Design, Synthesis, and In Vitro and In Vivo Bioactivity Studies of Hydrazide–Hydrazones of 2,4-Dihydroxybenzoic Acid
Abstract
:1. Introduction
2. Results
2.1. Chemistry
2.2. Microbiology—Antimicrobial Activity
2.3. In Vitro Assessment of the Antiproliferative Potential of the Tested Hydrazide–Hydrazones
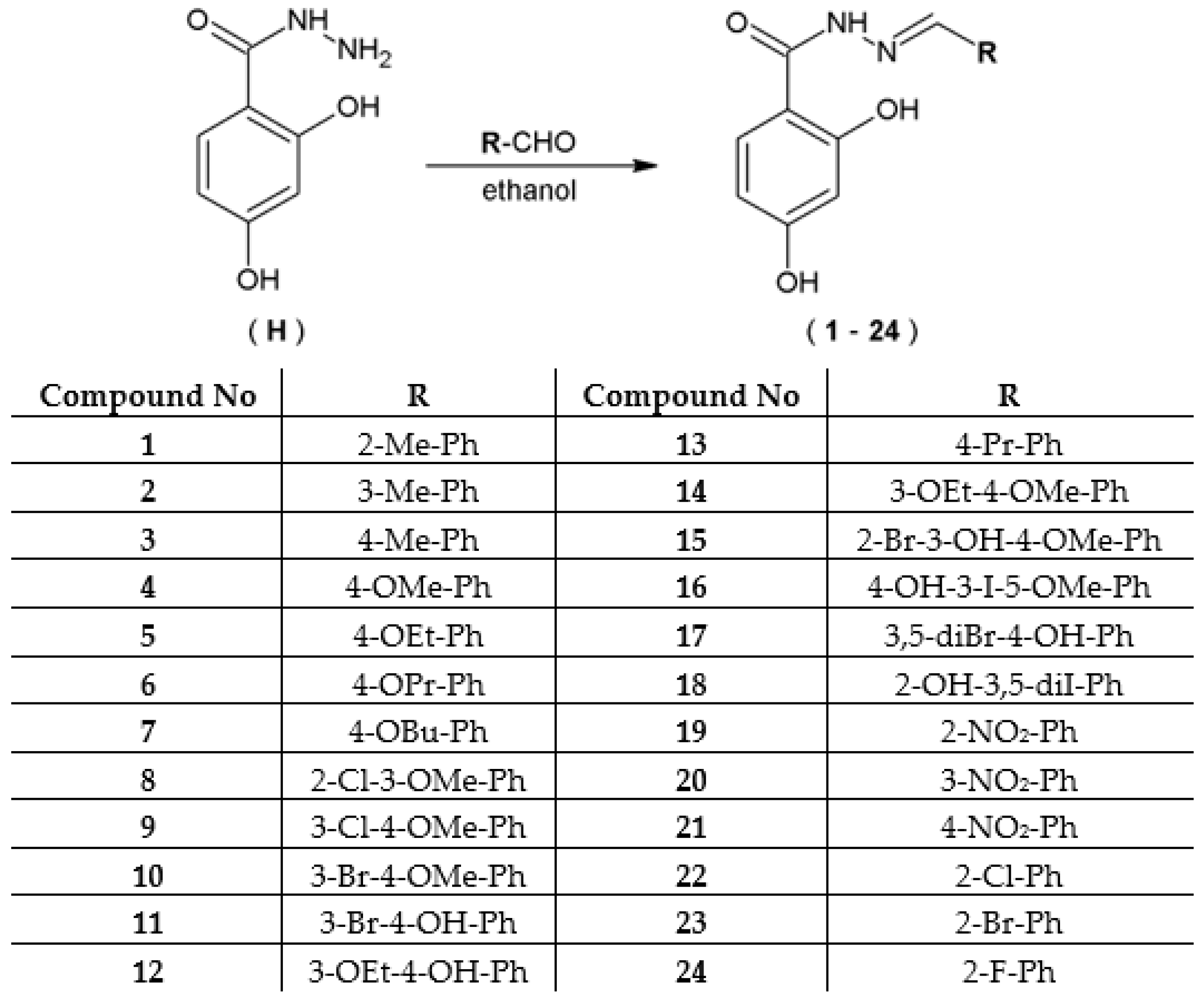
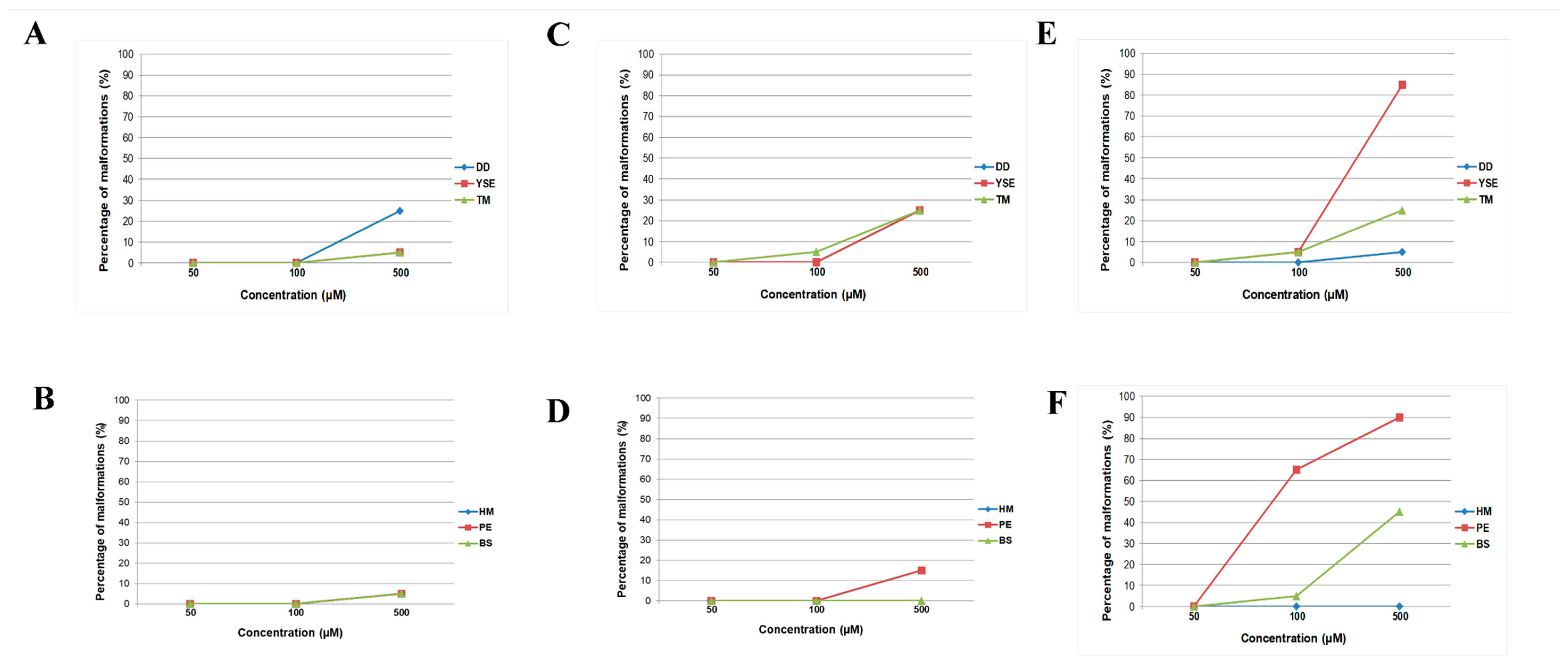
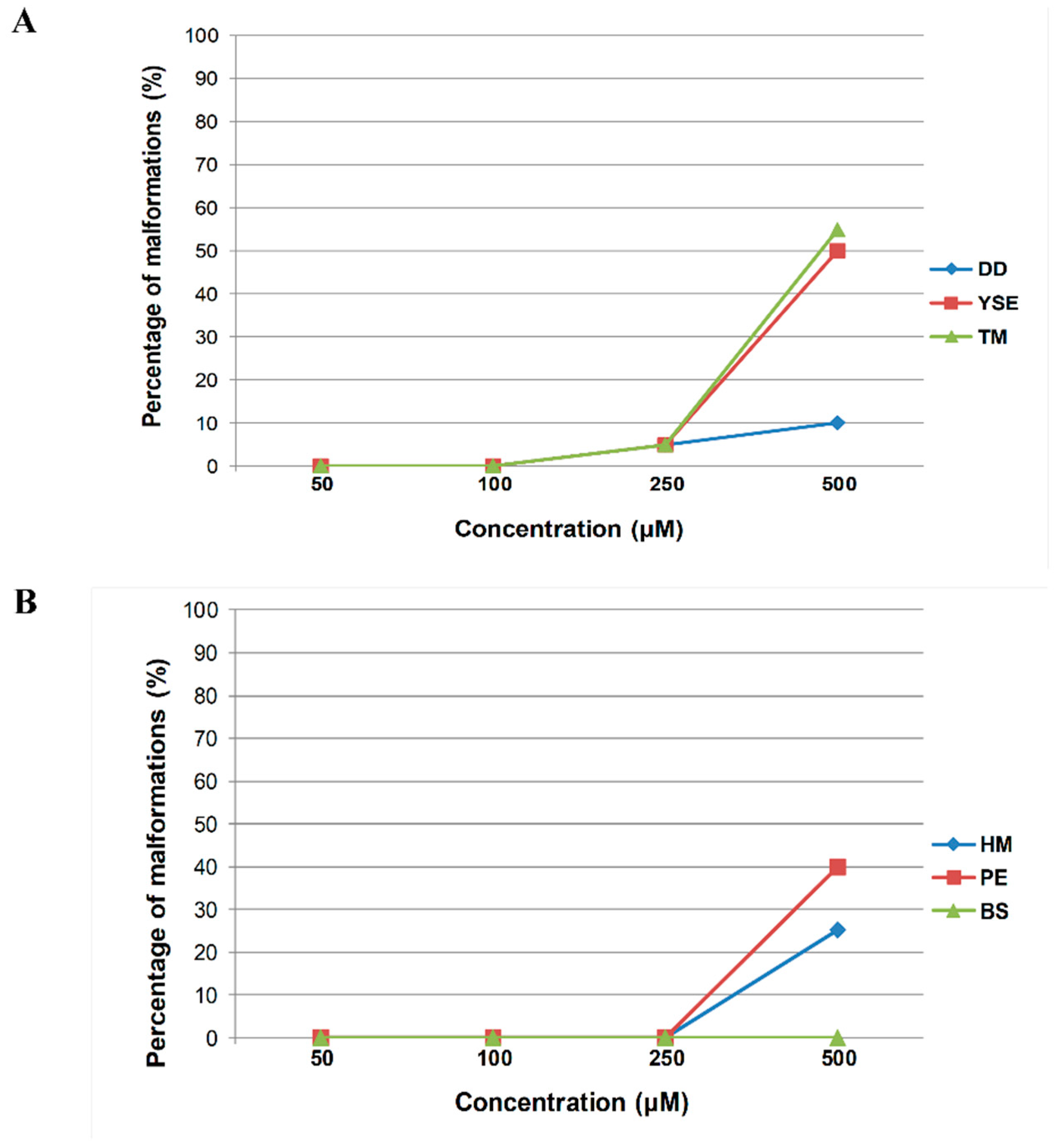
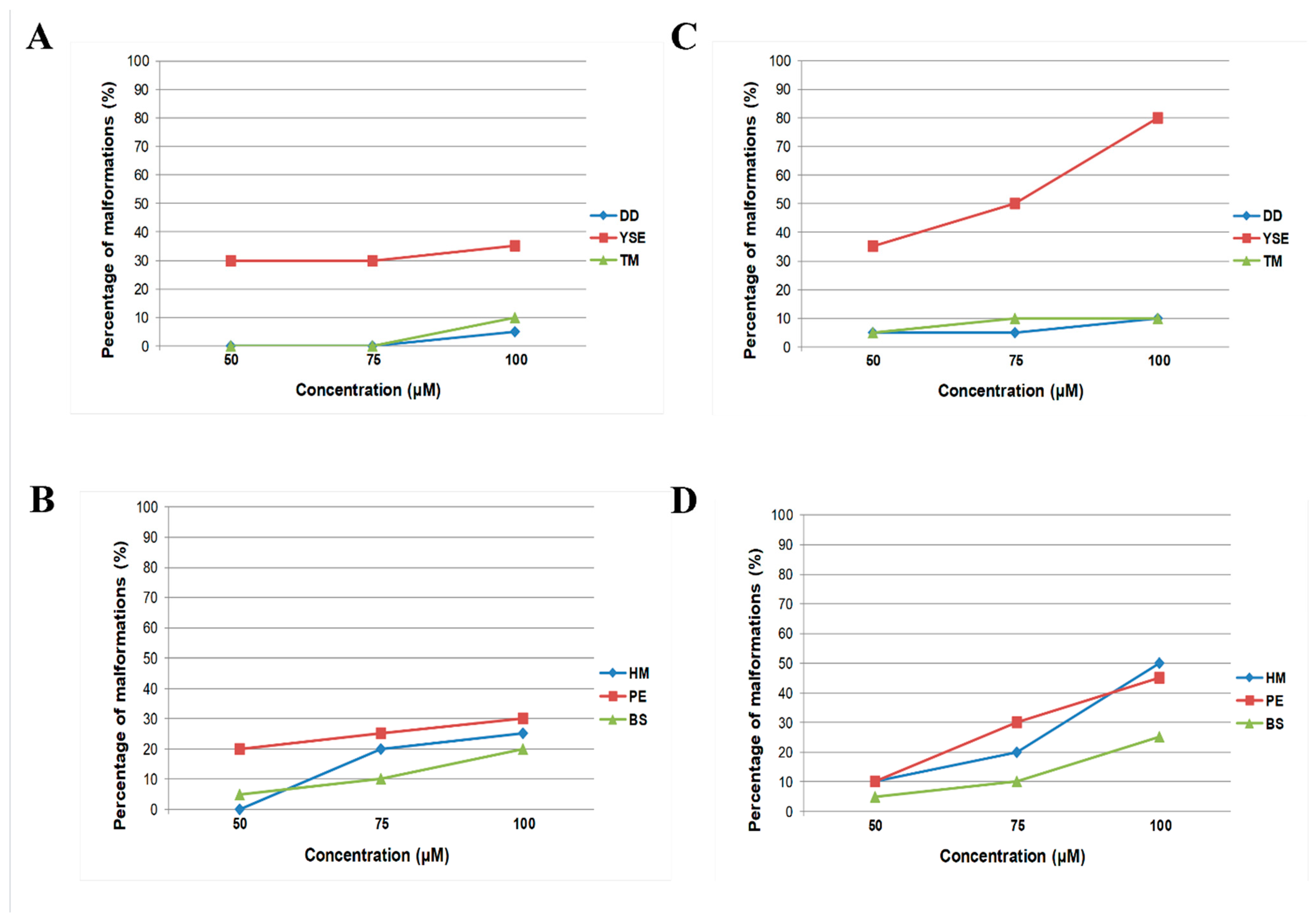
2.4. In Vivo Toxicity Assessment of Hydrazide–Hydrazones in the Zebrafish Model
3. Discussion
3.1. Chemistry
3.2. Microbiology—Antimicrobial Activity
3.3. Cytotoxicity
4. Materials and Methods
4.1. Chemistry
4.2. Procedure of the Synthesis of Hydrazide–Hydrazones of 2,4-Dihydroxybenzoic Acid
Detailed Physico-Chemical Properties of Hydrazide–Hydrazones of 2,4-Dihydroxybenzoic Acid (1–24)
- 2,4-dihydroxy-N-[(2-methylphenyl)methylidene]benzohydrazide (1)
- 2,4-dihydroxy-N-[(3-methylphenyl)methylidene]benzohydrazide (2)
- 2,4-dihydroxy-N-[(4-methylphenyl)methylidene]benzohydrazide (3)
- 2,4-dihydroxy-N-[(4-methoxyphenyl)methylidene]benzohydrazide (4)
- N-[(4-ethoxyphenyl)methylidene]-2,4-dihydroxybenzohydrazide (5)
- 2,4-dihydroxy-N-[(4-propoxyphenyl)methylidene]benzohydrazide (6)
- N-[(4-butoxyphenyl)methylidene]-2,4-dihydroxybenzohydrazide (7)
- N-[(2-chloro-3-methoxyphenyl)methylidene]-2,4-dihydroxybenzohydrazide (8)
- N-[(3-chloro-4-methoxyphenyl)methylidene]-2,4-dihydroxybenzohydrazide (9)
- N-[(3-bromo-4-methoxyphenyl)methylidene]-2,4-dihydroxybenzohydrazide (10)
- N-[(3-bromo-4-hydroxyphenyl)methylidene]-2,4-dihydroxybenzohydrazide (11)
- N-[(3-ethoxy-4-hydroxyphenyl)methylidene]-2,4-dihydroxybenzohydrazide (12)
- 2,4-dihydroxy-N-[(4-propylphenyl)methylidene]benzohydrazide (13)
- N-[(3-ethoxy-4-methoxyphenyl)methylidene]-2,4-dihydroxybenzohydrazide (14)
- N-[(2-bromo-3-hydroxy-4-methoxyphenyl)methylidene]-2,4-dihydroxybenzohydrazide (15)
- 2,4-dihydroxy-N-[(4-hydroxy-3-iodo-5-methoxyphenyl)methylidene]benzohydrazide (16)
- N-[(3,5-dibromo-4-hydroxyphenyl)methylidene]-2,4-dihydroxybenzohydrazide (17)
- 2,4-dihydroxy-N-[(2-hydroxy-3,5-diiodophenyl)methylidene]benzohydrazide (18)
- 2,4-dihydroxy-N-[(2-nitrophenyl)methylidene]benzohydrazide (19)
- 2,4-dihydroxy-N-[(3-nitrophenyl)methylidene]benzohydrazide (20)
- 2,4-dihydroxy-N-[(4-nitrophenyl)methylidene]benzohydrazide (21)
- N-[(2-chlorophenyl)methylidene]-2,4-dihydroxybenzohydrazide (22)
- N-[(2-bromophenyl)methylidene]-2,4-dihydroxybenzohydrazide (23)
- N-[(2-fluorophenyl)methylidene]-2,4-dihydroxybenzohydrazide (24)
4.3. Microbiology—Antimicrobial Activity
4.4. Cell Cultures
4.5. Breeding and Egg Collection of Danio rerio
4.6. Cytotoxicity Assay
4.7. Acute Toxicity Study in the Zebrafish Embryo Model
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Geddes-McAlister, J.; Shapiro, R.S. New pathogens, new tricks: Emerging, drug-resistant fungal pathogens and future prospects for antifungal therapeutics. Ann. N. Y. Acad. Sci. 2019, 1435, 57–78. [Google Scholar] [CrossRef]
- Wild, C.P.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- International Agency for Research on Cancer. Updated 2020. Lung Cancer Fact Sheet. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf (accessed on 31 July 2023).
- Debieuvre, D.; Molinier, O.; Falchero, L.; Locher, C.; Templement-Grangerat, D.; Meyer, N.; Morel, H.; Duval, Y.; Asselain, B.; Letierce, A.; et al. Lung cancer trends and tumor characteristic changes over 20 years (2000–2020): Results of three French consecutive nationwide prospective cohorts’ studies. Lancet Reg. Health Eur. 2022, 22, 100492. [Google Scholar] [CrossRef]
- American Cancer Society: Cancer Facts and Figures 2023. American Cancer Society. 2023. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html (accessed on 31 July 2023).
- Non-Small Cell Lung Cancer Treatment (PDQ®)–Health Professional Version. National Cancer Institute Website. Available online: https://www.cancer.gov/types/lung/hp/non-small-cell-lung-treatment-pdq (accessed on 31 July 2023).
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme-Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef]
- Ghiaseddin, A.P.; Shin, D.; Melnick, K.; Tran, D.D. Tumor Treating Fields in the Management of Patients with Malignant Gliomas. Curr. Treat. Options Oncol. 2020, 21, 76. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Updated 2020. Liver Cancer Fact Sheet. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf (accessed on 31 July 2023).
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Hepatocellular Carcinoma (HCC): Epidemiology, Etiology and Molecular Classification. Adv. Cancer Res. 2021, 149, 1–61. [Google Scholar] [CrossRef]
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Bahadoram, S.; Davoodi, M.; Hassanzadeh, S.; Bahadoram, M.; Barahman, M.; Mafakher, L. Renal cell carcinoma: An overview of the epidemiology, diagnosis, and treatment. G. Ital. Nefrol. 2022, 39. [Google Scholar]
- Gray, R.E.; Harris, G.T. Renal Cell Carcinoma: Diagnosis and Management. Am. Fam. Physician 2019, 99, 179–184. [Google Scholar] [PubMed]
- Rollas, S.; Küçükgüzel, Ş.G. Biological Activities of Hydrazone Derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef] [PubMed]
- Popiołek, Ł. Hydrazide–hydrazones as potential antimicrobial agents: Overview of the literature since 2010. Med. Chem. Res. 2017, 26, 287–301. [Google Scholar] [CrossRef]
- Popiołek, Ł. Updated Information on Antimicrobial Activity of Hydrazide–Hydrazones. Int. J. Mol. Sci. 2021, 22, 9389. [Google Scholar] [CrossRef] [PubMed]
- Popiołek, Ł. The bioactivity of benzenesulfonyl hydrazones: A short review. Biomed. Pharmacother. 2021, 141, 111851. [Google Scholar] [CrossRef]
- Popiołek, Ł. The application of hydrazones and hydrazide-hydrazones in the synthesis of bioactive azetidin-2-one derivatives: A mini review. Biomed. Pharmacother. 2023, 163, 114853. [Google Scholar] [CrossRef]
- Sharma, P.C.; Sharma, D.; Sharma, A.; Saini, N.; Goyal, R.; Ola, M.; Chawla, R.; Thakur, V.K. Hydrazone comprising compounds as promising anti-infective agents: Chemistry and structure-property relationship. Mater. Today Chem. 2020, 18, 100349. [Google Scholar] [CrossRef]
- Wang, H.; Ren, S.-X.; He, Z.-Y.; Wang, D.-L.; Yan, X.-N.; Feng, J.-T.; Zhang, X. Synthesis, Antifungal Activities and Qualitative Structure Activity Relationship of Carabrone Hydrazone Derivatives as Potential Antifungal Agents. Int. J. Mol. Sci. 2014, 15, 4257–4272. [Google Scholar] [CrossRef]
- Korcz, M.; Sączewski, F.; Bednarski, P.J.; Kornicka, A. Synthesis, Structure, Chemical Stability, and In Vitro Cytotoxic Properties of Novel Quinoline-3-Carbaldehyde Hydrazones Bearing a 1,2,4-Triazole or Benzotriazole Moiety. Molecules 2018, 23, 1497. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Rysz, B.; Biernasiuk, A.; Wujec, M. Synthesis of promising antimicrobial agents: Hydrazide-hydrazones of 5-nitrofuran-2-carboxylic acid. Chem. Biol. Drug. Des. 2020, 95, 260–269. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Tuszyńska, K.; Biernasiuk, A. Searching for novel antimicrobial agents among hydrazide-hydrazones of 4-iodosalicylic acid. Biomed. Pharmacother. 2022, 153, 113302. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, N.M.; Ghosh, S.; Shah, K. Novel bis(indolyl)hydrazide-hydrazones as potent cytotoxic agents. Bioorg. Med. Chem. 2012, 22, 212–215. [Google Scholar] [CrossRef]
- Nasr, T.; Bondock, S.; Youns, M. Anticancer activity of new coumarin substituted hydrazide-hydrazone derivatives. Eur. J. Med. Chem. 2014, 76, 539–548. [Google Scholar] [CrossRef]
- Yadagiri, B.; Holagunda, U.D.; Bantu, R.; Nagarapu, L.; Guguloth, V.; Polepally, S.; Jain, N. Rational design, synthesis and anti-proliferative evaluation of novel benzosuberone tethered with hydrazide-hydrazones. Bioorg. Med. Chem. 2014, 24, 5041–5044. [Google Scholar] [CrossRef]
- El-Faham, A.; Farooq, M.; Khattab, S.N.; Abutaha, N.; Wadaan, M.A.; Ghabbour, H.A.; Fun, H.-K. Synthesis, Characterization, and Anti-Cancer Activity of Some New N′-(2-Oxoindolin-3-ylidene)-2-propylpentane hydrazide-hydrazones Derivatives. Molecules 2015, 20, 14638–14655. [Google Scholar] [CrossRef]
- Nikolova-Mladenova, B.; Momekov, G.; Ivanov, D.; Bakalova, A. Design and drug-like properties of new 5-methoxysalicylaldehyde based hydrazones with anti-breast cancer activity. J. Appl. Biomed. 2017, 15, 233–240. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Piątkowska-Chmiel, I.; Gawrońska-Grzywacz, M.; Biernasiuk, A.; Izdebska, M.; Herbet, M.; Sysa, M.; Malm, A.; Dudka, J.; Wujec, M. New hydrazide-hydrazones and 1,3-thiazolidin-4-ones with 3-hydroxy-2-naphthoic moiety: Synthesis, in vitro and in vivo studies. Biomed. Pharmacother. 2018, 103, 1337–1347. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Patrejko, P.; Gawrońska-Grzywacz, M.; Biernasiuk, A.; Berecka-Rycerz, A.; Natorska-Chomicka, D.; Piątkowska-Chmiel, I.; Gumieniczek, A.; Dudka, J.; Wujec, M. Synthesis and in vitro bioactivity study of new hydrazide-hydrazones of 5-bromo-2-iodobenzoic acid. Biomed. Pharmacother. 2020, 130, 110526. [Google Scholar] [CrossRef]
- Horchani, M.; Sala, G.D.; Caso, A.; D’Aria, F.; Esposito, G.; Laurenzana, I.; Giancola, C.; Costantino, V.; Jannet, H.B.; Romdhane, A. Molecular Docking and Biophysical Studies for Antiproliferative Assessment of Synthetic Pyrazolo-Pyrimidinones Tethered with Hydrazide-Hydrazones. Int. J. Mol. Sci. 2021, 22, 2742. [Google Scholar] [CrossRef]
- Han, M.I.; Atalay, P.; Tunç, C.Ü.; Ünal, G.; Dayan, S.; Aydın, Ö.; Küçükgüzel, Ş.G. Design and synthesis of novel (S)-Naproxen hydrazide-hydrazones as potent VEGFR-2 inhibitors and their evaluation in vitro/in vivo breast cancer models. Bioorg. Med. Chem. 2021, 37, 116097. [Google Scholar] [CrossRef] [PubMed]
- McCalla, D.R.; Reuvers, A.; Kaiser, C. Mode of Action of Nitrofurazone. J. Bacteriol. 1970, 104, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.N.; Ghosh, S. Mechanism of Action of Furazolidone: Inter-Strand Cross-Linking in DNA & Liquid Holding Recovery of Vibrio cholerae Cells. Indian J. Biochem. Biophys. 1979, 16, 125–130. [Google Scholar] [PubMed]
- McOsker, C.C.; Fitzpatrick, P.M. Nitrofurantoin: Mechanism of Action and Implications for Resistance Development in Common Uropathogens. J. Antimicrob. Chemother. 1994, 33, 23–30. [Google Scholar] [CrossRef]
- Munoz-Davila, M.J. Role of Old Antibiotics in the Era of Antibiotic Resistance. Highlighted Nitrofurantoin for the Treatment of Lower Urinary Tract Infections. Antibiotics 2014, 3, 39–48. [Google Scholar] [CrossRef]
- Alam, M.S.; Sang-Un, C.; Dong-Ung, L. Synthesis, anticancer, and docking studies of salicyl-hydrazone analogues: A novel series of small potent tropomyosin receptor kinase A inhibitors. Bioorg. Med. Chem. 2017, 25, 389–396. [Google Scholar] [CrossRef]
- Cihan-Üstündağ, G.; Şatana, D.; Özhan, G.; Çapan, G. Indole-based hydrazide-hydrazones and 4-thiazolidinones: Synthesis and evaluation as antitubercular and anticancer agents. J. Enzym. Inhib. Med. Chem. 2016, 31, 369–380. [Google Scholar] [CrossRef]
- Kodisundaram, P.; Duraikannu, A.; Balasankar, T.; Ambure, P.S.; Roy, K. Cytotoxic and Antioxidant Activity of a Set of Hetero Bicylic Methylthiadiazole Hydrazones: A Structure-Activity Study. Int. J. Mol. Cell. Med. 2015, 4, 128–137. [Google Scholar]
- Vicini, P.; Incerti, M.; Doytchinova, I.A.; La Colla, P.; Busonera, B.; Loddo, R. Synthesis and antiproliferative activity of benzo[d]isothiazole hydrazones. Eur. J. Med. Chem. 2006, 41, 624–632. [Google Scholar] [CrossRef]
- Wardakhan, W.W.; El-Sayed, N.N.; Mohareb, R.M. Synthesis and anti-tumor evaluation of novel hydrazide and hydrazide-hydrazone derivatives. Acta Pharm. 2013, 63, 45–57. [Google Scholar] [CrossRef]
- Bingul, M.; Tan, O.; Gardner, C.R.; Sutton, S.K.; Arndt, G.M.; Marshall, G.M.; Cheung, B.B.; Kumar, N.; Black, D.S. Synthesis, Characterization and Anti-Cancer Activity of Hydrazide Derivatives Incorporating a Quinoline Moiety. Molecules 2016, 21, 916. [Google Scholar] [CrossRef]
- Patil, S.; Kuman, M.M.; Palvai, S.; Sengupta, P.; Basu, S. Impairing Powerhouse in Colon Cancer Cells by Hydrazide–Hydrazone-Based Small Molecule. ACS Omega 2018, 3, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Shaha, S.A.A.; Afifia, M.; Zulkefleec, M.; Sultana, S.; Wadood, A.; Rahim, F.; Ismail, N. Morpholine hydrazone scaffold: Synthesis, anticancer activity and docking studies. Chin. Chem. Lett. 2017, 28, 607–611. [Google Scholar] [CrossRef]
- Hristova-Avakumova, N.; Yoncheva, K.; Nikolova-Mladenova, B.; Traykov, T.; Momekov, G.; Hadjimitova, V. 3-methoxy aroylhydrazones—Free radicals scavenging, anticancer and cytoprotective potency. Redox Rep. 2017, 22, 408–417. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wang, X.; Shi, L.; Yin, W.; Yang, Z.; He, H.; Liang, Y. Synthesis, antitumor activity and mechanism of action of novel 1,3-thiazole derivatives containing hydrazide-hydrazone and carboxamide moiety. Bioorg. Med. Chem. Lett. 2016, 26, 3263–3270. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) (2003). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. EUCAST discussion document E. Dis 5.1. Clin. Microbiol. Infect. 2003, 9, 509–515. [Google Scholar]
- M27-S4; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012.
- DB-ALM Protocol n° 17: MTT Assay ECVAM. Available online: http://cidportal.jrc.ec.europa.eu/ftp/jrc-opendata/EURL-ECVAM/datasets/DBALM/LATEST/online/DBALM_docs/17_P_MTT%20Assay.pdf (accessed on 5 January 2023).
- OECD. OECD Guidelines for the Testing of Chemicals, Fish Embryo Acute Toxicity (FET) Test (no 236); OECD: Paris, France, 2013. [Google Scholar]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Spiegel, M.; Kapusta, K.; Kołodziejczyk, W.; Saloni, J.; Żbikowska, B.; Hill, G.A.; Sroka, Z. Antioxidant Activity of Selected Phenolic Acids–Ferric Reducing Antioxidant Power Assay and QSAR Analysis of the Structural Features. Molecules 2020, 25, 3088. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.F.R.; Froufe, H.J.C.; Abreu, R.M.V.; Martins, A.; Pintado, M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gołębiewska, E.; Świderski, G.; Męczyńska-Wielgosz, S.; Lewandowska, H.; Pietryczuk, A.; Cudowski, A.; Astel, A.; Świsłocka, R.; Samsonowicz, M.; et al. Plant-Derived and Dietary Hydroxybenzoic Acids—A Comprehensive Study of Structural, Anti-/Pro-Oxidant, Lipophilic, Antimicrobial, and Cytotoxic Activity in MDA-MB-231 and MCF-7 Cell Lines. Nutrients 2021, 13, 3107. [Google Scholar] [CrossRef]
- Nasr, T.; Bondock, S.; Rashed, H.M.; Fayad, W.; Youns, M.; Sakr, T.M. Novel hydrazide-hydrazone and amide substituted coumarin derivatives: Synthesis, cytotoxicity screening, microarray, radiolabeling and in vivo pharmacokinetic studies. Eur. J. Med. Chem. 2018, 151, 723–739. [Google Scholar] [CrossRef]
- Krátký, M.; Konečná, K.; Brablíková, M.; Janoušek, J.; Pflégr, V.; Maixnerová, J.; Trejtnar, F.; Vinšová, J. Iodinated 1,2-diacylhydrazines, benzohydrazide-hydrazones and their analogues as dual antimicrobial and cytotoxic agents. Bioorg. Med. Chem. 2021, 41, 116209. [Google Scholar] [CrossRef]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of Zebrafish in Drug Discovery Toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef]
- Osin, O.A.; Yu, T.; Cai, X.; Jiang, Y.; Peng, G.; Cheng, X.; Li, R.; Qin, Y.; Lin, S. Photocatalytic Degradation of 4-Nitrophenol by C, N-TiO2: Degradation Efficiency vs. Embryonic Toxicity of the Resulting Compounds. Front. Chem. 2018, 6, 192. [Google Scholar] [CrossRef]
- Yang, Y.; Li, T.; Yan, L.; Yu, Y.; Wang, S.; Li, C.; Wen, Y.; Zhao, Y. Investigation on the relationship between critical body residue and bioconcentration in zebrafish based on bio-uptake kinetics for five nitro-aromatics. Regul. Toxicol. Pharmacol. 2018, 98, 18–23. [Google Scholar] [CrossRef]
- Melong, N.; Steele, S.; MacDonald, M.; Holly, A.; Collins, C.C.; Zoubeidi, A.; Berman, J.N.; Dellaire, G. Enzalutamide inhibits testosterone-induced growth of human prostate cancer xenografts in zebrafish and can induce bradycardia. Sci. Rep. 2017, 7, 14698. [Google Scholar] [CrossRef]
- Kovács, R.; Csenki, Z.; Bakos, K.; Urbányi, B.; Horváth, A.; Garaj-Vrhovac, V.; Gajski, G.; Gerić, M.; Negreira, N.; de Alda, M.L.; et al. Assessment of toxicity and genotoxicity of low doses of 5-fluorouracil in zebrafish (Danio rerio) two-generation study. Water Res. 2015, 77, 201–212. [Google Scholar] [CrossRef]
- Suresh, D.M.; Sajan, D.; Diao, Y.-P.; Němec, I.; Joe, I.H.; Jothy, V.B. Structural conformations and density functional study on the intramolecular charge transfer on vibrational spectra of 2,4-dihydroxy-N’-(4-methoxybenzylidene)benzohydrazide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 110, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, F.; Smyth, T.J.; Ramachandran, V.N.; Smyth, W.F. A study of the antimicrobial activity of selected synthetic and naturally occurring quinolines. Int. J. Antimicrob. Agents 2010, 35, 30–38. [Google Scholar] [CrossRef] [PubMed]

| Compound Number | Yield (%) | Reaction Time (min) | Melting Point (°C) | Compound Number | Yield (%) | Reaction Time (min) | Melting Point (°C) |
|---|---|---|---|---|---|---|---|
| 1 | 83 | 16 | 250 | 13 | 23 | 40 | 197 |
| 2 | 96 | 15 | 205 | 14 | 70 | 31 | 230 |
| 3 | 98 | 16 | 248 | 15 | 39 | 33 | 244 |
| 4 | 95 | 18 | 263 | 16 | 28 | 39 | 255 |
| 5 | 94 | 17 | 248 | 17 | 66 | 37 | 260 |
| 6 | 95 | 20 | 251 | 18 | 57 | 40 | 220 |
| 7 | 98 | 23 | 246 | 19 | 68 | 33 | 238–240 |
| 8 | 58 | 26 | 252 | 20 | 72 | 36 | 246–248 |
| 9 | 63 | 30 | 256 | 21 | 59 | 36 | 252–254 |
| 10 | 81 | 31 | 260 | 22 | 63 | 37 | 244–246 |
| 11 | 98 | 29 | 232 | 23 | 75 | 40 | 243–245 |
| 12 | 96 | 36 | 240 | 24 | 78 | 40 | 251–253 |
| Species | MIC (MBC or MFC) [µg/mL] and {MBC/MIC or MFC/MIC} Values of the Tested Compounds and Reference Medicines | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 5 | 6 | 7 | 9 | CIP/VA * /NY ** | NIT | CFX | APC | ||
| Gram-positive bacteria | Staphylococcus aureus ATCC 43300 | 1000 (>1000) {>1} | 250 (500) {2} | - | - | 1000 (>1000) {>1} | - | 15.62 (62.5) {4} | 0.24 (0.24) {1} | 7.81 (15.62) | nd | nd |
| Staphylococcus aureus ATCC 29213 | 1000 (>1000) {>1} | 250 (250) {1} | - | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 31.25 (31.25) {1} | 0.48 (0.48) {1} | nd | nd | nd | |
| Staphylococcus epidermidis ATCC 12228 | - | 250 (500) {2} | - | - | 1000 (>1000) {>1} | 500 (1000) {2} | 62.5 (125) {2} | 0.12 (0.12) {1} | 3.91 (7.81) | 0.24 | nd | |
| Enterococcus faecalis ATCC 29212 | 1000 (>1000) {>1} | 500 (500) {1} | - | 1000 (>1000) {>1} | - | - | 125 (500) {4} | 0.98 * (1.95) {2} | nd | nd | nd | |
| Micrococcus luteus ATCC 10240 | 500 (>1000) {>2} | 31.25 (250) {8} | 500 (>1000) {>2} | 500 (>1000) {>2} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 125 (500) {4} | 0.98 (1.95) {2} | 62.5 (62.5) | 0.98 | nd | |
| Bacillus subtilis ATCC 6633 | 500 (>1000) {>2} | 62.5 (500) {8} | - | 500 (>1000) {>2} | 1000 (>1000) {>1} | - | 125 (500) {4} | 0.03 (0.03) {1} | 3.91 (3.91) | 15.62 | 62.5 | |
| Bacillus cereus ATCC 10876 | - | 125 (250) {2} | - | 500 (>1000) {>2} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 15.62 (62.5) {4} | 0.06 (0.12) {2} | 7.81 (15.62) | 31.25 | nd | |
| Gram-negative bacteria | Bordetella bronchiseptica ATCC 4617 | - | 1000 (>1000) {>1} | - | - | - | - | - | 0.98 (0.98) {1} | 125 (>1000) | nd | nd |
| Klebsiella pneumoniae ATCC 13883 | - | 1000 (>1000) {>1} | - | - | - | - | - | 0.12 (0.24) {2} | 15.62 (31.25) | nd | nd | |
| Proteus mirabilis ATCC 12453 | - | 1000 (>1000) {>1} | - | - | - | - | - | 0.03 (0.03) {1) | 62.5 (125) | nd | nd | |
| Salmonella typhimurium ATCC 14028 | - | 1000 (>1000) {>1} | - | - | - | - | - | 0.06 (0.06) {1} | 31.25 (62.5) | nd | nd | |
| Escherichia coli ATCC 25922 | - | 1000 (>1000) {>1} | - | - | - | - | - | 0.004 (0.008) {2} | 7.81 (15.62) | nd | nd | |
| Pseudomonas aeruginosa ATCC 9027 | - | - | - | - | - | - | - | 0.48 (0.98) {2} | nd | nd | nd | |
| Fungi | Candida albicans ATCC 2091 | - | - | - | 500 (>1000) {>2} | 500 (>1000) {>2} | 500 (>1000) {>2} | - | 0.24 ** (0.24) {1} | na | na | na |
| Candida albicans ATCC 10231 | - | - | - | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | - | 0.48 ** (0.48) {1} | na | na | na | |
| Candida parapsilosis ATCC 22019 | - | 500 (>1000) {>2} | - | - | 1000 (>1000) {>1} | - | - | 0.24 ** (0.48) {2} | na | na | na | |
| Candida glabrata ATCC 90030 | - | 250 (500) {2} | - | - | - | - | - | 0.24 ** (0.48) {2} | na | na | na | |
| Candida krusei ATCC 14243 | - | - | - | - | - | - | - | 0.24 ** (0.24) {1} | na | na | na | |
| Species | MIC (MBC or MFC) [µg/mL] and {MBC/MIC or MFC/MIC} Values of the Tested Compounds and Reference Medicines | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 11 | 13 | 14 | 15 | 16 | 17 | 18 | CIP/VA * /NY ** | NIT | CFX | APC | ||
| Gram-positive bacteria | Staphylococcus aureus ATCC 43300 | 31.25 (500) {16} | 500 (1000) {2} | 1000 (>1000) {>1} | - | - | - | 62.5 (>1000) {>16} | 3.91 (31.25) {8} | 0.24 (0.24) {1} | 7.81 (15.62) | nd | nd |
| Staphylococcus aureus ATCC 29213 | 15.62 (250) {16} | 250 (>1000) {>4} | 1000 (>1000) {>1} | - | - | 1000 (>1000) {>1} | 31.25 (>1000) {32} | 7.81 (15.62) {2} | 0.48 (0.48) {1} | nd | nd | nd | |
| Staphylococcus epidermidis ATCC 12228 | 1000 (1000) {>1} | 500 (1000) {2} | 1000 (>1000) {>1} | - | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 250 (>1000) {>8} | 0.98 (7.81) {8} | 0.12 (0.12) {1} | 3.91 (7.81) | 0.24 | nd | |
| Enterococcus faecalis ATCC 29212 | 250 (1000) {4} | 500 (>1000) {>2} | 1000 (>1000) {>1} | - | - | 1000 (>1000) {>1} | 500 (>1000) {>1} | 3.91 (15.62) {4} | 0.98 * (1.95) {2} | nd | nd | nd | |
| Micrococcus luteus ATCC 10240 | 250 (>1000) {>4} | 125 (>1000) {>8} | 1000 (>1000) {>1} | 125 (>1000) {>8} | 500 (>1000) {>2} | 250 (>1000) {>4} | 125 (>1000) {>8} | 0.48 (1.95) {4} | 0.98 (1.95) {2} | 62.5 (62.5) | 0.98 | nd | |
| Bacillus subtilis ATCC 6633 | 250 (>1000) {>4} | 500 (>1000) {>2} | 1000 (>1000) {>1} | - | - | 250 (>1000) {>4} | 125 (>1000) {>8} | 1.95 (3.91) {2} | 0.03 (0.03) {1} | 3.91 (3.91) | 15.62 | 62.5 | |
| Bacillus cereus ATCC 10876 | 125 (250) {2} | 250 (>1000) {>4} | 1000 (>1000) {>1} | 500 (>1000) {>2} | - | 1000 (>1000) {>1} | 250 (>1000) {>4} | 3.91 (15.62) {4} | 0.06 (0.12) {2} | 7.81 (15.62) | 31.25 | nd | |
| Gram-negative bacteria | Bordetella bronchiseptica ATCC 4617 | - | - | 1000 (>1000) {>1} | 1000 (1000) {1} | - | - | 1000 (>1000) {>1} | 500 (>1000) {>2} | 0.98 (0.98) {1} | 125 (>1000) | nd | nd |
| Klebsiella pneumoniae ATCC 13883 | - | - | 1000 (>1000) {>1} | - | - | - | 1000 (>1000) {>1} | 500 (>1000) {>2} | 0.12 (0.24) {2} | 15.62 (31.25) | nd | nd | |
| Proteus mirabilis ATCC 12453 | - | - | 1000 (>1000) {>1} | - | - | - | 1000 (>1000) {>1} | 500 (>1000) {>2} | 0.03 (0.03) {1) | 62.5 (125) | nd | nd | |
| Salmonella typhimurium ATCC 14028 | - | - | 1000 (>1000) {>1} | - | - | - | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 0.06 (0.06) {1} | 31.25 (62.5) | nd | nd | |
| Escherichia coli ATCC 25922 | - | - | 1000 (>1000) {>1} | - | - | - | 1000 (>1000) {>1} | 500 (>1000) {>2} | 0.004 (0.008) {2} | 7.81 (15.62) | nd | nd | |
| Pseudomonas aeruginosa ATCC 9027 | - | - | 1000 (>1000) {>1} | - | - | - | - | 1000 (>1000) {>1} | 0.48 (0.98) {2} | nd | nd | nd | |
| Fungi | Candida albicans ATCC 2091 | - | - | - | - | - | 250 (>1000) {>4} | 1000 (>1000) {>1} | 500 (>1000) {>2} | 0.24 ** (0.24) {1} | na | na | na |
| Candida albicans ATCC 10231 | - | - | 1000 (>1000) {>1} | 500 (>1000) {>2} | - | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 0.48 ** (0.48) {1} | na | na | na | |
| Candida parapsilosis ATCC 22019 | - | - | 1000 (>1000) {>1} | - | 1000 (>1000) {>1} | - | - | 500 (>1000) {>2} | 0.24 ** (0.48) {2} | na | na | na | |
| Candida glabrata ATCC 90030 | - | - | 1000 (>1000) {>1} | - | - | - | - | 1000 (>1000) {>1} | 0.24 ** (0.48) {2} | na | na | na | |
| Candida krusei ATCC 14243 | - | - | - | - | - | - | - | 1000 (>1000) {>1} | 0.24 ** (0.24) {1} | na | na | na | |
| Species | MIC (MBC or MFC) [µg/mL] and {MBC/MIC or MFC/MIC} Values of the Tested Compounds and Reference Medicines | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 19 | 20 | 21 | 22 | 23 | 24 | CIP/VA * /NY ** | NIT | CFX | APC | ||
| Gram-positive bacteria | Staphylococcus aureus ATCC 43300 | 500 (>1000) {>2} | - | 500 (>1000) {>2} | - | - | - | 0.24 (0.24) {1} | 7.81 (15.62) | nd | nd |
| Staphylococcus aureus ATCC 29213 | 1000 (>1000) {>1} | - | 500 (>1000) {>2} | - | - | - | 0.48 (0.48) {1} | nd | nd | nd | |
| Staphylococcus epidermidis ATCC 12228 | 500 (>1000) {>2} | - | 500 (>1000) {>2} | 1000 (>1000) {>1} | 500 (>1000) {>2} | - | 0.12 (0.12) {1} | 3.91 (7.81) | 0.24 | nd | |
| Enterococcus faecalis ATCC 29212 | 500 (>1000) {>2} | - | 500 (>1000) {>4} | - | - | 1000 (>1000) {>1} | 0.98 * (1.95) {2} | nd | nd | nd | |
| Micrococcus luteus ATCC 10240 | 1000 (>1000) {>1} | - | 1000 (>1000) {>1} | - | - | 1000 (>1000) {>1} | 0.98 (1.95) {2} | 62.5 (62.5) | 0.98 | nd | |
| Bacillus subtilis ATCC 6633 | 250 (>1000) {>4} | 250 (>1000) {>4} | 500 (>1000) {>2} | - | - | 500 (>1000) {>2} | 0.03 (0.03) {1} | 3.91 (3.91) | 15.62 | 62.5 | |
| Bacillus cereus ATCC 10876 | 250 (>1000) {>4} | - | 500 (>1000) {>2} | - | - | 1000 (>1000) {>1} | 0.06 (0.12) {2} | 7.81 (15.62) | 31.25 | nd | |
| Gram-negative bacteria | Bordetella bronchiseptica ATCC 4617 | 1000 (>1000) {>1} | - | - | - | - | - | 0.98 (0.98) {1} | 125 (>1000) | nd | nd |
| Klebsiella pneumonia ATCC 13883 | 500 (>1000) {>1} | - | - | - | - | - | 0.12 (0.24) {2} | 15.62 (31.25) | nd | nd | |
| Proteus mirabilis ATCC 12453 | 1000 (>1000) {>1} | - | - | - | - | - | 0.03 (0.03) {1) | 62.5 (125) | nd | nd | |
| Salmonella typhimurium ATCC 14028 | 1000 (>1000) {>1} | - | - | - | - | - | 0.06 (0.06) {1} | 31.25 (62.5) | nd | nd | |
| Escherichia coli ATCC 25922 | 1000 (>1000) {>1} | - | - | - | - | - | 0.004 (0.008) {2} | 7.81 (15.62) | nd | nd | |
| Pseudomonas aeruginosa ATCC 9027 | 1000 (>1000) {>1} | - | - | - | - | - | 0.48 (0.98) {2} | nd | nd | nd | |
| Fungi | Candida albicans ATCC 2091 | 500 (>1000) {>2} | - | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 0.24 ** (0.24) {1} | na | na | na |
| Candida albicans ATCC 10231 | 250 (>1000) {>4} | 1000 (>1000) {>1} | 500 (>1000) {>2} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 0.48 ** (0.48) {1} | na | na | na | |
| Candida parapsilosis ATCC 22019 | 250 (>1000) {>4} | 1000 (>1000) {>1} | 500 (>1000) {>2} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 0.24 ** (0.48) {2} | na | na | na | |
| Candida glabrata ATCC 90030 | 500 (>1000) {>2} | - | - | - | - | - | 0.24 ** (0.48) {2} | na | na | na | |
| Candida krusei ATCC 14243 | 500 (>1000) {>2} | - | 500 (>1000) {>2} | 1000 (>1000) {>1} | - | 1000 (>1000) {>1} | 0.24 ** (0.24) {1} | na | na | na | |
| Compound | R | IC50 in HEK-293, µM | IC50 in 769-P, µM | Selectivity Index a | IC50 in HepG2, µM | Selectivity Index a | IC50 in H1563, µM | Selectivity Index a | IC50 in LN-229, µM | Selectivity Index a |
|---|---|---|---|---|---|---|---|---|---|---|
| 19 | 2-NO2-Ph | 1.49 × 105 | 45.42 | 3.29 × 103 | >500.00 | nd | 65.57 | 2.27 × 103 | 130.17 | 1.14 × 103 |
| 20 | 3-NO2-Ph | 1.34 × 104 | >500.00 | nd | >500.00 | nd | 70.94 | 1.89 × 102 | >500.00 | nd |
| 21 | 4-NO2-Ph | 4.45 × 1010 | 12.39 | 3.59 × 109 | 7.81 | 5.69 × 109 | >500.00 | nd | 0.77 | 5.78 × 109 |
| 22 | 2-Cl-Ph | 314.68 | 262.48 | 1.20 | nd | nd | 216.73 | 1.45 | 110.53 | 2.85 |
| 23 | 2-Br-Ph | 9.18 × 104 | >500.00 | nd | nd | nd | 101.14 | 9.08 × 102 | 156.77 | 5.85 × 102 |
| 24 | 2-F-Ph | 386.73 | >500.00 | nd | >500.00 | nd | 199.69 | 1.94 | 199.32 | 1.94 |
| Compound | R | LC50, µM | LC50, mg/L |
|---|---|---|---|
| 19 | 2-NO2-Ph | 1.88 × 105 | 5.68 × 104 |
| 20 | 3-NO2-Ph | 3.48 × 104 | 1.05 × 104 |
| 21 | 4-NO2-Ph | 370.00 | 110.00 |
| 22 | 2-Cl-Ph | 56.56 | 16.44 |
| 23 | 2-Br-Ph | 85.62 | 30.41 |
| 24 | 2-F-Ph | 4.24 × 103 | 1.16 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popiołek, Ł.; Gawrońska-Grzywacz, M.; Dziduch, A.; Biernasiuk, A.; Piątkowska-Chmiel, I.; Herbet, M. Design, Synthesis, and In Vitro and In Vivo Bioactivity Studies of Hydrazide–Hydrazones of 2,4-Dihydroxybenzoic Acid. Int. J. Mol. Sci. 2023, 24, 17481. https://doi.org/10.3390/ijms242417481
Popiołek Ł, Gawrońska-Grzywacz M, Dziduch A, Biernasiuk A, Piątkowska-Chmiel I, Herbet M. Design, Synthesis, and In Vitro and In Vivo Bioactivity Studies of Hydrazide–Hydrazones of 2,4-Dihydroxybenzoic Acid. International Journal of Molecular Sciences. 2023; 24(24):17481. https://doi.org/10.3390/ijms242417481
Chicago/Turabian StylePopiołek, Łukasz, Monika Gawrońska-Grzywacz, Aleksandra Dziduch, Anna Biernasiuk, Iwona Piątkowska-Chmiel, and Mariola Herbet. 2023. "Design, Synthesis, and In Vitro and In Vivo Bioactivity Studies of Hydrazide–Hydrazones of 2,4-Dihydroxybenzoic Acid" International Journal of Molecular Sciences 24, no. 24: 17481. https://doi.org/10.3390/ijms242417481






