Pomegranate (Punica granatum L.) and Its Rich Ellagitannins as Potential Inhibitors in Ulcerative Colitis
Abstract
:1. Introduction
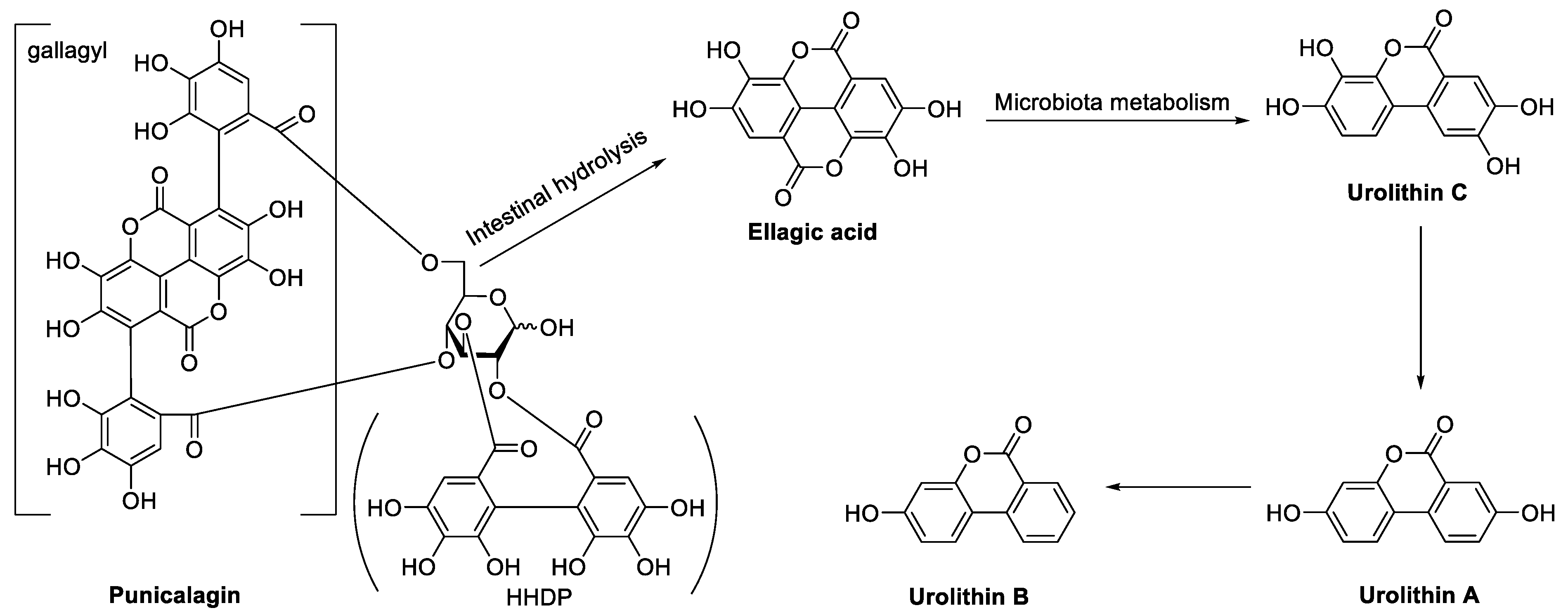
2. In Vivo Metabolic Process of Ellagitannins in Pomegranate
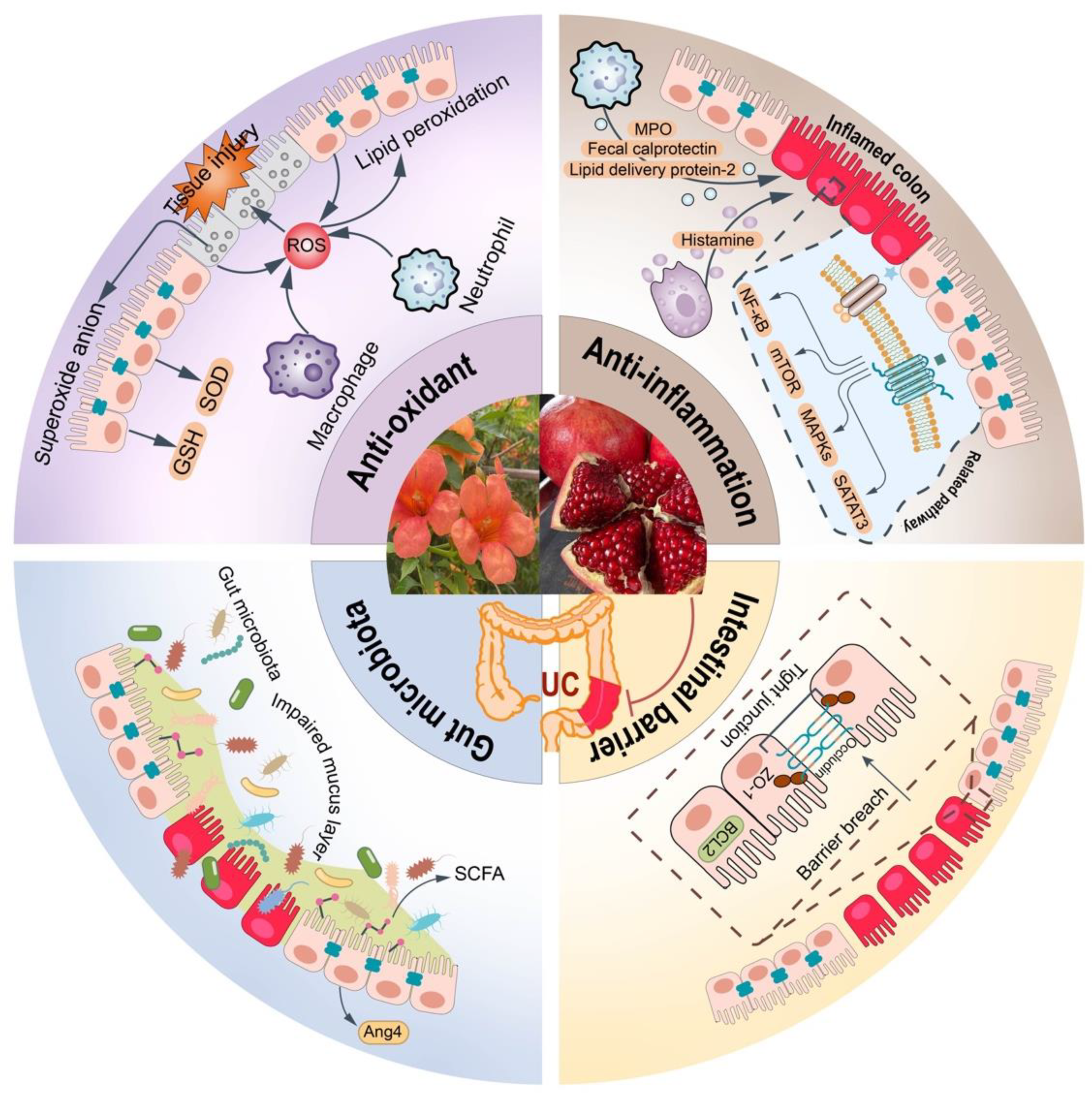
3. Pharmacological Effect and Mechanism of Pomegranate and Its Ellagitannins on UC
3.1. Antioxidant Effects and Mechanisms
3.1.1. Clearing Free Radicals Directly
3.1.2. Inhibiting Lipid Peroxidation
3.1.3. Activating the Activity of Antioxidant Enzyme
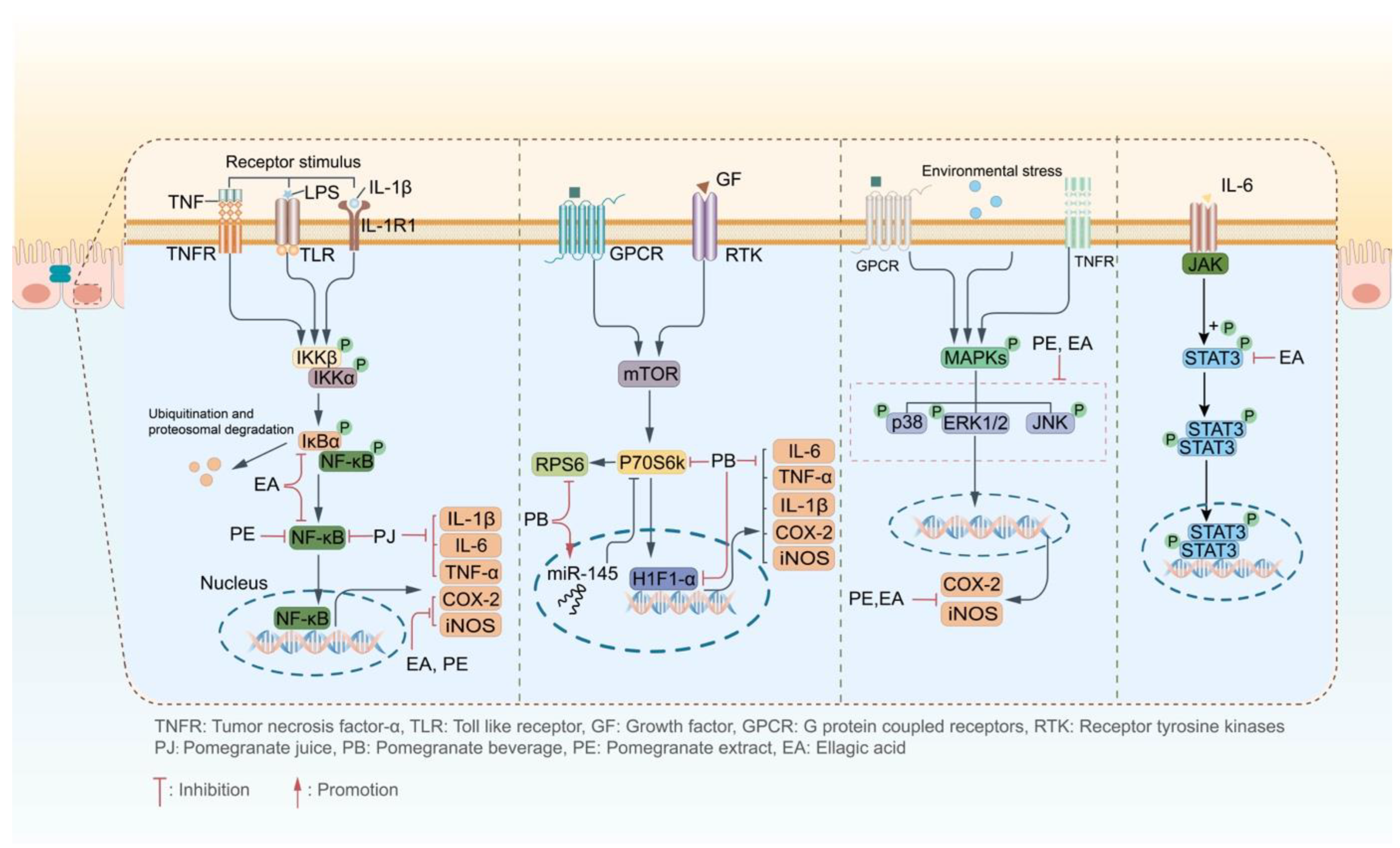
3.2. Anti-Inflammatory Effects and Mechanisms
3.2.1. Regulating Immune Response
Inhibition of Neutrophil Infiltration
Inhibition of Mast Cell Degranulation
3.2.2. Influencing on the Inflammatory Signaling Pathway
Regulation on the NF-κB-Signaling Pathway
Regulation on the p70S6K-Signaling Pathway
Regulation on the MAPK-Signaling Pathway
Regulation on the STAT3-Signaling Pathway
3.3. Intestinal Barrier Improvement Effects and Mechanisms
3.3.1. Promoting the Expression of Tight Junction Protein
3.3.2. Inhibiting Apoptosis of Intestinal Epithelial Cells
3.4. Regulation of Gut Microbiota
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 74. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Kotze, P.G.; Spinelli, A. Surgery in ulcerative colitis: When? how? Best Pract. Res. Clin. Gastroenterol. 2018, 32–33, 71–78. [Google Scholar] [CrossRef]
- Le, Q.; Liou, D.Z.; Murrell, Z.; Fleshner, P. Does a history of postoperative ileus predispose to recurrent ileus after multistage ileal pouch-anal anastomosis? Tech. Coloproctol. 2013, 17, 383–388. [Google Scholar] [CrossRef]
- Vasconcelos, P.C.; Andreo, M.A.; Vilegas, W.; Hiruma-Lima, C.A.; Pellizzon, C.H. Effect of Mouriri pusa tannins and flavonoids on prevention and treatment against experimental gastric ulcer. J. Ethnopharmacol. 2010, 131, 146–153. [Google Scholar] [CrossRef]
- González-Quilen, C.; Rodríguez-Gallego, E.; Beltrán-Debón, R.; Pinent, M.; Ardévol, A.; Blay, M.T.; Terra, X. Health-promoting properties of proanthocyanidins for intestinal dysfunction. Nutrients 2020, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Castillo, C.M.S.; Caroca, R.; Lazo-Vélez, M.A.; Antonyak, H.; Polishchuk, A.; Lysiuk, R.; Oliinyk, P.; De Masi, L.; et al. Ellagic acid: A review on its natural sources, chemical stability, and therapeutic potential. Oxid. Med. Cell. Longev. 2022, 2022, 3848084. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Lamy, E.; Pinheiro, C.; Rodrigues, L.; Silva, F.C.E.; Rui, G. Determinants of Tannin-Rich Food and Beverage Consumption: Oral Perception vs. Psychosocial Aspects; Combs, C.A., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2016; pp. 29–58. [Google Scholar]
- Tow, W.K.; Chee, P.Y.; Sundralingam, U.; Palanisamy, U.D. The therapeutic relevance of urolithins, intestinal metabolites of ellagitannin-rich food: A systematic review of in vivo studies. Nutrients 2022, 14, 3494. [Google Scholar] [CrossRef]
- Bao, L.; Wang, H.; Wu, J.; Bai, T.; Chen, H.; Wang, X. Simultaneous determinnation of eight components in Zhachong Shisanwei Pills by QAMS. Zhongcaoyao 2021, 52, 3249–3256. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Hosseinzadeh, H. Protective effects of pomegranate (Punica granatum) and its main components against natural and chemical toxic agents: A comprehensive review. Phytomedicine 2023, 109, 154581. [Google Scholar] [CrossRef] [PubMed]
- Maphetu, N.; Unuofin, J.O.; Masuku, N.P.; Olisah, C.; Lebelo, S.L. Medicinal uses, pharmacological activities, phytochemistry, and the molecular mechanisms of Punica granatum L. (pomegranate) plant extracts: A review. Biomed. Pharmacother. 2022, 153, 113256. [Google Scholar] [CrossRef] [PubMed]
- Yisimayili, Z.; Chao, Z. A review on phytochemicals, metabolic profiles and pharmacokinetics studies of the different parts (juice, seeds, peel, flowers, leaves and bark) of pomegranate (Punica granatum L.). Food Chem. 2022, 395, 133600. [Google Scholar] [CrossRef] [PubMed]
- Gilardi, D.; Fiorino, G.; Genua, M.; Allocca, M.; Danese, S. Complementary and alternative medicine in inflammatory bowel diseases: What is the future in the field of herbal medicine? Expert Rev. Gastroent. 2014, 8, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Jaggi, A.S.; Singh, N. Exploring the ameliorative potential of Punica granatum in dextran sulfate sodium induced ulcerative colitis in mice. Phytother. Res. 2009, 23, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Rudiansyah, M.; Abdalkareem Jasim, S.; Azizov, B.S.; Samusenkov, V.; Kamal Abdelbasset, W.; Yasin, G.; Mohammad, H.J.; Jawad, M.A.; Mahmudiono, T.; Hosseini-Fard, S.R.; et al. The emerging microbiome-based approaches to IBD therapy: From SCFAs to urolithin A. J. Dig. Dis. 2022, 23, 412–434. [Google Scholar] [CrossRef] [PubMed]
- Helm, R.F.; Ranatunga, T.D.; Chandra, M. Lignin hydrolyzable tannin interactions in wood. J. Agric. Food Chem. 1997, 45, 3100–3106. [Google Scholar] [CrossRef]
- Cerdá, B.; Llorach, R.; Cerón, J.J.; Espín, J.C.; Tomás-Barberán, F.A. Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. Eur. J. Nutr. 2003, 42, 18–28. [Google Scholar] [CrossRef]
- Yang, X.; Tomás-Barberán, F.A. Tea is a significant dietary source of ellagitannins and ellagic acid. J. Agric. Food Chem. 2019, 67, 5394–5404. [Google Scholar] [CrossRef]
- Seeram, N.P.; Henning, S.M.; Zhang, Y.; Suchard, M.; Li, Z.; Heber, D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J. Nutr. 2006, 136, 2481–2485. [Google Scholar] [CrossRef]
- García-Villalba, R.; Giménez-Bastida, J.A.; Cortés-Martín, A.; Ávila-Gálvez, M.Á.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C.; González-Sarrías, A. Urolithins: A comprehensive update on their metabolism, bioactivity, and associated gut microbiota. Mol. Nutr. Food Res. 2022, 66, e2101019. [Google Scholar] [CrossRef] [PubMed]
- Xian, W.; Yang, S.; Deng, Y.; Yang, Y.; Chen, C.; Li, W.; Yang, R. Distribution of urolithins metabotypes in healthy chinese youth: Difference in gut microbiota and predicted metabolic pathways. J. Agric. Food Chem. 2021, 69, 13055–13065. [Google Scholar] [CrossRef]
- Larrosa, M.; González-Sarrías, A.; Yáñez-Gascón, M.J.; Selma, M.V.; Azorín-Ortuño, M.; Toti, S.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010, 8, 717–725. [Google Scholar] [CrossRef]
- Seeram, N.P.; Lee, R.; Heber, D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin. Chim. Acta 2004, 348, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, B.; Ojha, S.; Belur, P.D.; Bhongade, B.; Raj, V.; Collin, P.D.; Adrian, T.E.; Subramanya, S.B. Phytochemical drug candidates for the modulation of peroxisome proliferator-activated receptor γ in inflammatory bowel diseases. Phytother. Res. 2020, 34, 1530–1549. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Yin, S.; Ma, H.; Jiang, Q.; Ma, Y. Effects of punicalagin on intestinal mucosal injury in experimental colitis mice through SIRT1/PGC-1α/NRF1 pathway. Immunol. J. 2022, 38, 655–664. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kanatsu, K.; Iino, T.; Kato, S.; Jeong, Y.I.; Shibata, N.; Takada, K.; Takeuchi, K. Protection against dextran sulfate sodium-induced colitis by microspheres of ellagic acid in rats. Life Sci. 2002, 71, 827–839. [Google Scholar] [CrossRef]
- Shah, T.A.; Parikh, M.; Patel, K.V.; Patel, K.G.; Joshi, C.G.; Gandhi, T.R. Evaluation of the effect of Punica granatum juice and punicalagin on NFκB modulation in inflammatory bowel disease. Mol. Cell. Biochem. 2016, 419, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Al-Gubory, K.H.; Blachier, F.; Faure, P.; Garrel, C. Pomegranate peel extract decreases small intestine lipid peroxidation by enhancing activities of major antioxidant enzymes. J. Sci. Food Agric. 2016, 96, 3462–3468. [Google Scholar] [CrossRef]
- Riaz, A.; Khan, R.A.; Afroz, S.; Mallick, N. Prophylactic and therapeutic effect of Punica granatum in trinitrobenzene sulfonic acid induced inflammation in rats. Pak. J. Pharm. Sci. 2017, 30, 155–162. [Google Scholar]
- Scaioli, E.; Belluzzi, A.; Ricciardiello, L.; Del Rio, D.; Rotondo, E.; Mena, P.; Derlindati, E.; Danesi, F. Pomegranate juice to reduce fecal calprotectin levels in inflammatory bowel disease patients with a high risk of clinical relapse: Study protocol for a randomized controlled trial. Trials 2019, 20, 327. [Google Scholar] [CrossRef]
- Yang, J.; Germano, P.M.; Oh, S.; Wang, S.; Wang, J.; Lee, R.; Paige, H.; Yang, S.; Henning, S.M.; Zhong, J.; et al. Pomegranate extract improves colitis in IL-10 knockout mice fed a high fat high sucrose diet. Mol. Nutr. Food Res. 2022, 66, 2100730. [Google Scholar] [CrossRef]
- Marín, M.; María Giner, R.; Ríos, J.L.; Recio, M.C. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J. Ethnopharmacol. 2013, 150, 925–934. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Sánchez-Hidalgo, M.; Cárdeno, A.; Aparicio-Soto, M.; Sánchez-Fidalgo, S.; Villegas, I.; de la Lastra, C.A. Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats. Pharmacol. Res. 2012, 66, 235–242. [Google Scholar] [CrossRef]
- Kim, H.; Banerjee, N.; Ivanov, I.; Pfent, C.M.; Prudhomme, K.R.; Bisson, W.H.; Dashwood, R.H.; Talcott, S.T.; Mertens-Talcott, S.U. Comparison of anti-inflammatory mechanisms of mango (Mangifera indica L.) and pomegranate (Punica granatum L.) in a preclinical model of colitis. Mol. Nutr. Food Res. 2016, 60, 1912–1923. [Google Scholar] [CrossRef]
- Kim, H.; Banerjee, N.; Sirven, M.A.; Minamoto, Y.; Markel, M.E.; Suchodolski, J.S.; Talcott, S.T.; Mertens-Talcott, S.U. Pomegranate polyphenolics reduce inflammation and ulceration in intestinal colitis-involvement of the miR-145/p70S6K1/HIF1α axis in vivo and in vitro. J. Nutr. Biochem. 2017, 43, 107–115. [Google Scholar] [CrossRef]
- Zhao, R.; Long, X.; Yang, J.; Du, L.; Zhang, X.; Li, J.; Hou, C. Pomegranate peel polyphenols reduce chronic low-grade inflammatory responses by modulating gut microbiota and decreasing colonic tissue damage in rats fed a high-fat diet. Food Funct. 2019, 10, 8273–8285. [Google Scholar] [CrossRef]
- Wu, S.; Wang, X.; Zhang, W.; Meng, W.; Xia, Y.; Zhang, R.; Zhang, Y. Effects of pomegranate juice on DSS-induced ulcerative colitis in mice. Food Sci. 2023, 44, 1–13. [Google Scholar]
- Smith, A.D.; George, N.S.; Cheung, L.; Bhagavathy, G.V.; Luthria, D.L.; John, K.M.; Bhagwat, A.A. Pomegranate peel extract reduced colonic damage and bacterial translocation in a mouse model of infectious colitis induced by Citrobacter rodentium. Nutr. Res. 2020, 73, 27–37. [Google Scholar] [CrossRef] [PubMed]
- George, N.S.; Cheung, L.; Luthria, D.L.; Santin, M.; Dawson, H.D.; Bhagwat, A.A.; Smith, A.D. Pomegranate peel extract alters the microbiome in mice and dysbiosis caused by Citrobacter rodentium infection. Food Sci. Nutr. 2019, 7, 2565–2576. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid. Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef] [PubMed]
- Larabi, A.; Barnich, N.; Nguyen, H.T.T. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy 2020, 16, 38–51. [Google Scholar] [CrossRef]
- Kasai, K.; Yoshimura, M.; Koga, T.; Arii, M.; Kawasaki, S. Effects of oral administration of ellagic acid-rich pomegranate extract on ultraviolet-induced pigmentation in the human skin. J. Nutr. Sci. Vitaminol. 2006, 52, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Mkaouar, S.; Krichen, F.; Bahloul, N.; Allaf, F.; Kechaou, N. Enhancement of bioactive compounds and antioxidant activities of olive (Olea europaea L.) leaf extract by instant controlled pressure drop. Food Bioprocess Technol. 2018, 11, 1222–1229. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Liang, J.; Li, J.; Zhao, W.; Liu, Y. Effect of pomegranate peel polyphenols on lipid peroxidation in vitro. Shipin Yu Shengwu Jishu Xuebao 2012, 31, 159–165. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Zafrilla, P.; Ferreres, F.; Tomás-Barberán, F.A. Effect of processing and storage on the antioxidant ellagic acid derivatives and flavonoids of red raspberry (Rubus idaeus) jams. J. Agric. Food Chem. 2001, 49, 3651–3655. [Google Scholar] [CrossRef]
- Rathinam, V.A.K.; Chan, F.K. Inflammasome, inflammation, and tissue homeostasis. Trends Mol. Med. 2018, 24, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Feehan, K.T.; Gilroy, D.W. Is resolution the end of inflammation? Trends Mol. Med. 2019, 25, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Marano, C.; Zhang, H.; Yang, F.; Sandborn, W.J.; Sands, B.E.; Feagan, B.G.; Rubin, D.T.; Peyrin-Biroulet, L.; Friedman, J.R.; et al. Relationship between combined histologic and endoscopic endpoints and efficacy of ustekinumab treatment in patients with ulcerative colitis. Gastroenterology 2020, 159, 2052–2064. [Google Scholar] [CrossRef] [PubMed]
- Mutua, V.; Gershwin, L.J. A review of neutrophil extracellular traps (NETs) in disease: Potential anti-nets therapeutics. Clin. Rev. Allergy Immunol. 2021, 61, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Ayling, R.M.; Kok, K. Fecal Calprotectin. Adv. Clin. Chem. 2018, 87, 161–190. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Ramos, A.; Viana, G.C.S.; de Macedo Brigido, M.; Almeida, J.F. Neutrophil extracellular traps in inflammatory bowel diseases: Implications in pathogenesis and therapeutic targets. Pharmacol. Res. 2021, 171, 105779. [Google Scholar] [CrossRef] [PubMed]
- Shivji, S.; Conner, J.R.; Kirsch, R. Mast cell evaluation in gastrointestinal biopsies: Should we be counting? A critical review and practical guide for the surgical pathologist. Histopathology 2023, 82, 960–973. [Google Scholar] [CrossRef]
- Ballout, J.; Diener, M. Interactions between rat submucosal neurons and mast cells are modified by cytokines and neurotransmitters. Eur. J. Nutr. 2019, 864, 172713. [Google Scholar] [CrossRef]
- Dou, D.; Chen, L.; Di, H.; Song, Z.; Li, S.; Bu, X.; Dai, Q.; Wang, S.; Li, J.X.; Zhu, X.; et al. Vasopressin augments TNBS-induced colitis through enteric neuronal V1a receptor-mediated COX-2-dependent prostaglandin release from mast cells in mice. Neurogastroent. Motil. 2019, 31, 13493. [Google Scholar] [CrossRef]
- Abraham, C.; Abreu, M.T.; Turner, J.R. Pattern recognition receptor signaling and cytokine networks in microbial defenses and regulation of intestinal barriers: Implications for inflammatory bowel disease. Gastroenterology 2022, 162, 1602–1616.e6. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Ma, L.L.; Zhang, C.; Lin, J.Z.; Han, L.; He, Y.N.; Xie, C.G. Exploring the mechanism of indigo naturalis in the treatment of ulcerative colitis based on TLR4/MyD88/NF-κB signaling pathway and gut microbiota. Front. Pharmacol. 2021, 12, 674416. [Google Scholar] [CrossRef] [PubMed]
- Capece, D.; Verzella, D.; Flati, I.; Arboretto, P.; Cornice, J.; Franzoso, G. NF-κB: Blending metabolism, immunity, and inflammation. Trends Immunol. 2022, 43, 757–775. [Google Scholar] [CrossRef] [PubMed]
- Papoutsopoulou, S.; Campbell, B.J. Epigenetic modifications of the nuclear factor kappa B signalling pathway and its impact on inflammatory bowel disease. Curr. Pharm. Des. 2021, 27, 3702–3713. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, W. Regulation effect of p-mTOR/p70S6K signaling pathway in inflammation of acute necrotizing pancreatitis. Shandong Yiyao 2018, 58, 17–20. [Google Scholar] [CrossRef]
- Hassan-Zahraee, M.; Ye, Z.; Xi, L.; Baniecki, M.L.; Li, X.; Hyde, C.L.; Zhang, J.; Raha, N.; Karlsson, F.; Quan, J.; et al. Antitumor necrosis factor-like ligand 1A therapy targets tissue inflammation and fibrosis pathways and reduces gut pathobionts in ulcerative colitis. Inflamm. Bowel Dis. 2022, 28, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Rieder, F. Hypoxia-inducible factor 1-alpha stabilizers in the treatment of inflammatory bowel diseases: Oxygen as a novel IBD therapy? J. Crohns Colitis 2022, 16, 1924–1932. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Jiang, H.X.; Shi, Q.Y.; Qiu, X.; Wei, X.; Zhang, X.L.; Qin, S.Y. miR-145 inhibits Th9 cell differentiation by suppressing activation of the PI3K/Akt/mTOR/p70S6K/HIF-1α pathway in malignant ascites from liver cancer. OncoTargets Ther. 2020, 13, 3789–3800. [Google Scholar] [CrossRef]
- Zheng, S.; Xue, T.; Wang, B.; Guo, H.; Liu, Q. Chinese medicine in the treatment of ulcerative colitis: The mechanisms of signaling pathway regulations. Am. J. Chin. Med. 2022, 50, 1781–1798. [Google Scholar] [CrossRef]
- Li, B.L.; Zhao, D.Y.; Du, P.L.; Wang, X.T.; Yang, Q.; Cai, Y.R. Luteolin alleviates ulcerative colitis through SHP-1/STAT3 pathway. J. Inflamm. Res. 2021, 70, 705–717. [Google Scholar] [CrossRef]
- van der Post, S.; Jabbar, K.S.; Birchenough, G.; Arike, L.; Akhtar, N.; Sjovall, H.; Johansson, M.E.V.; Hansson, G.C. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut 2019, 68, 2142–2151. [Google Scholar] [CrossRef]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend your fences: The epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 33–46. [Google Scholar] [CrossRef]
- Manzano, S.; Williamson, G. Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells. Mol. Nutr. Food Res. 2010, 54, 1773–1780. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, L.; Jiang, S.; Qian, D.; Duan, J. Excessive apoptosis in ulcerative colitis: Crosstalk between apoptosis, ROS, ER stress, and intestinal homeostasis. Inflamm. Bowel Dis. 2022, 28, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Scaldaferri, F.; Gerardi, V.; Lopetuso, L.R.; Del Zompo, F.; Mangiola, F.; Boškoski, I.; Bruno, G.; Petito, V.; Laterza, L.; Cammarota, G.; et al. Gut microbial flora, prebiotics, and probiotics in IBD: Their current usage and utility. BioMed Res. Int. 2013, 2013, 435268. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Dheer, R.; Davies, J.M.; Quintero, M.A.; Damas, O.M.; Deshpande, A.R.; Kerman, D.H.; Sawyer, W.P.; Pignac-Kobinger, J.; Ban, Y.; Fernandez, I.; et al. Microbial signatures and innate immune gene expression in lamina propria phagocytes of inflammatory bowel disease patients. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 387–402. [Google Scholar] [CrossRef]
- Tajasuwan, L.; Kettawan, A.; Rungruang, T.; Wunjuntuk, K.; Prombutara, P. Role of dietary defatted rice bran in the modulation of gut microbiota in AOM/DSS-induced colitis-associated colorectal cancer rat model. Nutrients 2023, 15, 1528. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Mullineaux-Sanders, C.; Sanchez-Garrido, J.; Hopkins, E.G.D.; Shenoy, A.R.; Barry, R.; Frankel, G. Citrobacter rodentium-host-microbiota interactions: Immunity, bioenergetics and metabolism. Nat. Rev. Microbiol. 2019, 17, 701–715. [Google Scholar] [CrossRef]
- Sultana, M.F.; Abo, H.; Kawashima, H. Human and mouse angiogenins: Emerging insights and potential opportunities. Front. Microbiol. 2022, 13, 1022945. [Google Scholar] [CrossRef]
- Kuhn, K.A.; Schulz, H.M.; Regner, E.H.; Severs, E.L.; Hendrickson, J.D.; Mehta, G.; Whitney, A.K.; Ir, D.; Ohri, N.; Robertson, C.E.; et al. Bacteroidales recruit IL-6-producing intraepithelial lymphocytes in the colon to promote barrier integrity. Mucosal Immunol. 2018, 11, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Rufino, M.N.; da Costa, A.L.; Jorge, E.N.; Paiano, V.F.; Camparoto, M.L.; Keller, R.; Bremer-Neto, H. Synbiotics improve clinical indicators of ulcerative colitis: Systematic review with meta-analysis. Nutr. Rev. 2022, 80, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.A.; Befrits, R. Colorectal cancer in Crohn’s disease-review of a 56-year experience in Karolinska Institute University Hospital. J. Environ. Pathol. Tox. 2008, 27, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Haskey, N.; Gibson, D.L. An examination of diet for the maintenance of remission in inflammatory bowel disease. Nutrients 2017, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Masci, A.; Coccia, A.; Lendaro, E.; Mosca, L.; Paolicelli, P.; Cesa, S. Evaluation of different extraction methods from pomegranate whole fruit or peels and the antioxidant and antiproliferative activity of the polyphenolic fraction. Food Chem. 2016, 202, 59–69. [Google Scholar] [CrossRef]
- Salunkhe, D.K.; Jadhav, S.J.; Kadam, S.S.; Chavan, J.K. Chemical, biochemical, and biological significance of polyphenols in cereals and legumes. Crit. Rev. Food Sci. 1982, 17, 277–305. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Andreu-Sánchez, S.; Vogl, T.; Hu, S.; Vich Vila, A.; Gacesa, R.; Leviatan, S.; Kurilshikov, A.; Klompus, S.; Kalka, I.N.; et al. Phage-display immunoprecipitation sequencing of the antibody epitope repertoire in inflammatory bowel disease reveals distinct antibody signatures. Immunity 2023, 56, 1393–1409.e6. [Google Scholar] [CrossRef]
- Israeli, E.; Grotto, I.; Gilburd, B.; Balicer, R.D.; Goldin, E.; Wiik, A.; Shoenfeld, Y. Anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies as predictors of inflammatory bowel disease. Gut 2005, 54, 1232–1236. [Google Scholar] [CrossRef]


| Activity | Source | Model | Effect | Ref. |
|---|---|---|---|---|
| Antioxidation | Pomegranate juice (PJ) | – | ↓ABTS, ↓DPPH, ↓DMPD, ↓FRAP | [28] |
| Punicalagin | Mice/DSS-induced colitis | ↓ROS | [29] | |
| Pomegranate flower hydroalcohol extract (PFHE), ellagic acid-rich fraction of pomegranate flower (EAOPF) | Mice/DSS-induced colitis | ↓Superoxide anion level | [17] | |
| PFHE, EAOPF | Mice/DSS-induced colitis | ↓TBARS | [17] | |
| Pomegranate extract (PE) | Rats/DSS-induced colitis | ↓TBARS | [25] | |
| EA | Rats/DSS-induced colitis | ↓TBARS | [30] | |
| PJ, punicalagin | Rats/DNBS-induced colitis | ↓MDA | [31] | |
| Pomegranate peel extract (PPE) | Mice | ↓MDA | [32] | |
| Punicalagin | Mice/DSS-induced colitis | ↓MDA | [29] | |
| PPE | Mice | ↑SOD1, ↑SOD2 | [32] | |
| PJ, punicalagin | Rats/DNBS-induced colitis | ↑SOD | [31] | |
| PJ | Rats/TNBS-induced colitis | ↑GSH | [33] | |
| Antiinflammation | PJ | IBD patients | ↓FC | [34] |
| PE | Mice/IL-10 deficient colitis model | ↓LCN2 | [35] | |
| PJ | Rats/TNBS-induced colitis | ↓MPO | [33] | |
| PJ, punicalagin | Rats/DNBS-induced colitis | ↓MPO | [31] | |
| EA | Mice/DSS-induced colitis | ↓MPO | [36] | |
| PE, EA | Rats/TNBS-induced colitis | ↓MPO | [37] | |
| PFHE, EAOPF | Mice/DSS-induced colitis | ↓MPO | [17] | |
| PFHE, EAOPF | Mice/DSS-induced colitis | ↓Histamine | [17] | |
| PJ | Rats/DNBS-induced colitis | ↓NF-κB, ↓IL-6, ↓IL-β, ↓TNF-α | [31] | |
| EA | Mice/DSS-induced colitis | ↓p-IκBα/IκBα, ↓NF-κB, ↓COX-2, ↓iNOS | [36] | |
| PE, EA | Rats/TNBS-induced colitis | ↓NF-κB, ↓COX-2, ↓iNOS | [37] | |
| Pomegranate beverage (PB) | Rats/DSS-induced colitis | ↓p70S6K, ↓RPS6, ↓TNF-α, ↓IL-1β, ↓IL-6 | [38] | |
| PB | Rats/DSS-induced colitis | ↑miR-145, ↓p70S6K1, ↓HIF-α, ↓TNF-α, ↓IL-1β, ↓COX-2, ↓iNOS | [39] | |
| PE, EA | Rats/TNBS-induced colitis | ↓p-JNK/JNK, ↓p-p38/p38, ↓p-ERK1/2/ERK1/2, ↓COX-2, ↓iNOS | [37] | |
| PB | Rats/DSS-induced colitis | ↓MAPK1, ↓MAP2K2, ↓SFN, ↓CDC42 | [38] | |
| EA | Mice/DSS-induced colitis | ↓p38 MAPK | [36] | |
| EA | Mice/DSS-induced colitis | ↓p-STAT3, ↓IL-6 | [36] | |
| Improvement of intestinal barrier | pomegranate peel polyphenols (PPP), punicalagin | LPS-induced Caco-2 | ↑ZO-1 | [40] |
| Punicalagin | Mice/DSS-induced colitis | ↑ZO-1, ↑Occludin, ↑Bcl-2 | [29] | |
| Regulation of intestinal microbiota | PJ | Mice/DSS-induced colitis | ↑Short chain fatty acid | [41] |
| methanol extract of pomegranate peel (PPE-M) | Mice/Cr-induced colitis | ↑Ang4 | [42] | |
| 80% methanol extract of pomegranate peel (PPE-80 M) | Mice/Cr-induced colitis | ↑Bacteroidetes, ↓Firmicutes | [43] | |
| PE | Rats/DSS-induced colitis | ↑Lactobacilli, ↑Bifidobacterium | [25] | |
| PE | Mice/IL-10 deficient colitis model | ↑Akkermansia, ↓Paeniclostridium, ↓Clostridium_sensu_stricto_1 | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Ruan, J.; Huang, J.; Yang, D.; Yu, H.; Wu, Y.; Zhang, Y.; Wang, T. Pomegranate (Punica granatum L.) and Its Rich Ellagitannins as Potential Inhibitors in Ulcerative Colitis. Int. J. Mol. Sci. 2023, 24, 17538. https://doi.org/10.3390/ijms242417538
Li H, Ruan J, Huang J, Yang D, Yu H, Wu Y, Zhang Y, Wang T. Pomegranate (Punica granatum L.) and Its Rich Ellagitannins as Potential Inhibitors in Ulcerative Colitis. International Journal of Molecular Sciences. 2023; 24(24):17538. https://doi.org/10.3390/ijms242417538
Chicago/Turabian StyleLi, Huimin, Jingya Ruan, Jiayan Huang, Dingshan Yang, Haiyang Yu, Yuzheng Wu, Yi Zhang, and Tao Wang. 2023. "Pomegranate (Punica granatum L.) and Its Rich Ellagitannins as Potential Inhibitors in Ulcerative Colitis" International Journal of Molecular Sciences 24, no. 24: 17538. https://doi.org/10.3390/ijms242417538




