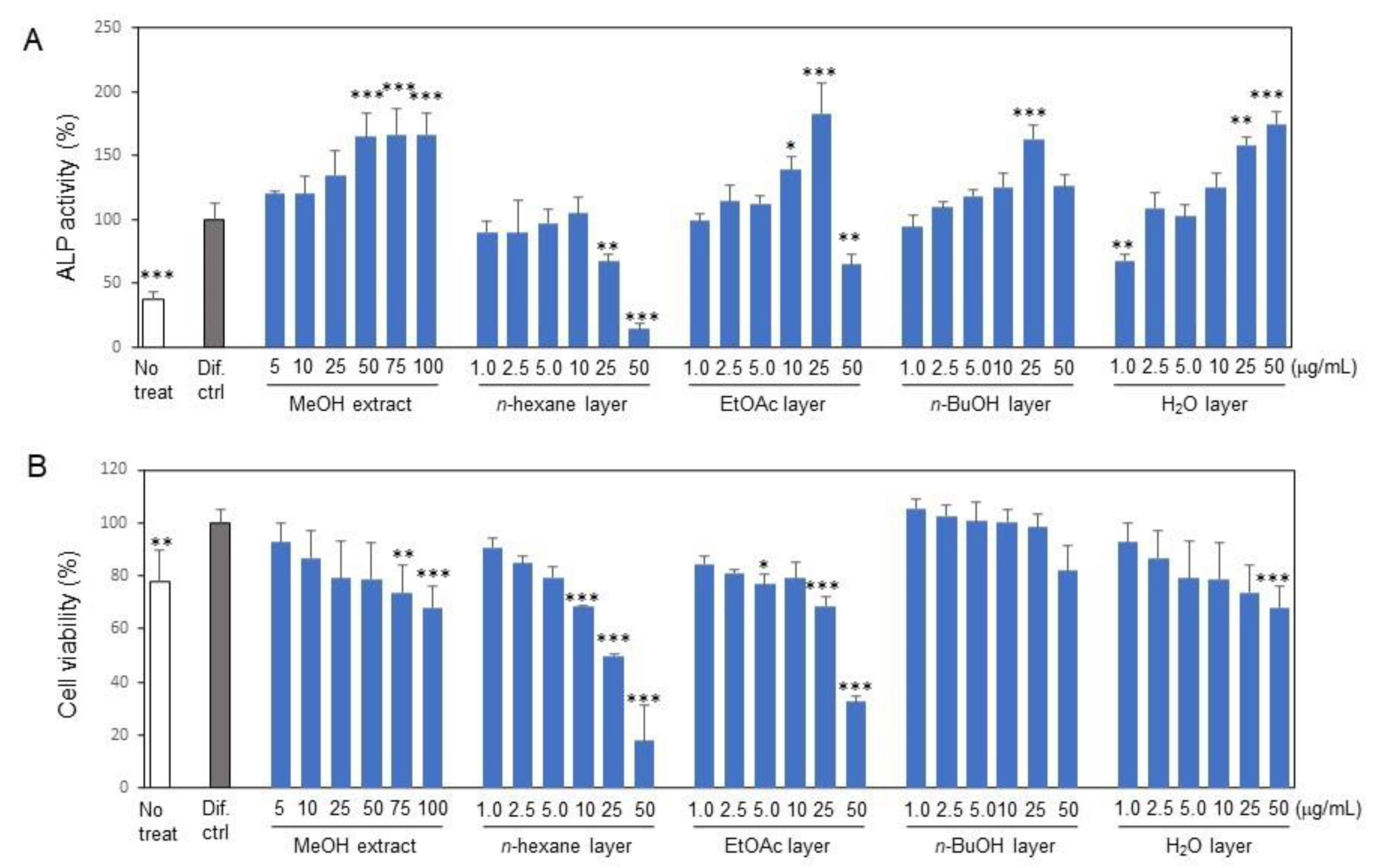
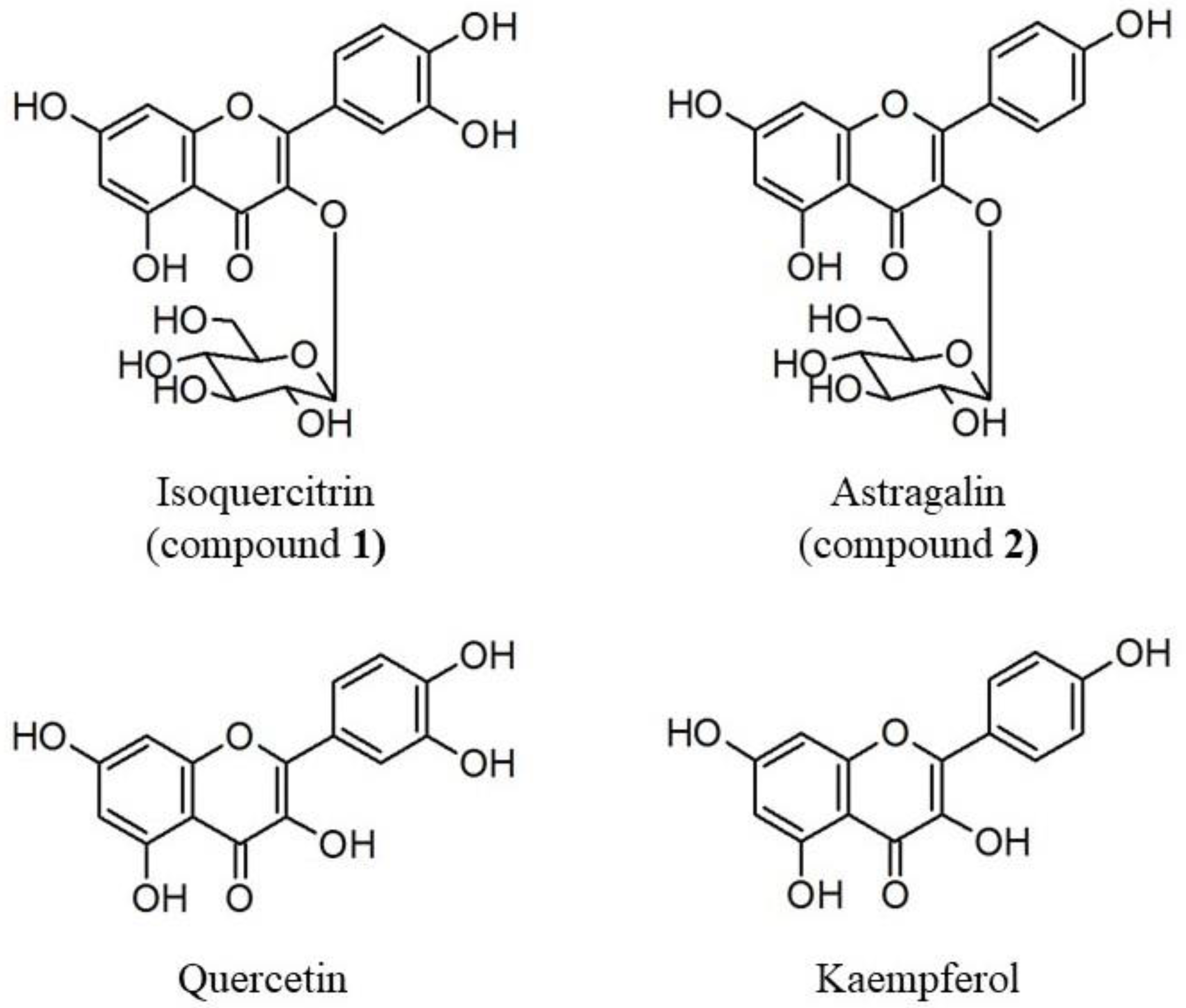
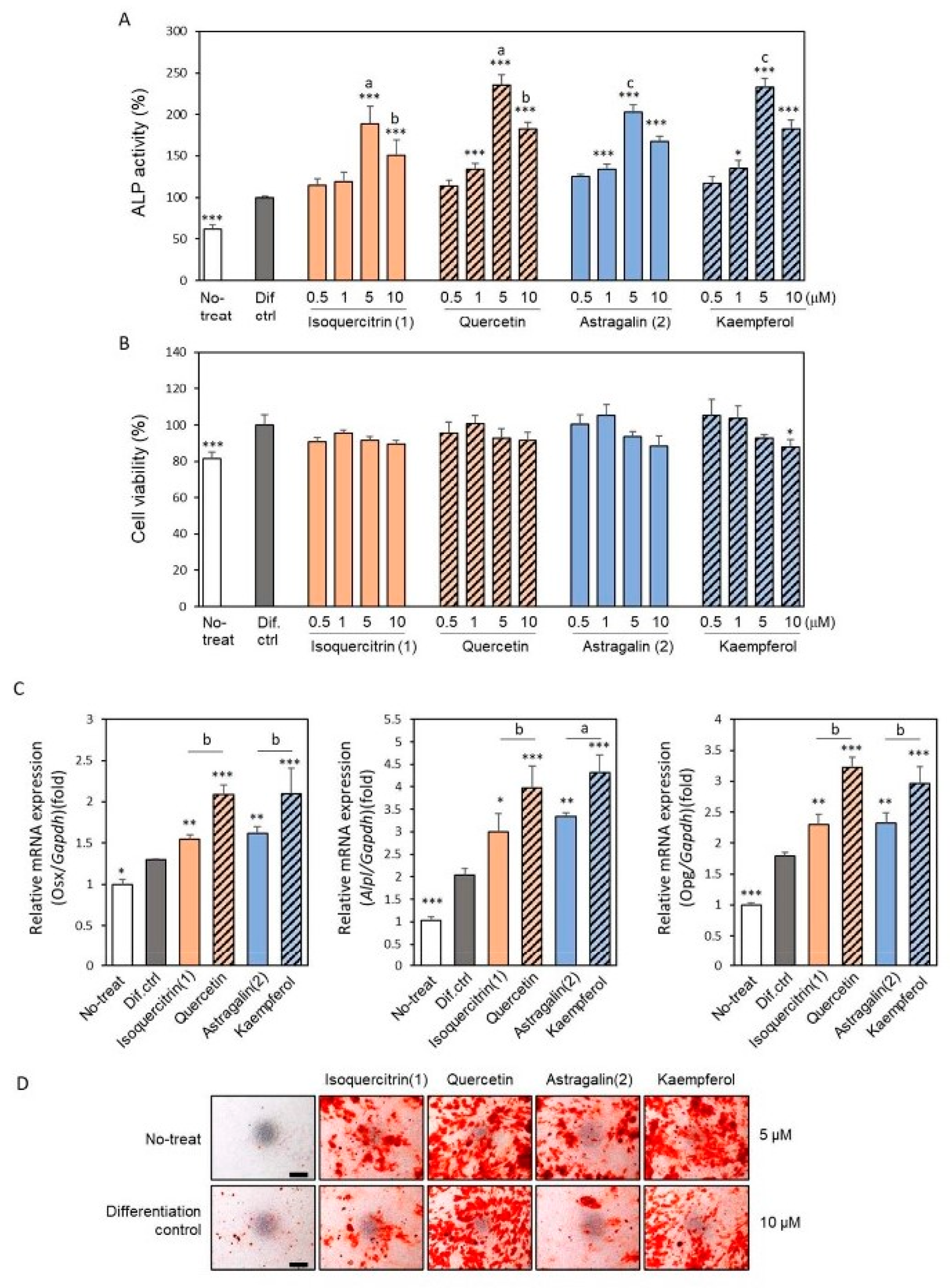
2.1. Isolation of Osteoblast Differentiation-Promoting Compounds from E. polyandra
2.2. Effects of the Isolated Compounds on RANKL-Induced Osteoclastogenesis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction, Solvent Fractionation, and Column Chromatography
4.3. Osteoblast Differentiation
4.4. ALP Activity
4.5. Cell Viability Analysis
4.6. Mineralization Analysis
4.7. Osteoclast Differentiation
4.8. TRAP Activity Assay and Staining
4.9. RNA Extraction and Quantitative PCR (qPCR)
4.10. Instrumental Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manolagas, S.C. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000, 21, 115–137. [Google Scholar] [CrossRef]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner. Res. 2007, 22, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Advisory Task Force on Bisphosphonate-Related Osteonecrosis of the Jaws. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J. Oral Maxillofac. Surg. 2007, 65, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Bord, S.; Ireland, D.C.; Beavan, S.R.; Compston, J.E. The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone 2003, 32, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Streicher, C.; Heyny, A.; Andrukhova, O.; Haigl, B.; Slavic, S.; Schüler, C.; Kollmann, K.; Kantner, I.; Sexl, V.; Kleiter, M.; et al. Estrogen Regulates Bone Turnover by Targeting RANKL Expression in Bone Lining Cells. Sci. Rep. 2017, 7, 6460. [Google Scholar] [CrossRef]
- Colditz, G.A. Relationship between estrogen levels, use of hormone replacement therapy, and breast cancer. J. Natl. Cancer Inst. 1998, 90, 814–823. [Google Scholar] [CrossRef]
- Torre, E. Molecular signaling mechanisms behind polyphenol-induced bone anabolism. Phytochem. Rev. 2017, 16, 1183–1226. [Google Scholar] [CrossRef]
- Clarkson, T.B.; Anthony, M.S.; Williams, J.K.; Honoré, E.K.; Cline, J.M. The potential of soybean phytoestrogens for postmenopausal hormone replacement therapy. Proc. Soc. Exp. Biol. Med. 1998, 217, 365–368. [Google Scholar] [CrossRef]
- Morabito, N.; Crisafulli, A.; Vergara, C.; Gaudio, A.; Lasco, A.; Frisina, N.; D’Anna, R.; Corrado, F.; Pizzoleo, M.A.; Cincotta, M.; et al. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: A randomized double-blind placebo-controlled study. J. Bone Miner. Res. 2002, 17, 1904–1912. [Google Scholar] [CrossRef]
- Fujioka, M.; Uehara, M.; Wu, J.; Adlercreutz, H.; Suzuki, K.; Kanazawa, K.; Takeda, K.; Yamada, K.; Ishimi, Y. Equol, a metabolite of daidzein, inhibits bone loss in ovariectomized mice. J. Nutr. 2004, 134, 2623–2627. [Google Scholar] [CrossRef] [PubMed]
- Notoya, K.; Yoshida, K.; Tsukuda, R.; Taketomi, S. Effect of ipriflavone on expression of markers characteristic of the osteoblast phenotype in rat bone marrow stromal cell culture. J. Bone Miner. Res. 1994, 9, 395–400. [Google Scholar] [CrossRef]
- Cao, L.; Wang, J.; Zhang, Y.; Tian, F.; Wang, C. Osteoprotective effects of flavonoids: Evidence from in vivo and in vitro studies (Review). Mol. Med. Rep. 2022, 25, 200. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Chang, J.K.; Tsai, C.H.; Chien, T.T.; Kuo, P.L. Myricetin induces human osteoblast differentiation through bone morphogenetic protein-2/p38 mitogen-activated protein kinase pathway. Biochem. Pharmacol. 2007, 73, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ohba, S.; Shinkai, M.; Chung, U.I.; Nagamune, T. Icariin induces osteogenic differentiation in vitro in a BMP- and Runx2-dependent manner. Biochem. Biophys. Res. Commun. 2008, 369, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Cheng, T.L.; Lin, S.Y.; Ho, C.J.; Chyu, J.Y.; Yang, R.S.; Chen, C.H.; Shen, C.L. Osteoprotective roles of green tea catechins. Antioxidants 2020, 9, 1136. [Google Scholar] [CrossRef] [PubMed]
- Royal Botanic Gardens, Kew. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:554480-1#publications (accessed on 10 December 2023).
- Goto, M.; Imai, S.; Murata, T.; Fujioka, S. Glycosides from the leaves of Euptelea polyandra Sieb. et Zucc. Chem. Pharm. Bull. 1964, 12, 516–518. [Google Scholar] [CrossRef]
- Goto, M.; Imai, S.; Murata, T.; Noguchi, T.; Fujioka, S. Anti-microbial glycosides of Euptelea polyandra Sieb. et Zucc. I. Isolation, constitutions and anti-microbial activities of eupteleside A and eupteleoside B. Yakugaku Zasshi 1970, 90, 736–743. (In Japanese) [Google Scholar] [CrossRef]
- Murata, T.; Imai, S.; Imanishi, M.; Goto, M. Anti-microbial glycosides of Euptelea polyandra Sieb. et Zucc. II. The structure of eupteleogenin. Yakugaku Zasshi. 1970, 90, 744–751. (In Japanese) [Google Scholar] [CrossRef]
- Konoshima, T.; Takasaki, M.; Kozuka, M.; Tokuda, H. Studies on inhibitors of skin-tumor promotion, I. Inhibitory effects of triterpenes from Euptelea polyandra on Epstein-Barr virus activation. J. Nat. Prod. 1987, 50, 1167–1170. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Murakami, T.; Oominami, H.; Matsuda, H. Bioactive saponins and glycosides. XVI. Nortriterpene oligoglycosides with gastroprotective activity from the fresh leaves of Euptelea polyandra Sieb. et Zucc. (1): Structures of Eupteleasaponins I, II, III, IV, V, and V acetate. Chem. Pharm. Bull. 2000, 48, 1045–1050. [Google Scholar] [CrossRef]
- Murakami, T.; Oominami, H.; Matsuda, H.; Yoshikawa, M. Bioactive saponins and glycosides. XVIII. Nortriterpene and triterpene oligoglycosides from the fresh leaves of Euptelea polyandra Sieb. et Zucc. (2): Structures of eupteleasaponins VI, VI acetate, VII, VIII, IX, X, XI, and XII. Chem. Pharm. Bull. 2001, 49, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Fukami, S.; Tomomura, M.; Tomomura, A.; Shirataki, Y. Screening for natural medicines effective for the treatment of osteoporosis. J. Nat. Med. 2019, 73, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Lin, Y.; Lv, R.; Yan, H.; Yu, J.; Zhao, H.; Wang, X.; Wang, D. An Efficient method for the preparative isolation and purification of flavonoids from Leaves of Crataegus pinnatifida by HSCCC and Pre-HPLC. Molecules 2017, 22, 767. [Google Scholar] [CrossRef]
- Han, J.T.; Bang, M.H.; Chun, O.K.; Kim, D.O.; Lee, C.Y.; Baek, N.I. Flavonol glycosides from the aerial parts of Aceriphyllum rossii and their antioxidant activities. Arch. Pharm. Res. 2004, 27, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yang, S.; Shao, J.; Li, Y.P. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front Biosci. 2007, 12, 3068–3092. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, D.S.; Kim, S.; Rho, J. Regulation of Osteoblast Differentiation by Cytokine Networks. Int. J. Mol. Sci. 2021, 22, 2851. [Google Scholar] [CrossRef] [PubMed]
- Fayed, H.A.; Barakat, B.M.; Elshaer, S.S.; Abdel-Naim, A.B.; Menze, E.T. Antiosteoporotic activities of isoquercitrin in ovariectomized rats: Role of inhibiting hypoxia inducible factor-1 alpha. Eur. J. Pharmacol. 2019, 865, 172785. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Wang, Y.; Lu, C.; Zheng, D.; Zhang, J. Isoquercitrin, a flavonoid glucoside, exerts a positive effect on osteogenesis in vitro and in vivo. Chem. Biol. Interact. 2019, 297, 85–94. [Google Scholar] [CrossRef]
- Liu, L.; Wang, D.; Qin, Y.; Xu, M.; Zhou, L.; Xu, W.; Liu, X.; Ye, L.; Yue, S.; Zheng, Q.; et al. Astragalin promotes osteoblastic differentiation in MC3T3-E1 cells and bone formation in vivo. Front. Endocrinol. 2019, 10, 228. [Google Scholar] [CrossRef]
- Imtiyaz, Z.; Lin, Y.T.; Liang, F.Y.; Chiou, W.F.; Lee, M.H. Compounds isolated from Wikstroemia taiwanensis regulate bone remodeling by modulating osteoblast and osteoclast activities. Front. Pharmacol. 2021, 12, 670254. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Ima-Nirwana, S. Quercetin as an agent for protecting the bone: A review of the current evidence. Int. J. Mol. Sci. 2020, 21, 6448. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, P.; Jagadeesan, R.; Sekaran, S.; Dhanasekaran, A.; Vimalraj, S. Flavonoids: Classification, function, and molecular mechanisms involved in bone remodeling. Front. Endocrinol. 2021, 12, 779638. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Feng, Y.; Zhang, X.; Gong, L.; Liu, J.; Li, Y.; Liao, H. Antiosteoporosis studies of 20 medicine food homology plants containing quercetin, rutin, and kaempferol: TCM characteristics, in vivo and in vitro activities, potential mechanisms, and food functions. Evid. Based Complement Alternat. Med. 2022, 2022, 5902293. [Google Scholar] [CrossRef]
- Xi, Y.; Shen, J.; Li, X.; Bao, Y.; Zhao, T.; Li, B.; Zhang, X.; Wang, J.; Bao, Y.; Gao, J.; et al. Regulatory effects of quercetin on bone homeostasis: Research updates and future perspectives. Am. J. Chin. Med. 2023, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.Y.; Ima-Nirwana, S. The osteoprotective effects of kaempferol: The evidence from in vivo and in vitro studies. Drug Des. Devel. Ther. 2019, 13, 3497–3514. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent progress regarding kaempferol for the treatment of various diseases. Exp. Ther. Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Lara-Castillo, N. Estrogen Signaling in Bone. Appl. Sci. 2021, 11, 4439. [Google Scholar] [CrossRef]
- Kiyama, R. Estrogenic flavonoids and their molecular mechanisms of action. J. Nutr. Biochem. 2023, 114, 109250. [Google Scholar] [CrossRef] [PubMed]
- Prouillet, C.; Mazière, J.C.; Mazière, C.; Wattel, A.; Brazier, M.; Kamel, S. Stimulatory effect of naturally occurring flavonols quercetin and kaempferol on alkaline phosphatase activity in MG-63 human osteoblasts through ERK and estrogen receptor pathway. Biochem. Pharmacol. 2004, 67, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, K.L.; Farman, H.; Henning, P.; Lionikaite, V.; Movérare-Skrtic, S.; Wu, J.; Ryberg, H.; Koskela, A.; Gustafsson, J.Å.; Tuukkanen, J.; et al. The role of membrane ERα signaling in bone and other major estrogen responsive tissues. Sci. Rep. 2016, 6, 29473. [Google Scholar] [CrossRef] [PubMed]
- Bonnelye, E.; Aubin, J.E. An energetic orphan in an endocrine tissue: A revised perspective of the function of estrogen receptor-related receptor alpha in bone and cartilage. J. Bone Miner. Res. 2013, 28, 225–233. [Google Scholar] [CrossRef]
- Qattan, M.Y.; Khan, M.I.; Alharbi, S.H.; Verma, A.K.; Al-Saeed, F.A.; Abduallah, A.M.; Al Areefy, A.A. Therapeutic Importance of Kaempferol in the Treatment of Cancer through the Modulation of Cell Signaling Pathways. Molecules 2022, 27, 8864. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, R.; Shirataki, Y.; Tomomura, A.; Bandow, K.; Sakagami, H.; Tomomura, M. Isolation of Pro-Osteogenic Compounds from Euptelea polyandra That Reciprocally Regulate Osteoblast and Osteoclast Differentiation. Int. J. Mol. Sci. 2023, 24, 17479. https://doi.org/10.3390/ijms242417479
Suzuki R, Shirataki Y, Tomomura A, Bandow K, Sakagami H, Tomomura M. Isolation of Pro-Osteogenic Compounds from Euptelea polyandra That Reciprocally Regulate Osteoblast and Osteoclast Differentiation. International Journal of Molecular Sciences. 2023; 24(24):17479. https://doi.org/10.3390/ijms242417479
Chicago/Turabian StyleSuzuki, Ryuichiro, Yoshiaki Shirataki, Akito Tomomura, Kenjiro Bandow, Hiroshi Sakagami, and Mineko Tomomura. 2023. "Isolation of Pro-Osteogenic Compounds from Euptelea polyandra That Reciprocally Regulate Osteoblast and Osteoclast Differentiation" International Journal of Molecular Sciences 24, no. 24: 17479. https://doi.org/10.3390/ijms242417479






