Extraciliary OFD1 Is Involved in Melanocyte Survival through Cell Adhesion to ECM via Paxillin
Abstract
:1. Introduction
2. Results
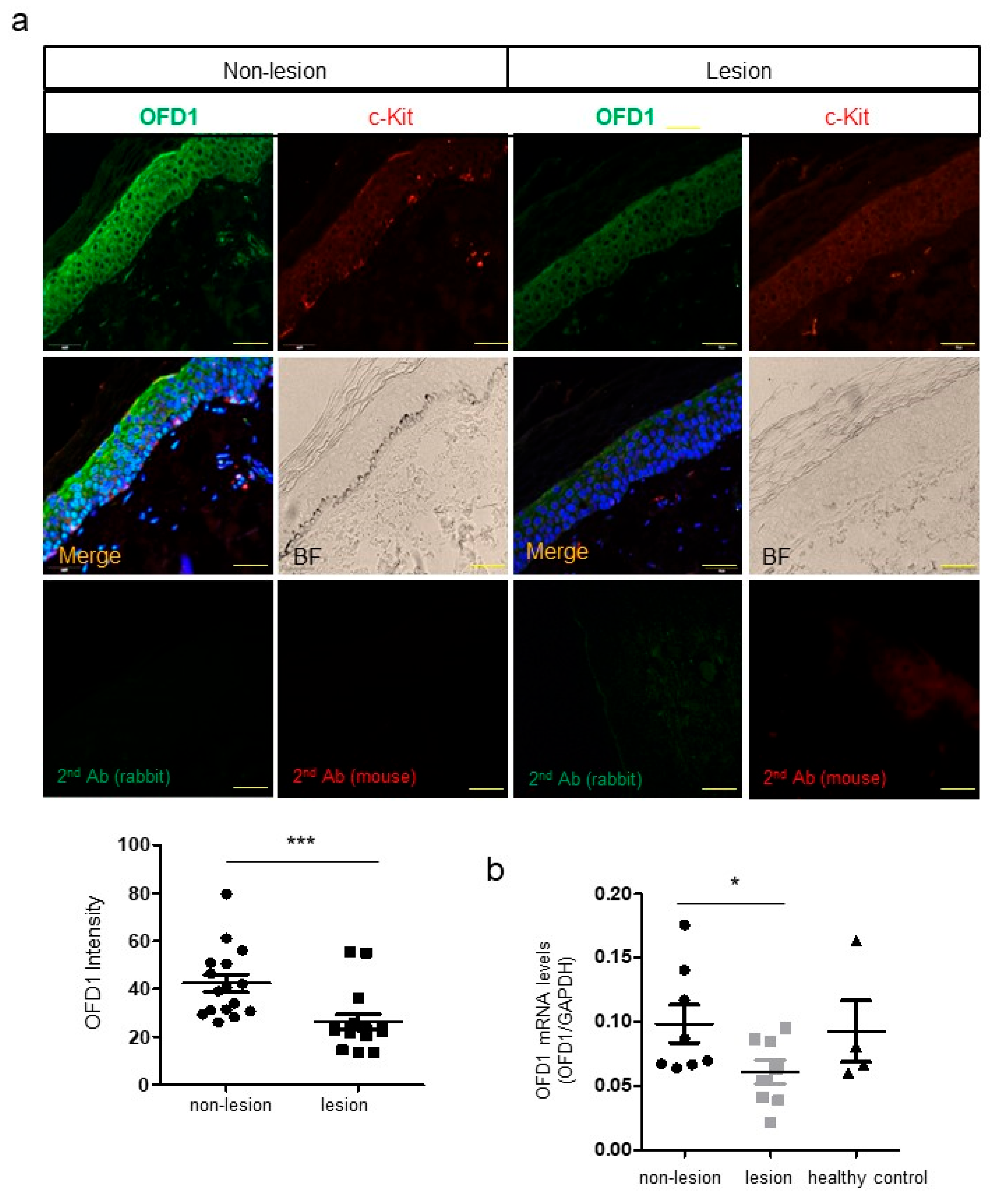
2.1. OFD1 Is Downregulated in Lesional Epidermis of Patients with Vitiligo
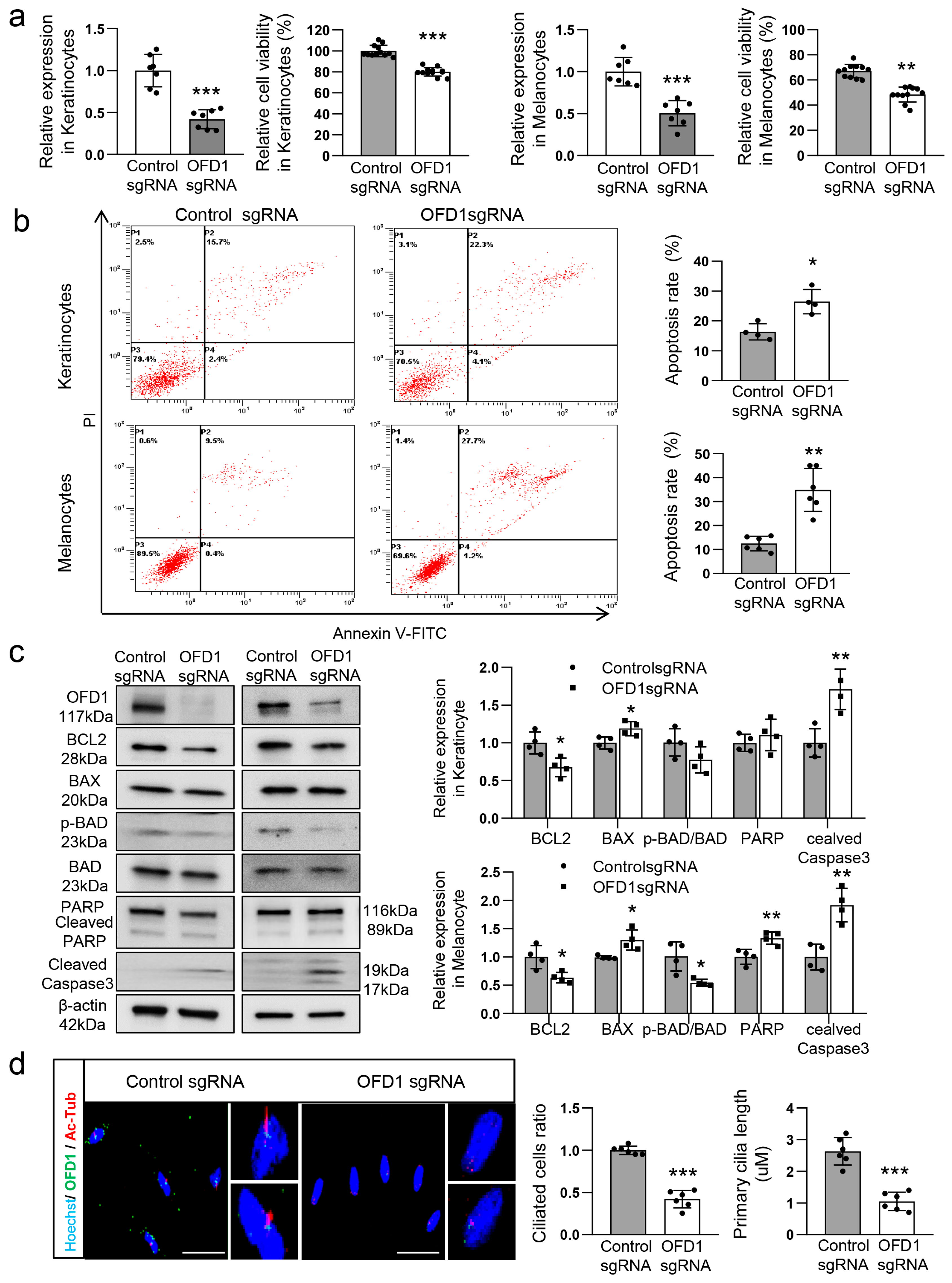
2.2. OFD1 Is Involved in Ciliogenesis and Apoptosis of Keratinocytes and Melanocytes
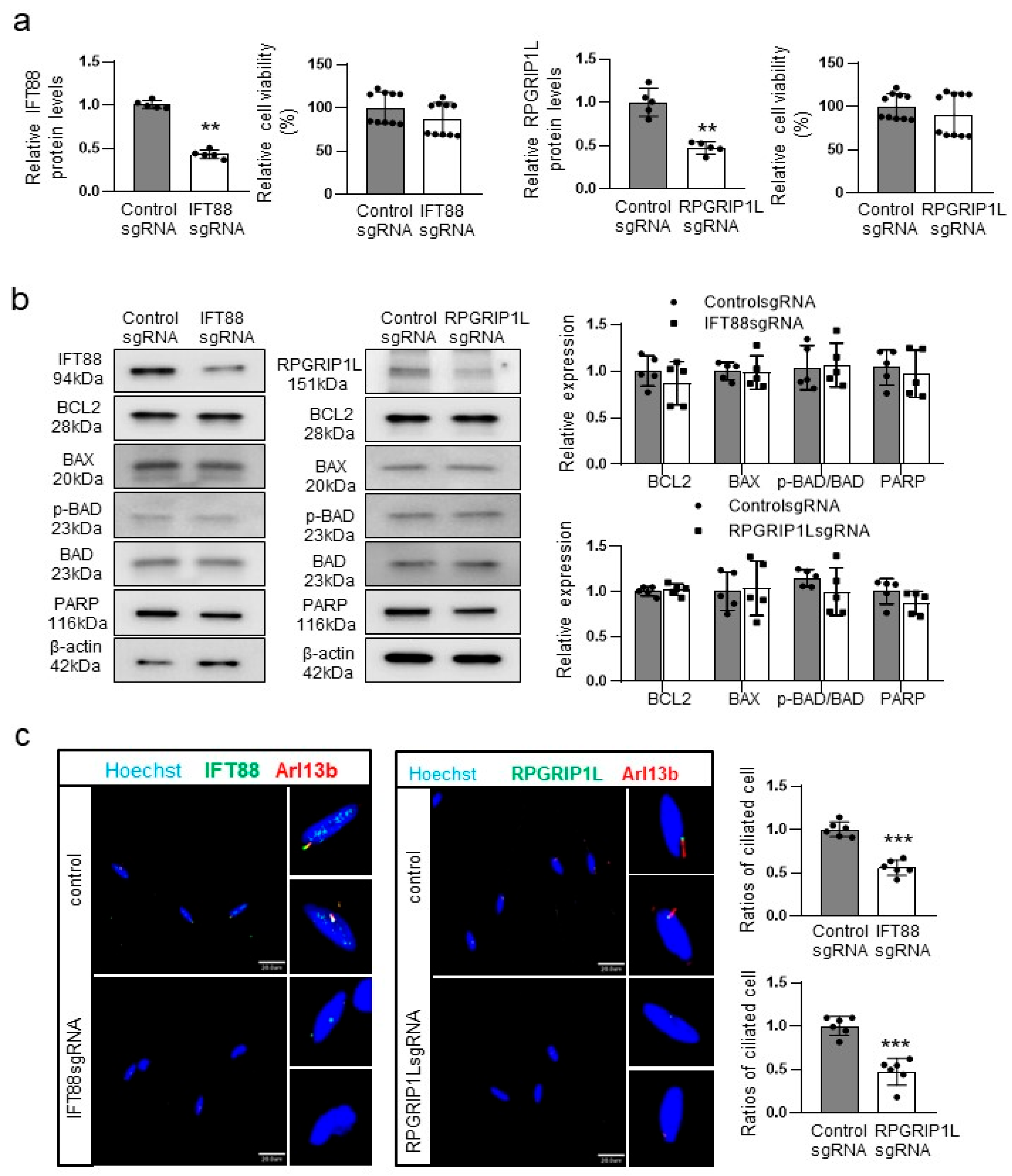
2.3. Knockdown of IFT88 or RPGRIP1L Impairs Ciliogenesis without Increasing Melanocyte Apoptosis
2.4. Knockdown of OFD1, IFT88, or RPGRIP1L Reduces Hedgehog Signaling Pathways in Melanocytes
2.5. OFD1 Knockdown Reduces Interactions with the Extracellular Matrix (ECM) via Paxillin
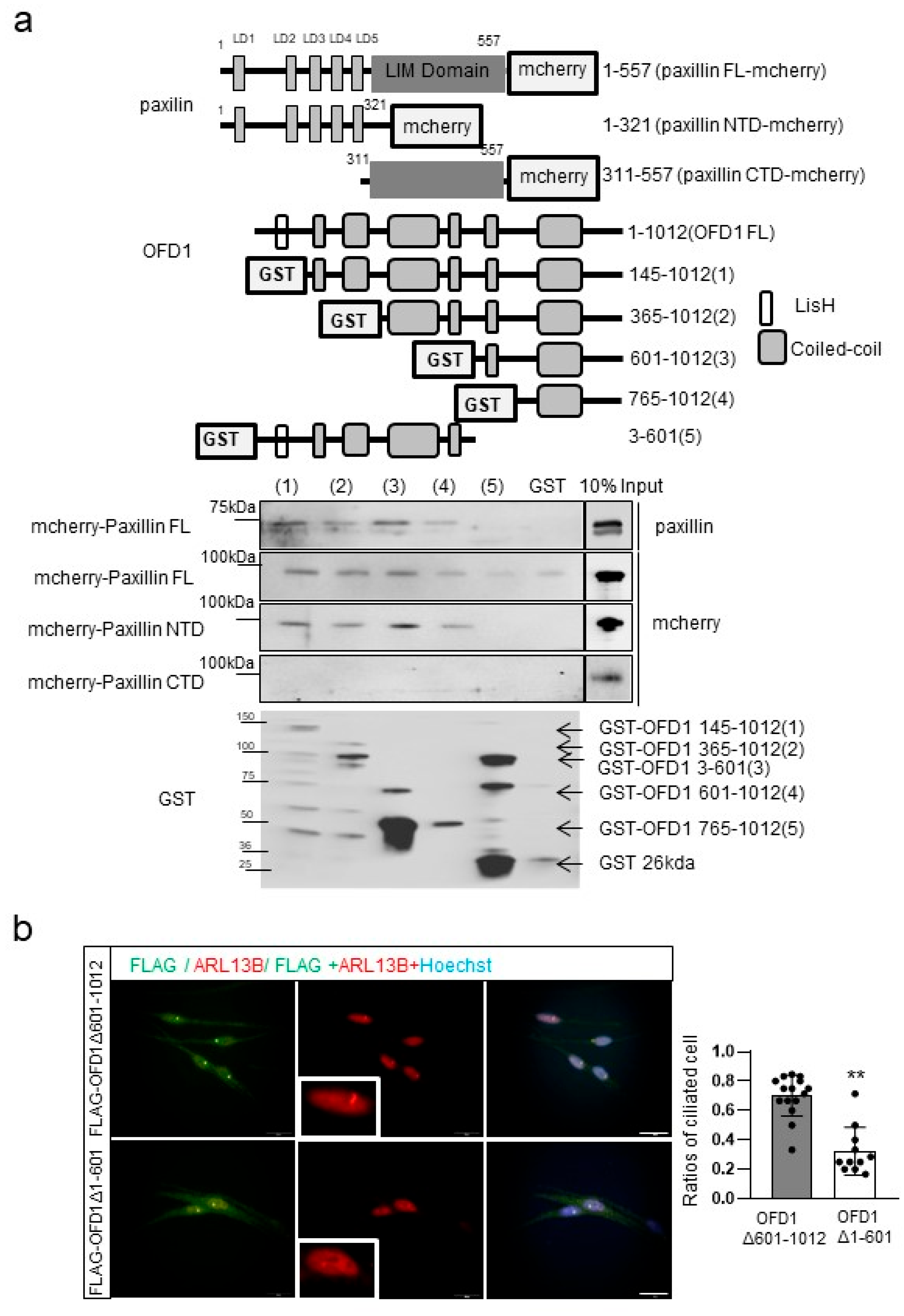
2.6. OFD1 Domains Involved in Paxillin Binding Differ from Those Participating in Ciliogenesis
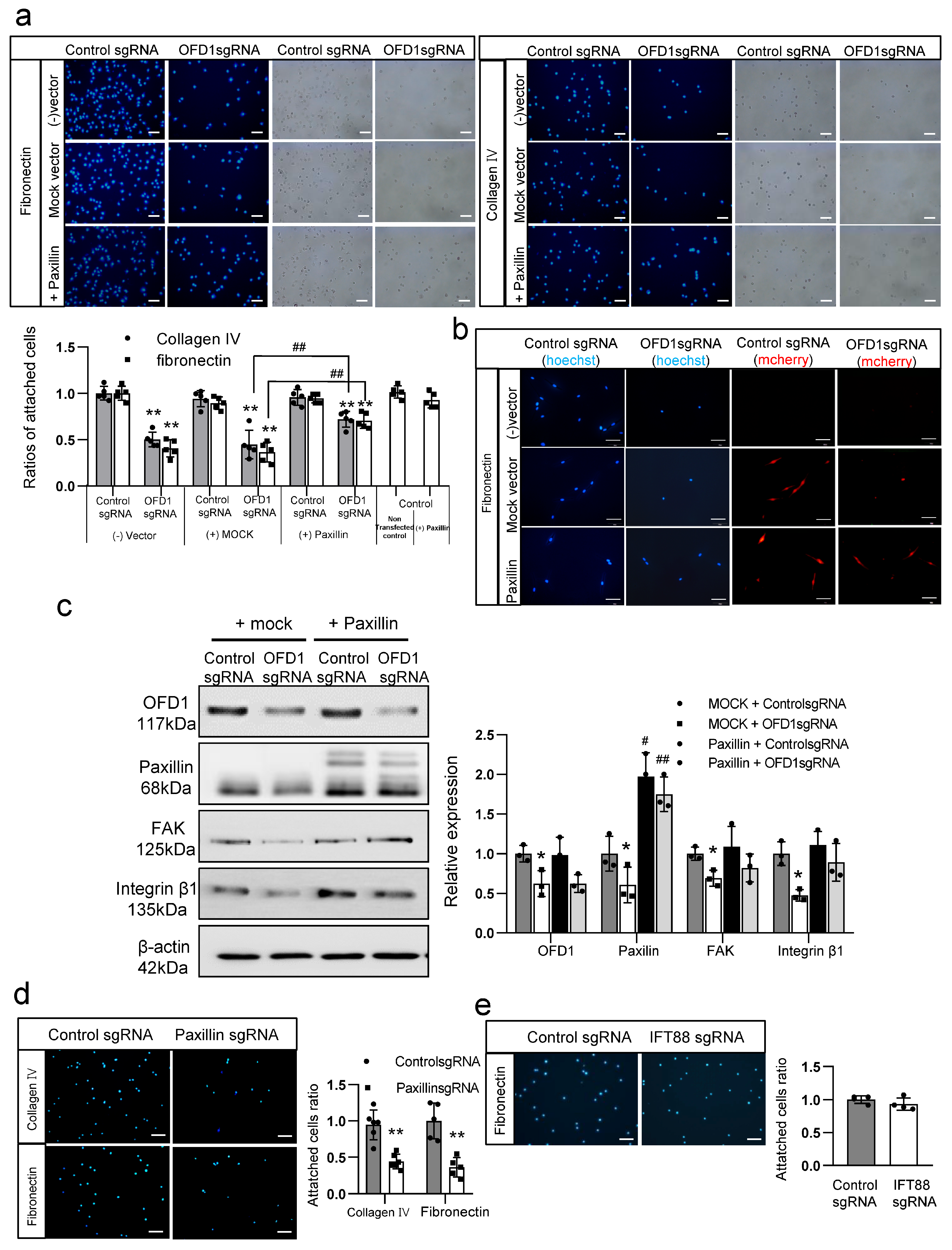
2.7. Downregulation of Paxillin by OFD1 Knockdown Inhibits Melanocyte Adhesion to the ECM
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Normal Human Skin Keratinocyte and Melanocyte Culture
4.3. Knockdown of OFD1, IFT88, or RIGRIP1L and Overexpression of OFD1 or PXN
4.4. Real-Time PCR
4.5. Western Blot Analysis
4.6. Immunoprecipitation
4.7. Immunofluorescent and Confocal Microscopy
4.8. Plasmid Construction
4.9. GST Pull-Down Assay
4.10. Cell Viability Test
4.11. Flow Cytometry Analysis
4.12. Cell Adhesion Assay
4.13. Statistical Analysis
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saif, G.A.B.; Alshammary, A.F.; Ali Khan, I. Evaluation of CAT Variants A-89T, C389T, and C419T in Patients with Vitiligo in the Saudi Population. Medicina 2023, 59, 708. [Google Scholar] [CrossRef] [PubMed]
- Laddha, N.C.; Dwivedi, M.; Mansuri, M.S.; Gani, A.R.; Ansarullah, M.; Ramachandran, A.; Dalai, S.; Begum, R. Vitiligo: Interplay between oxidative stress and immune system. Exp. Dermatol. 2013, 22, 245–250. [Google Scholar] [CrossRef] [PubMed]
- van Reeuwijk, J.; Arts, H.H.; Roepman, R. Scrutinizing ciliopathies by unraveling ciliary interaction networks. Hum. Mol. Genet. 2011, 20, R149–R157. [Google Scholar] [CrossRef]
- Wang, S.; Dong, Z. Primary cilia and kidney injury: Current research status and future perspectives. Am. J. Physiol.-Ren. Physiol. 2013, 305, F1085–F1098. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.M.; Leaper, M.J.; Bayliss, R. The primary cilium: Guardian of organ development and homeostasis. Organogenesis 2014, 10, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Pala, R.; Alomari, N.; Nauli, S.M. Primary cilium-dependent signaling mechanisms. Int. J. Mol. Sci. 2017, 18, 2272. [Google Scholar] [CrossRef] [PubMed]
- Hosio, M.; Jaks, V.; Lagus, H.; Vuola, J.; Ogawa, R.; Kankuri, E. Primary ciliary signaling in the skin—Contribution to wound healing and scarring. Front. Cell Dev. Biol. 2020, 8, 578384. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, F.; Benzing, T.; Katsanis, N. Ciliopathies. N. Engl. J. Med. 2011, 364, 1533–1543. [Google Scholar] [CrossRef]
- Warfvinge, K. Single cilia in human epidermis are susceptible to challenge. Acta Derm.-Venereol. 1995, 75, 446–448. [Google Scholar] [CrossRef]
- Reiter, J.F.; Leroux, M.R. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 2017, 18, 533–547. [Google Scholar] [CrossRef]
- Lang, U.E.; Torres, R.; Cheung, C.; Vladar, E.K.; McCalmont, T.H.; Kim, J.; Judson-Torres, R.L. Ciliation index is a useful diagnostic tool in challenging spitzoid melanocytic neoplasms. J. Investig. Dermatol. 2020, 140, 1401–1409.e1402. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Lu, C.; Sheng, F.; Li, C.; Li, H.; Ding, N.; Chen, Y.; Zhang, T.; Yang, K.; Xu, Y. Analysis of potential genes associated with primary cilia in bladder cancer. Cancer Manag. Res. 2018, 10, 3047–3056. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.I.; Zullo, A.; Barra, A.; Bimonte, S.; Messaddeq, N.; Studer, M.; Dollé, P.; Franco, B. Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nat. Genet. 2006, 38, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Singla, V.; Reiter, J.F. The primary cilium as the cell’s antenna: Signaling at a sensory organelle. Science 2006, 313, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Bruel, A.-L.; Franco, B.; Duffourd, Y.; Thevenon, J.; Jego, L.; Lopez, E.; Deleuze, J.-F.; Doummar, D.; Giles, R.H.; Johnson, C.A. Fifteen years of research on oral–facial–digital syndromes: From 1 to 16 causal genes. J. Med. Genet. 2017, 54, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Pezzella, N.; Bove, G.; Tammaro, R.; Franco, B. OFD1: One gene, several disorders. Am. J. Med. Genet. Part C Semin. Med. Genet. 2022, 190, 57–71. [Google Scholar]
- Wang, J.; Chen, X.; Wang, F.; Zhang, J.; Li, P.; Li, Z.; Xu, J.; Gao, F.; Jin, C.; Tian, H. OFD1, as a ciliary protein, exhibits neuroprotective function in photoreceptor degeneration models. PLoS ONE 2016, 11, e0155860. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.B.; Rosenbaum, J.L. Chapter two intraflagellar transport (IFT): Role in ciliary assembly, resorption and signalling. Curr. Top. Dev. Biol. 2008, 85, 23–61. [Google Scholar]
- Mahuzier, A.; Gaudé, H.-M.; Grampa, V.; Anselme, I.; Silbermann, F.; Leroux-Berger, M.; Delacour, D.; Ezan, J.; Montcouquiol, M.; Saunier, S. Dishevelled stabilization by the ciliopathy protein Rpgrip1l is essential for planar cell polarity. J. Cell Biol. 2012, 198, 927–940. [Google Scholar] [CrossRef]
- Lee, A.-Y.; Kim, N.-H.; Choi, W.-I.; Youm, Y.-H. Less keratinocyte-derived factors related to more keratinocyte apoptosis in depigmented than normally pigmented suction-blistered epidermis may cause passive melanocyte death in vitiligo. J. Investig. Dermatol. 2005, 124, 976–983. [Google Scholar] [CrossRef]
- Lee, A.-Y. Role of keratinocytes in the development of vitiligo. Ann. Dermatol. 2012, 24, 115–125. [Google Scholar] [CrossRef]
- Christensen, S.T.; Pedersen, S.F.; Satir, P.; Veland, I.R.; Schneider, L. The primary cilium coordinates signaling pathways in cell cycle control and migration during development and tissue repair. Curr. Top. Dev. Biol. 2008, 85, 261–301. [Google Scholar] [PubMed]
- Seeger-Nukpezah, T.; Golemis, E.A. The extracellular matrix and ciliary signaling. Curr. Opin. Cell Biol. 2012, 24, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Ballestrem, C.; Erez, N.; Kirchner, J.; Kam, Z.; Bershadsky, A.; Geiger, B. Molecular mapping of tyrosine-phosphorylated proteins in focal adhesions using fluorescence resonance energy transfer. J. Cell Sci. 2006, 119, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Deakin, N.O.; Turner, C.E. Paxillin comes of age. J. Cell Sci. 2008, 121, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.A.; Prosser, S.L.; Romio, L.; Hirst, R.A.; O’Callaghan, C.; Woolf, A.S.; Fry, A.M. Centriolar satellites are assembly points for proteins implicated in human ciliopathies, including oral-facial-digital syndrome 1. J. Cell Sci. 2011, 124, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Shaner, N.C.; Campbell, R.E.; Steinbach, P.A.; Giepmans, B.N.; Palmer, A.E.; Tsien, R.Y. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004, 22, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Singla, V.; Romaguera-Ros, M.; Garcia-Verdugo, J.M.; Reiter, J.F. Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Dev. Cell 2010, 18, 410–424. [Google Scholar] [CrossRef]
- McGlashan, S.R.; Jensen, C.G.; Poole, C.A. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J. Histochem. Cytochem. 2006, 54, 1005–1014. [Google Scholar] [CrossRef]
- Wu, J.; Du, H.; Wang, X.; Mei, C.; Sieck, G.C.; Qian, Q. Characterization of primary cilia in human airway smooth muscle cells. Chest 2009, 136, 561–570. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Gauthier, Y.; Andre, M.C.; Taïeb, A. A critical appraisal of vitiligo etiologic theories. Is melanocyte loss a melanocytorrhagy? Pigment Cell Res. 2003, 16, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Gupta, S.; Parsad, D. Melanocyte Adhesion and apoptosis in Vitiligo: Linking puzzle blocks. Curr. Mol. Med. 2023, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.A.; Liang, H.; Cassidy, L.L. Developmental regulation of focal contact protein expression in human melanocytes. Pigment Cell Res. 1995, 8, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Ghata, J.; Cowley, B.D., Jr. Polycystic kidney disease. Compr. Physiol. 2011, 7, 945–975. [Google Scholar]
- Prattichizzo, C.; Macca, M.; Novelli, V.; Giorgio, G.; Barra, A.; Franco, B. Mutational spectrum of the oral-facial-digital type I syndrome: A study on a large collection of patients. Hum. Mutat. 2008, 29, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Coene, K.L.; Roepman, R.; Doherty, D.; Afroze, B.; Kroes, H.Y.; Letteboer, S.J.; Ngu, L.H.; Budny, B.; van Wijk, E.; Gorden, N.T. OFD1 is mutated in X-linked Joubert syndrome and interacts with LCA5-encoded lebercilin. Am. J. Hum. Genet. 2009, 85, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Macca, M.; Franco, B. The molecular basis of oral-facial-digital syndrome, type 1. Am. J. Med. Genet. Part C Semin. Med. Genet. 2009, 151C, 318–325. [Google Scholar]
- Sero, J.E.; German, A.E.; Mammoto, A.; Ingber, D.E. Paxillin controls directional cell motility in response to physical cues. Cell Adhes. Migr. 2012, 6, 502–508. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, M.S.; Park, H.J.; Lee, H.; Yun, J.I.; Lim, H.W.; Lee, S.T. Screening of integrins localized on the surface of human epidermal melanocytes. In Vitro Cell. Dev. Biol.-Anim. 2020, 56, 435–443. [Google Scholar] [CrossRef]
- Stenman, S.; Vaheri, A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J. Exp. Med. 1978, 147, 1054–1064. [Google Scholar] [CrossRef]
- Kumar, R.; Parsad, D.; Kanwar, A. Role of apoptosis and melanocytorrhagy: A comparative study of melanocyte adhesion in stable and unstable vitiligo. Br. J. Dermatol. 2011, 164, 187–191. [Google Scholar] [CrossRef]
- Abdou, A.G.; Maraee, A.H.; Shoeib, M.A.E.-M.; Elbana, R. Immunolocalization of tenascin-C in vitiligo. Appl. Immunohistochem. Mol. Morphol. 2012, 20, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Ricard, A.S.; Pain, C.; Daubos, A.; Ezzedine, K.; Lamrissi-Garcia, I.; Bibeyran, A.; Guyonnet-Dupérat, V.; Taieb, A.; Cario-André, M. Study of CCN3 (NOV) and DDR1 in normal melanocytes and vitiligo skin. Exp. Dermatol. 2012, 21, 411–416. [Google Scholar] [CrossRef]
- Zhang, R.; Premi, S.; Kilic, S.S.; Bacchiocchi, A.; Halaban, R.; Brash, D.E. Clonal growth of human melanocytes using cell-free extracellular matrix. Pigment Cell Melanoma Res. 2013, 26, 925. [Google Scholar] [CrossRef]
- Bin, B.-H.; Kim, D.-K.; Kim, N.-H.; Choi, E.-J.; Bhin, J.; Kim, S.T.; Gho, Y.S.; Lee, A.-Y.; Lee, T.R.; Cho, E.-G. Fibronectin-containing extracellular vesicles protect melanocytes against ultraviolet radiation-induced cytotoxicity. J. Investig. Dermatol. 2016, 136, 957–966. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.-H.; Lee, C.H.; Lee, A.-Y. Extraciliary OFD1 Is Involved in Melanocyte Survival through Cell Adhesion to ECM via Paxillin. Int. J. Mol. Sci. 2023, 24, 17528. https://doi.org/10.3390/ijms242417528
Kim N-H, Lee CH, Lee A-Y. Extraciliary OFD1 Is Involved in Melanocyte Survival through Cell Adhesion to ECM via Paxillin. International Journal of Molecular Sciences. 2023; 24(24):17528. https://doi.org/10.3390/ijms242417528
Chicago/Turabian StyleKim, Nan-Hyung, Chang Hoon Lee, and Ai-Young Lee. 2023. "Extraciliary OFD1 Is Involved in Melanocyte Survival through Cell Adhesion to ECM via Paxillin" International Journal of Molecular Sciences 24, no. 24: 17528. https://doi.org/10.3390/ijms242417528




