“B” Regulatory Subunits of PP2A: Their Roles in Plant Development and Stress Reactions
Abstract
:1. Introduction
2. Roles and Functioning of B Subunits
2.1. Subcellular Localizations of B Subunits
2.2. Regulation of Plant Metabolism by B Subunits
2.3. Regulation of Cell Cycle/division and Whole-Plant Development by B Subunits
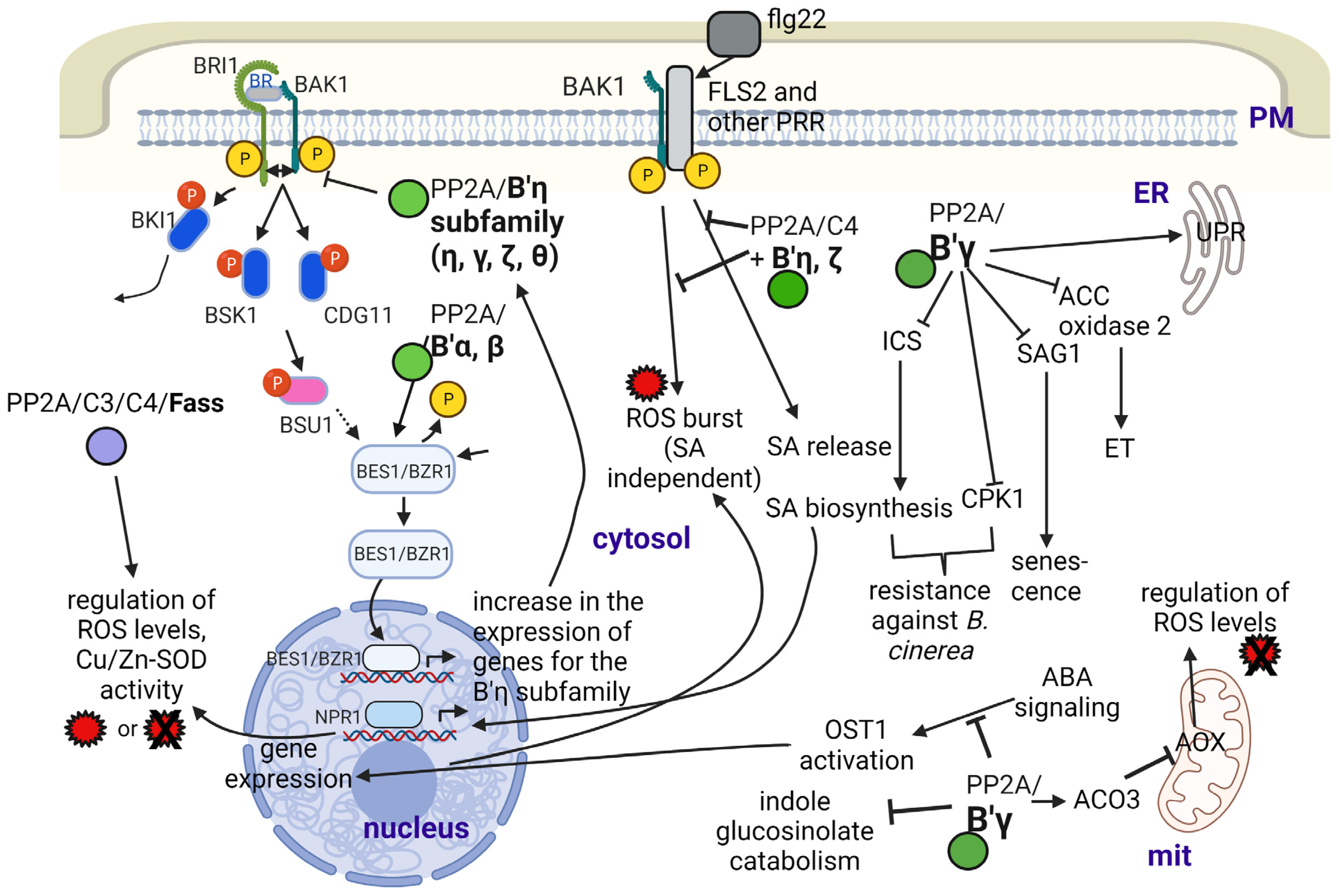
2.4. Regulation of Plant Responses to Abiotic and Biotic Stresses
3. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, R.D.; Walker, J.C. Plant Protein Phosphatases. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 101–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, S. Protein Phosphatases in Plants. Annu. Rev. Plant Biol. 2003, 54, 63–92. [Google Scholar] [CrossRef]
- Shi, Y. Serine/Threonine Phosphatases: Mechanism through Structure. Cell 2009, 139, 468–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virshup, D.M.; Shenolikar, S. From Promiscuity to Precision: Protein Phosphatases Get a Makeover. Mol. Cell 2009, 33, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Csordás Tóth, É.; Vissi, E.; Kovács, I.; Szöke, A.; Ariño, J.; Gergely, P.; Dudits, D.; Dombrádi, V. Protein Phosphatase 2A Holoenzyme and Its Subunits from Medicago Sativa. Plant Mol. Biol. 2000, 43, 527–536. [Google Scholar] [CrossRef]
- Farkas, I.; Dombrádi, V.; Miskei, M.; Szabados, L.; Koncz, C. Arabidopsis PPP Family of Serine/Threonine Phosphatases. Trends Plant Sci. 2007, 12, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, M.N.M.; Grossi, C.; Ulloa, R.M.; Capiati, D.A. The Protein Phosphatase 2A Catalytic Subunit StPP2Ac2b Enhances Susceptibility to Phytophthora Infestans and Senescence in Potato. PLoS ONE 2022, 17, e0275844. [Google Scholar] [CrossRef]
- Uhrig, R.G.; Labandera, A.-M.; Moorhead, G.B. Arabidopsis PPP Family of Serine/Threonine Protein Phosphatases: Many Targets but Few Engines. Trends Plant Sci. 2013, 18, 505–513. [Google Scholar] [CrossRef]
- Freytag, C.; Máthé, C.; Rigó, G.; Nodzyński, T.; Kónya, Z.; Erdődi, F.; Cséplő, Á.; Pózer, E.; Szabados, L.; Kelemen, A.; et al. Microcystin-LR, a Cyanobacterial Toxin Affects Root Development by Changing Levels of PIN Proteins and Auxin Response in Arabidopsis Roots. Chemosphere 2021, 276, 130183. [Google Scholar] [CrossRef]
- Máthé, C.; M-Hamvas, M.; Vasas, G.; Garda, T.; Freytag, C. Subcellular Alterations Induced by Cyanotoxins in Vascular Plants—A Review. Plants 2021, 10, 984. [Google Scholar] [CrossRef]
- Zwiewka, M.; Bilanovičová, V.; Seifu, Y.W.; Nodzyński, T. The Nuts and Bolts of PIN Auxin Efflux Carriers. Front. Plant Sci. 2019, 10, 985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durian, G.; Jeschke, V.; Rahikainen, M.; Vuorinen, K.; Gollan, P.J.; Brosché, M.; Salojärvi, J.; Glawischnig, E.; Winter, Z.; Li, S.; et al. PROTEIN PHOSPHATASE 2A-B′γ Controls Botrytis Cinerea Resistance and Developmental Leaf Senescence. Plant Physiol. 2020, 182, 1161–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durian, G.; Rahikainen, M.; Alegre, S.; Brosché, M.; Kangasjärvi, S. Protein Phosphatase 2A in the Regulatory Network Underlying Biotic Stress Resistance in Plants. Front. Plant Sci. 2016, 7, 812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Máthé, C.; Garda, T.; Freytag, C.; M-Hamvas, M. The Role of Serine-Threonine Protein Phosphatase PP2A in Plant Oxidative Stress Signaling—Facts and Hypotheses. Int. J. Mol. Sci. 2019, 20, 3028. [Google Scholar] [CrossRef] [Green Version]
- Kataya, A.R.; Heidari, B.; Lillo, C. Protein Phosphatase 2A Regulatory Subunits Affecting Plant Innate Immunity, Energy Metabolism, and Flowering Time—Joint Functions among B’η Subfamily Members. Plant Signal. Behav. 2015, 10, e1026024. [Google Scholar] [CrossRef] [Green Version]
- Kataya, A.R.A.; Heidari, B.; Hagen, L.; Kommedal, R.; Slupphaug, G.; Lillo, C. Protein Phosphatase 2A Holoenzyme Is Targeted to Peroxisomes by Piggybacking and Positively Affects Peroxisomal β-Oxidation. Plant Physiol. 2015, 167, 493–506. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.-T.; Ahn, H.-K.; Pai, H.-S. The Subfamily II Catalytic Subunits of Protein Phosphatase 2A (PP2A) Are Involved in Cortical Microtubule Organization. Planta 2018, 248, 1551–1567. [Google Scholar] [CrossRef]
- Ballesteros, I.; Domínguez, T.; Sauer, M.; Paredes, P.; Duprat, A.; Rojo, E.; Sanmartín, M.; Sánchez-Serrano, J.J. Specialized Functions of the PP2A Subfamily II Catalytic Subunits PP2A-C3 and PP2A-C4 in the Distribution of Auxin Fluxes and Development in Arabidopsis. Plant J. 2013, 73, 862–872. [Google Scholar] [CrossRef]
- Creighton, M.T.; Sanmartín, M.; Kataya, A.R.A.; Averkina, I.O.; Heidari, B.; Nemie-Feyissa, D.; Sánchez-Serrano, J.J.; Lillo, C. Light Regulation of Nitrate Reductase by Catalytic Subunits of Protein Phosphatase 2A. Planta 2017, 246, 701–710. [Google Scholar] [CrossRef]
- Spinner, L.; Gadeyne, A.; Belcram, K.; Goussot, M.; Moison, M.; Duroc, Y.; Eeckhout, D.; Winne, N.D.; Schaefer, E.; Slijke, E.V.D.; et al. A Protein Phosphatase 2A Complex Spatially Controls Plant Cell Division. Nat. Commun. 2013, 4, 1863. [Google Scholar] [CrossRef] [Green Version]
- Bheri, M.; Mahiwal, S.; Sanyal, S.K.; Pandey, G.K. Plant Protein Phosphatases: What Do We Know about Their Mechanism of Action? FEBS J. 2021, 288, 756–785. [Google Scholar] [CrossRef] [PubMed]
- Bheri, M.; Pandey, G.K. Protein Phosphatases Meet Reactive Oxygen Species in Plant Signaling Networks. Environ. Exp. Bot. 2019, 161, 26–40. [Google Scholar] [CrossRef]
- Camilleri, C.; Azimzadeh, J.; Pastuglia, M.; Bellini, C.; Grandjean, O.; Bouchez, D. The Arabidopsis TONNEAU2 Gene Encodes a Putative Novel Protein Phosphatase 2A Regulatory Subunit Essential for the Control of the Cortical Cytoskeleton. Plant Cell 2002, 14, 833–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Ruiz, R.A.; Jürgens, G. Mutations in the FASS Gene Uncouple Pattern Formation and Morphogenesis in Arabidopsis Development. Development 1994, 120, 2967–2978. [Google Scholar] [CrossRef] [PubMed]
- Matre, P.; Meyer, C.; Lillo, C. Diversity in Subcellular Targeting of the PP2A B′η Subfamily Members. Planta 2009, 230, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Heidari, B.; Matre, P.; Nemie-Feyissa, D.; Meyer, C.; Rognli, O.A.; Møller, S.G.; Lillo, C. Protein Phosphatase 2A B55 and A Regulatory Subunits Interact with Nitrate Reductase and Are Essential for Nitrate Reductase Activation. Plant Physiol. 2011, 156, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Jonassen, E.M.; Heidari, B.; Nemie-Feyissa, D.; Matre, P.; Lillo, C. Protein Phosphatase 2A Regulatory Subunits Are Starting to Reveal Their Functions in Plant Metabolism and Development. Plant Signal. Behav. 2011, 6, 1216–1218. [Google Scholar] [CrossRef] [Green Version]
- Tsugama, D.; Liu, S.; Fujino, K.; Takano, T. B-Family Subunits of Protein Phosphatase 2A Are Necessary for Pollen Development but Not for Female Gametophyte Development in Arabidopsis. Biochem. Biophys. Res. Commun. 2018, 505, 176–180. [Google Scholar] [CrossRef]
- Shao, Z.; Zhao, B.; Kotla, P.; Burns, J.G.; Tran, J.; Ke, M.; Chen, X.; Browning, K.S.; Qiao, H. Phosphorylation Status of Bβ Subunit Acts as a Switch to Regulate the Function of Phosphatase PP2A in Ethylene-Mediated Root Growth Inhibition. New Phytol. 2022, 236, 1762–1778. [Google Scholar] [CrossRef]
- Heidari, B.; Nemie-Feyissa, D.; Kangasjärvi, S.; Lillo, C. Antagonistic Regulation of Flowering Time through Distinct Regulatory Subunits of Protein Phosphatase 2A. PLoS ONE 2013, 8, e67987. [Google Scholar] [CrossRef]
- Yuan, G.; Ahootapeh, B.H.; Komaki, S.; Schnittger, A.; Lillo, C.; De Storme, N.; Geelen, D. PROTEIN PHOSHATASE 2A B’α and β Maintain Centromeric Sister Chromatid Cohesion during Meiosis in Arabidopsis. Plant Physiol. 2018, 178, 317–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Liu, M.; Yuan, M.; Oses-Prieto, J.A.; Cai, X.; Sun, Y.; Burlingame, A.L.; Wang, Z.-Y.; Tang, W. The Brassinosteroid-Activated BRI1 Receptor Kinase Is Switched off by Dephosphorylation Mediated by Cytoplasm-Localized PP2A B′ Subunits. Mol. Plant 2016, 9, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Yuan, M.; Wang, R.; Yang, Y.; Wang, C.; Oses-Prieto, J.A.; Kim, T.-W.; Zhou, H.-W.; Deng, Z.; Gampala, S.S.; et al. PP2A Activates Brassinosteroid-Responsive Gene Expression and Plant Growth by Dephosphorylating BZR1. Nat. Cell Biol. 2011, 13, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Rasool, B.; Karpinska, B.; Konert, G.; Durian, G.; Denessiouk, K.; Kangasjärvi, S.; Foyer, C.H. Effects of Light and the Regulatory B-Subunit Composition of Protein Phosphatase 2A on the Susceptibility of Arabidopsis Thaliana to Aphid (Myzus Persicae) Infestation. Front. Plant Sci. 2014, 5, 405. [Google Scholar] [CrossRef] [Green Version]
- Rasool, B.; Karpinska, B.; Pascual, J.; Kangasjärvi, S.; Foyer, C.H. Catalase, Glutathione, and Protein Phosphatase 2A-Dependent Organellar Redox Signalling Regulate Aphid Fecundity under Moderate and High Irradiance. Plant Cell Environ. 2020, 43, 209–222. [Google Scholar] [CrossRef]
- Li, S.; Mhamdi, A.; Trotta, A.; Kangasjärvi, S.; Noctor, G. The Protein Phosphatase Subunit PP2A-B′γ Is Required to Suppress Day Length-Dependent Pathogenesis Responses Triggered by Intracellular Oxidative Stress. New Phytol. 2014, 202, 145–160. [Google Scholar] [CrossRef]
- Waadt, R.; Manalansan, B.; Rauniyar, N.; Munemasa, S.; Booker, M.A.; Brandt, B.; Waadt, C.; Nusinow, D.A.; Kay, S.A.; Kunz, H.-H.; et al. Identification of Open Stomata1-Interacting Proteins Reveals Interactions with Sucrose Non-Fermenting1-Related Protein Kinases2 and with Type 2A Protein Phosphatases That Function in Abscisic Acid Responses. Plant Physiol. 2015, 169, 760–779. [Google Scholar] [CrossRef] [Green Version]
- Segonzac, C.; Macho, A.P.; Sanmartín, M.; Ntoukakis, V.; Sánchez-Serrano, J.J.; Zipfel, C. Negative Control of BAK1 by Protein Phosphatase 2A during Plant Innate Immunity. EMBO J. 2014, 33, 2069–2079. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Shen, G.; Wijewardene, I.; Cai, Y.; Esmaeili, N.; Sun, L.; Zhang, H. The B’ζ Subunit of Protein Phosphatase 2A Negatively Regulates Ethylene Signaling in Arabidopsis. Plant Physiol. Biochem. 2021, 169, 81–91. [Google Scholar] [CrossRef]
- Tsugama, D.; Yoon, H.S.; Fujino, K.; Liu, S.; Takano, T. Protein Phosphatase 2A Regulates the Nuclear Accumulation of the Arabidopsis BZIP Protein VIP1 under Hypo-Osmotic Stress. J. Exp. Bot. 2019, 70, 6101–6112. [Google Scholar] [CrossRef] [Green Version]
- Freytag, C.; Garda, T.; Kónya, Z.; M-Hamvas, M.; Tóth-Várady, B.; Juhász, G.P.; Ujlaky-Nagy, L.; Kelemen, A.; Vasas, G.; Máthé, C. B″ and C Subunits of PP2A Regulate the Levels of Reactive Oxygen Species and Superoxide Dismutase Activities in Arabidopsis. Plant Physiol. Biochem. 2023, 195, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Leivar, P.; Antolín-Llovera, M.; Ferrero, S.; Closa, M.; Arró, M.; Ferrer, A.; Boronat, A.; Campos, N. Multilevel Control of Arabidopsis 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase by Protein Phosphatase 2A. Plant Cell 2011, 23, 1494–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drevensek, S.; Goussot, M.; Duroc, Y.; Christodoulidou, A.; Steyaert, S.; Schaefer, E.; Duvernois, E.; Grandjean, O.; Vantard, M.; Bouchez, D.; et al. The Arabidopsis TRM1–TON1 Interaction Reveals a Recruitment Network Common to Plant Cortical Microtubule Arrays and Eukaryotic Centrosomes. Plant Cell 2012, 24, 178–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaefer, E.; Belcram, K.; Uyttewaal, M.; Duroc, Y.; Goussot, M.; Legland, D.; Laruelle, E.; de Tauzia-Moreau, M.-L.; Pastuglia, M.; Bouchez, D. The Preprophase Band of Microtubules Controls the Robustness of Division Orientation in Plants. Science 2017, 356, 186–189. [Google Scholar] [CrossRef]
- Rasmussen, C.G.; Bellinger, M. An Overview of Plant Division-Plane Orientation. New Phytol. 2018, 219, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.S.; Fujino, K.; Liu, S.; Takano, T.; Tsugama, D. The B″-Family Subunits of Protein Phosphatase 2A Are Necessary for in-Vitro Dephosphorylation of the Arabidopsis Mechanosensory Transcription Factor VIP1. Biochem. Biophys. Res. Commun. 2021, 534, 353–358. [Google Scholar] [CrossRef]
- Azimzadeh, J.; Nacry, P.; Christodoulidou, A.; Drevensek, S.; Camilleri, C.; Amiour, N.; Parcy, F.; Pastuglia, M.; Bouchez, D. Arabidopsis TONNEAU1 Proteins Are Essential for Preprophase Band Formation and Interact with Centrin. Plant Cell 2008, 20, 2146–2159. [Google Scholar] [CrossRef] [Green Version]
- Van Damme, D. Division Plane Determination during Plant Somatic Cytokinesis. Curr. Opin. Plant Biol. 2009, 12, 745–751. [Google Scholar] [CrossRef]
- Ren, H.; Rao, J.; Tang, M.; Li, Y.; Dang, X.; Lin, D. PP2A Interacts with KATANIN to Promote Microtubule Organization and Conical Cell Morphogenesis. J. Integr. Plant Biol. 2022, 64, 1514–1530. [Google Scholar] [CrossRef]
- Smertenko, A.P.; Chang, H.-Y.; Sonobe, S.; Fenyk, S.I.; Weingartner, M.; Bögre, L.; Hussey, P.J. Control of the AtMAP65-1 Interaction with Microtubules through the Cell Cycle. J. Cell Sci. 2006, 119, 3227–3237. [Google Scholar] [CrossRef] [Green Version]
- McClinton, R.S.; Sung, Z.R. Organization of Cortical Microtubules at the Plasma Membrane in Arabidopsis. Planta 1997, 201, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Kirik, A.; Ehrhardt, D.W.; Kirik, V. TONNEAU2/FASS Regulates the Geometry of Microtubule Nucleation and Cortical Array Organization in Interphase Arabidopsis Cells. Plant Cell 2012, 24, 1158–1170. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.J.; Gallagher, K.; Smith, L.G. Discordia1 and Alternative Discordia1 Function Redundantly at the Cortical Division Site to Promote Preprophase Band Formation and Orient Division Planes in Maize. Plant Cell 2009, 21, 234–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ábrahám, E.; Yu, P.; Farkas, I.; Darula, Z.; Varga, E.; Lukács, N.; Ayaydin, F.; Medzihradszky, K.F.; Dombrádi, V.; Dudits, D.; et al. The B″ Regulatory Subunit of Protein Phosphatase 2A Mediates the Dephosphorylation of Rice Retinoblastoma-Related Protein-1. Plant Mol. Biol. 2015, 87, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Averkina, I.O.; Harris, M.; Asare, E.O.; Hourdin, B.; Paponov, I.A.; Lillo, C. Pinpointing Regulatory Protein Phosphatase 2A Subunits Involved in Beneficial Symbiosis between Plants and Microbes. BMC Plant Biol. 2021, 21, 183. [Google Scholar] [CrossRef]
- Pascual, J.; Rahikainen, M.; Angeleri, M.; Alegre, S.; Gossens, R.; Shapiguzov, A.; Heinonen, A.; Trotta, A.; Durian, G.; Winter, Z.; et al. ACONITASE 3 Is Part of TheANAC017 Transcription Factor-Dependent Mitochondrial Dysfunction Response. Plant Physiol. 2021, 186, 1859–1877. [Google Scholar] [CrossRef]
- Haynes, J.G.; Hartung, A.J.; Hendershot III, J.D.; Passingham, R.S.; Rundle, S.J. Molecular Characterization of the B′ Regulatory Subunit Gene Family of Arabidopsis Protein Phosphatase 2A. Eur. J. Biochem. 1999, 260, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Latorre, K.A.; Harris, D.M.; Rundle, S.J. Differential Expression of Three Arabidopsis Genes Encoding the B′ Regulatory Subunit of Protein Phosphatase 2A. Eur. J. Biochem. 1997, 245, 156–163. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R.; Blumwald, E. The Roles of ROS and ABA in Systemic Acquired Acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Pernas, M.; García-Casado, G.; Rojo, E.; Solano, R.; Sánchez-Serrano, J.J. A Protein Phosphatase 2A Catalytic Subunit Is a Negative Regulator of Abscisic Acid Signalling1. Plant J. 2007, 51, 763–778. [Google Scholar] [CrossRef]
- Fuchs, J.; Demidov, D.; Houben, A.; Schubert, I. Chromosomal Histone Modification Patterns—From Conservation to Diversity. Trends Plant Sci. 2006, 11, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Fan, X.-H.; Wang, Q.; Yin, Z.-G.; Sheng, X.-W.; Chen, J.; Zhou, Y.-B.; Chen, M.; Ma, Y.-Z.; Ma, J.; et al. Genomic Analysis of Soybean PP2A-B′′ Family and Its Effects on Drought and Salt Tolerance. Front. Plant Sci. 2022, 12, 3435. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, B.; Feng, G.; Mao, X.; Li, A.; Chang, X.; Jing, R. TaPP2AbBʺ-γ, a Wheat Regulatory Subunit of PP2A Enhanced Abiotic Stress Tolerance. Plant Growth Regul. 2019, 89, 345–355. [Google Scholar] [CrossRef]
- Konert, G.; Rahikainen, M.; Trotta, A.; Durian, G.; Salojärvi, J.; Khorobrykh, S.; Tyystjärvi, E.; Kangasjärvi, S. Subunits B′γ and B′ζ of Protein Phosphatase 2A Regulate Photo-Oxidative Stress Responses and Growth in Arabidopsis Thaliana. Plant Cell Environ. 2015, 38, 2641–2651. [Google Scholar] [CrossRef] [Green Version]
- Konert, G.; Trotta, A.; Kouvonen, P.; Rahikainen, M.; Durian, G.; Blokhina, O.; Fagerstedt, K.; Muth, D.; Corthals, G.L.; Kangasjärvi, S. Protein Phosphatase 2A (PP2A) Regulatory Subunit B′γ Interacts with Cytoplasmic ACONITASE 3 and Modulates the Abundance of AOX1A and AOX1D in Arabidopsis Thaliana. New Phytol. 2015, 205, 1250–1263. [Google Scholar] [CrossRef]
- Trotta, A.; Konert, G.; Rahikainen, M.; Aro, E.-M.; Kangasjärvi, S. Knock-down of Protein Phosphatase 2A Subunit B’γ Promotes Phosphorylation of CALRETICULIN 1 in Arabidopsis Thaliana. Plant Signal. Behav. 2011, 6, 1665–1668. [Google Scholar] [CrossRef] [Green Version]
- Trotta, A.; Wrzaczek, M.; Scharte, J.; Tikkanen, M.; Konert, G.; Rahikainen, M.; Holmström, M.; Hiltunen, H.-M.; Rips, S.; Sipari, N.; et al. Regulatory Subunit B′γ of Protein Phosphatase 2A Prevents Unnecessary Defense Reactions under Low Light in Arabidopsis. Plant Physiol. 2011, 156, 1464–1480. [Google Scholar] [CrossRef] [Green Version]
- Mhamdi, A. The Protein Phosphatase PP2A-B′γ Takes Control over Salicylic Acid to Suppress Defense and Premature Senescence. Plant Physiol. 2020, 182, 681–682. [Google Scholar] [CrossRef]
- Degrave, A.; Siamer, S.; Boureau, T.; Barny, M.-A. The AvrE Superfamily: Ancestral Type III Effectors Involved in Suppression of Pathogen-Associated Molecular Pattern-Triggered Immunity. Mol. Plant Pathol. 2015, 16, 899–905. [Google Scholar] [CrossRef]
- Siamer, S.; Guillas, I.; Shimobayashi, M.; Kunz, C.; Hall, M.N.; Barny, M.-A. Expression of the Bacterial Type III Effector DspA/E in Saccharomyces Cerevisiae Down-Regulates the Sphingolipid Biosynthetic Pathway Leading to Growth Arrest. J. Biol. Chem. 2014, 289, 18466–18477. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Ham, J.H.; Hage, R.; Zhao, W.; Soto-Hernández, J.; Lee, S.Y.; Paek, S.-M.; Kim, M.G.; Boone, C.; Coplin, D.L.; et al. Direct and Indirect Targeting of PP2A by Conserved Bacterial Type-III Effector Proteins. PLoS Pathog. 2016, 12, e1005609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahikainen, M.; Trotta, A.; Alegre, S.; Pascual, J.; Vuorinen, K.; Overmyer, K.; Moffatt, B.; Ravanel, S.; Glawischnig, E.; Kangasjärvi, S. PP2A-B′γ Modulates Foliar Trans-Methylation Capacity and the Formation of 4-Methoxy-Indol-3-Yl-Methyl Glucosinolate in Arabidopsis Leaves. Plant J. 2017, 89, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.M.; Gonda, S.; Vasas, G. A Review on the Phytochemical Composition and Potential Medicinal Uses of Horseradish (Armoracia Rusticana) Root. Food Rev. Int. 2013, 29, 261–275. [Google Scholar] [CrossRef]

| Subunit Family | Subunit Name | Subcellular Localization | Catalytic Subunit Partner | Function/Mechanism of Action | References |
|---|---|---|---|---|---|
| B | Bα, Bβ | cytosol, nucleus | unknown for Bα | male gametophyte development interacts with nitrate reductase to activate (dephosphorylate) it and promotes fertility; delay of flowering | [13,26,27,28,29,30] |
| Bβ | plasma membrane-bound? | C4 | ethylene-induced root growth inhibition | [29]; | |
| B′ | B′α, B′β | nucleus, cytosol | C5? | sister chromatid cohesion during meiosis; dephosphorylation of BZR1 and promoting BR signaling | [6,31,32,33] |
| B′γ, δ, ε, ζ, κ, θ, η | nucleus, nucleolus, cytosol | not known for all of them (C4 for B′η, ζ) | interaction of PP2A with BRI1 to dephosphorylate and inactivate it; therefore, they negatively regulate brassinolide signaling | [13,25,32,33] | |
| B′γ | cytosol, nucleus, plasma membrane | not studied here | promotion of flowering; modulation of PR, e.g., against Botrytis cinerea; regulation of resistance against aphid fecundity; inhibition of premature leaf senescence | [12,13,30,34,35,36] | |
| B′ζ, η | cytoplasm, nucleus, nucleolus, mitochondria; for η, plasma membrane as well | C4 | inactivates the PRR system BAK1-Flg22 to reduce plant immune response | [25,33,37,38] | |
| B′ζ | mitochondria, cytosol | C4 | dephosphorylation of CTR1 to prevent ethylene signaling; regulation of resistance against aphid fecundity; | [13,25,35,39] | |
| B′θ | nucleus, cytosol, peroxisomes | C2, C5 | β-oxidation of IBA and fatty acids; ABA signaling via the OST1 (SnRK2) | [15,16,25] | |
| B″ | TON2/FASS | CMTs, PPB, cytosol | C3, C4 | regulation of MT assembly in mitotic and non-mitotic cells; control of ROS levels, Cu/Zn SOD activity and phosphorylation of histone H2AX; dephosphorylation of the VIP1 bZIP TF for the defense against mechanical stress in roots | [20,40,41] |
| B″α, B″β | cytosol, nucleus | not studied here | regulation of the mevalonate pathway under normal and stress conditions to influence isoprenoid/plant hormone metabolism | [13,42] | |
| B″δ | cytosol? | not studied here | dephosphorylation of the VIP1 bZIP TF for the defense against mechanical stress in roots | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Máthé, C.; Freytag, C.; Kelemen, A.; M-Hamvas, M.; Garda, T. “B” Regulatory Subunits of PP2A: Their Roles in Plant Development and Stress Reactions. Int. J. Mol. Sci. 2023, 24, 5147. https://doi.org/10.3390/ijms24065147
Máthé C, Freytag C, Kelemen A, M-Hamvas M, Garda T. “B” Regulatory Subunits of PP2A: Their Roles in Plant Development and Stress Reactions. International Journal of Molecular Sciences. 2023; 24(6):5147. https://doi.org/10.3390/ijms24065147
Chicago/Turabian StyleMáthé, Csaba, Csongor Freytag, Adrienn Kelemen, Márta M-Hamvas, and Tamás Garda. 2023. "“B” Regulatory Subunits of PP2A: Their Roles in Plant Development and Stress Reactions" International Journal of Molecular Sciences 24, no. 6: 5147. https://doi.org/10.3390/ijms24065147





