Impact of Pesticide Residues on the Gut-Microbiota–Blood–Brain Barrier Axis: A Narrative Review
Abstract
:1. Introduction
2. Formation, Composition, and Role of Gut Microbiota
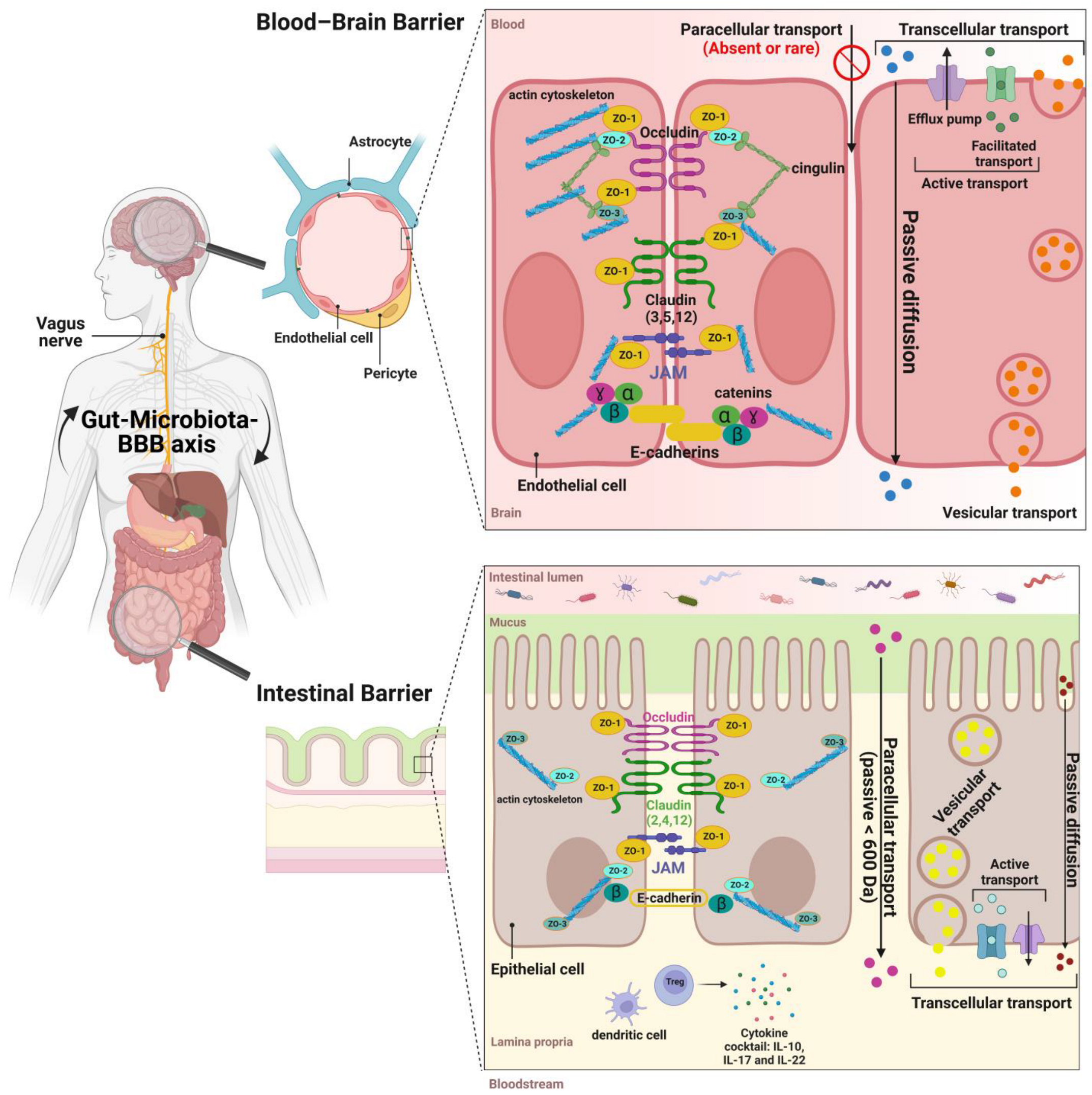
3. The Structure and Functions of the Intestinal Barrier
4. The Structure and Functions of the Blood–Brain Barrier
5. Gut-Microbiota-BBB Communication
6. Pesticide Residues Exposure and Effects on the Gut and BBB
6.1. Chlorpyrifos (CPF)
6.1.1. CPF Utilizations until 2022
6.1.2. CPF Mechanism of Toxicity and Toxicokinetic
6.1.3. CPF Biotransformation by Intestinal and Soil Bacteria
6.1.4. CPF Effects on Gut-Microbiota-BBB Axis
6.1.5. CPF Molecular Pathways Underlying Its Effects
7. Beneficial Modulation of the Gut-Microbiota–Brain Axis
8. Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- Mostafalou, S.; Abdollahi, M. Pesticides: An Update of Human Exposure and Toxicity. Arch. Toxicol. 2016, 91, 549–599. [Google Scholar] [CrossRef] [PubMed]
- Rathod, A.L.; Garg, R.K. Chlorpyrifos Poisoning and Its Implications in Human Fatal Cases: A Forensic Perspective with Reference to Indian Scenario. J. Forensic Leg. Med. 2017, 47, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Akashe, M.M.; Pawade, U.V.; Nikam, A.V. Classification of Pesticides: A Review. Int. J. Res. Ayurveda Pharm. 2018, 9, 144–150. [Google Scholar] [CrossRef]
- WHO. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification, 2019 Edition; WHO: Geneva, Switzerland, 2020. Available online: https://www.who.int/publications/i/item/9789240005662 (accessed on 5 March 2023).
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Abubakar, Y.; Tijjani, H.; Egbuna, C.; Adetunji, C.O.; Kala, S.; Kryeziu, T.L.; Patrick-Iwuanyanwu, K.C. Pesticides, History, and Classification. In Natural Remedies for Pest, Disease and Weed Control; Academic Press: Cambridge, MA, USA, 2019; pp. 29–42. [Google Scholar] [CrossRef]
- Doroudian, A.; Emadi, M.; Hosseinzadeh, R.; Maghami, P. Biological and Molecular Effects of Pesticides on Human Health; IntechOpen: London, UK, 2022; pp. 225–240. [Google Scholar]
- Meng, H.; Leong, W.; Leong, K.W.; Chen, C.; Zhao, Y. Walking the Line: The Fate of Nanomaterials at Biological Barriers. Biomaterials 2018, 174, 41–53. [Google Scholar] [CrossRef]
- Finbloom, J.A.; Sousa, F.; Stevens, M.M.; Desai, T.A. Engineering the Drug Carrier Biointerface to Overcome Biological Barriers to Drug Delivery. Adv. Drug Deliv. Rev. 2020, 167, 89–108. [Google Scholar] [CrossRef]
- Cong, Y.; Baimanov, D.; Zhou, Y.; Chen, C.; Wang, L. Penetration and Translocation of Functional Inorganic Nanomaterials into Biological Barriers. Adv. Drug Deliv. Rev. 2022, 191, 114615. [Google Scholar] [CrossRef] [PubMed]
- Antonini, M.; Lo Conte, M.; Sorini, C.; Falcone, M. How the Interplay between the Commensal Microbiota, Gut Barrier Integrity, and Mucosal Immunity Regulates Brain Autoimmunity. Front. Immunol. 2019, 10, 1937. [Google Scholar] [CrossRef] [Green Version]
- Vancamelbeke, M.; Vermeire, S. The Intestinal Barrier: A Fundamental Role in Health and Disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Saint-Pol, J.; Gosselet, F.; Duban-Deweer, S.; Pottiez, G.; Karamanos, Y. Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles. Cells 2020, 9, 851. [Google Scholar] [CrossRef] [Green Version]
- Benz, F.; Liebner, S. Structure and Function of the Blood-Brain Barrier (BBB). Handb. Exp. Pharmacol. 2020, 37, 13–25. [Google Scholar]
- Daneman, R.; Rescigno, M. Review the Gut Immune Barrier and the Blood-Brain Barrier: Are They So Different? Immunity 2009, 31, 722–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takiishi, T.; Ideli, C.; Fenero, M.; Olsen, N.; Câmara, S. Intestinal Barrier and Gut Microbiota: Shaping Our Immune Responses throughout Life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Chen, Z.; Li, G. Immune Response and Blood-Brain Barrier Dysfunction during Viral Neuroinvasion. Innate Immun. 2020, 27, 109–117. [Google Scholar] [CrossRef]
- Ma, T.Y.; Nighot, P.; Al-Sadi, R. Tight Junctions and the Intestinal Barrier, 6th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 1–2, ISBN 9780128124260. [Google Scholar]
- Versele, R.; Corsi, M.; Fuso, A.; Sevin, E.; Businaro, R.; Gosselet, F.; Fenart, L.; Candela, P. Ketone Bodies Promote Amyloid-Β1–40 Clearance in a Human in Vitro Blood–Brain Barrier Model. Int. J. Mol. Sci. 2020, 21, 934. [Google Scholar] [CrossRef] [Green Version]
- Shindler, A.E.; Hill-Yardin, E.L.; Petrovski, S.; Cunningham, A.C.; Bishop, N.; Franks, A.E. Potential Determinants of Gastrointestinal Dysfunction in Autism Spectrum Disorders. Rev. J. Autism Dev. Disord. 2020, 7, 182–196. [Google Scholar] [CrossRef]
- Anwar, H.; Irfan, S.; Hussain, G.; Naeem Faisal, M.; Muzaffar, H.; Mustafa, I.; Mukhtar, I.; Malik, S.; Irfan Ullah, M. Gut Microbiome: A New Organ System in Body. Parasitol. Microbiol. Res. 2020, 1, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [Green Version]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host In Fl Ammasome In Fl Uence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Jin, C.; Zeng, Z.; Fu, Z.; Jin, Y. Oral Imazalil Exposure Induces Gut Microbiota Dysbiosis and Colonic Inflammation in Mice. Chemosphere 2016, 160, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Djekkoun, N.; Depeint, F.; Guibourdenche, M.; El Khayat El Sabbouri, H.; Corona, A.; Rhazi, L.; Gay-Queheillard, J.; Rouabah, L.; Hamdad, F.; Bach, V.; et al. Chronic Perigestational Exposure to Chlorpyrifos Induces Perturbations in Gut Bacteria and Glucose and Lipid Markers in Female Rats and Their Offspring. Toxics 2022, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Toor, D.; Wasson, M.K.; Kumar, P.; Karthikeyan, G.; Kaushik, N.K.; Goel, C.; Singh, S.; Kumar, A.; Prakash, H. Dysbiosis Disrupts Gut Immune Homeostasis and Promotes Gastric Diseases. Int. J. Mol. Sci. 2019, 20, 2432. [Google Scholar] [CrossRef] [Green Version]
- Martinez, J.E.; Kahana, D.D.; Ghuman, S.; Wilson, H.P.; Wilson, J.; Kim, S.C.J.; Lagishetty, V.; Jacobs, J.P.; Sinha-Hikim, A.P.; Friedman, T.C. Unhealthy Lifestyle and Gut Dysbiosis: A Better Understanding of the Effects of Poor Diet and Nicotine on the Intestinal Microbiome. Front. Endocrinol. 2021, 12, 667066. [Google Scholar] [CrossRef]
- Santhiravel, S.; Bekhit, A.E.D.A.; Mendis, E.; Jacobs, J.L.; Dunshea, F.R.; Rajapakse, N.; Ponnampalam, E.N. The Impact of Plant Phytochemicals on the Gut Microbiota of Humans for a Balanced Life. Int. J. Mol. Sci. 2022, 23, 8124. [Google Scholar] [CrossRef] [PubMed]
- Joly Condette, C.; Elion Dzon, B.; Hamdad, F.; Biendo, M.; Bach, V.; Khorsi-Cauet, H. Use of Molecular Typing to Investigate Bacterial Translocation from the Intestinal Tract of Chlorpyrifos-Exposed Rats. Gut Pathog. 2016, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- Guibourdenche, M.; El Khayat El Sabbouri, H.; Djekkoun, N.; Khorsi-Cauet, H.; Bach, V.; Anton, P.M.; Gay-Quéheillard, J. Programming of Intestinal Homeostasis in Male Rat Offspring after Maternal Exposure to Chlorpyrifos and/or to a High Fat Diet. Sci. Rep. 2021, 11, 11420. [Google Scholar] [CrossRef]
- Galea, I. The Blood-Brain Barrier in Systemic Infection and in Fl Ammation. Cell. Mol. Immunol. 2021, 18, 2489–2501. [Google Scholar] [CrossRef]
- Djekkoun, N.; Lalau, J.D.; Bach, V.; Depeint, F.; Khorsi-Cauet, H. Chronic Oral Exposure to Pesticides and Their Consequences on Metabolic Regulation: Role of the Microbiota. Eur. J. Nutr. 2021, 60, 4131–4149. [Google Scholar] [CrossRef]
- Joly Condette, C.; Bach, V.; Mayeur, C.; Gay-Quéheillard, J.; Khorsi-Cauet, H. Chlorpyrifos Exposure during Perinatal Period Affects Intestinal Microbiota Associated with Delay of Maturation of Digestive Tract in Rats. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 30–40. [Google Scholar] [CrossRef]
- Reygner, J.; Condette, C.J.; Bruneau, A.; Delanaud, S.; Rhazi, L.; Depeint, F.; Abdennebi-Najar, L.; Bach, V.; Mayeur, C.; Khorsi-Cauet, H. Changes in Composition and Function of Human Intestinal Microbiota Exposed to Chlorpyrifos in Oil as Assessed by the SHIME® Model. Int. J. Environ. Res. Public Health 2016, 13, 1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safi-Stibler, S.; Gabory, A. Seminars in Cell & Developmental Biology Epigenetics and the Developmental Origins of Health and Disease: Parental Environment Signalling to the Epigenome, Critical Time Windows and Sculpting the Adult Phenotype. Semin. Cell Dev. Biol. 2020, 97, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.; de Vos, W.M. Early Life Colonization of the Human Gut: Microbes Matter Everywhere. Curr. Opin. Microbiol. 2018, 44, 70–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-datchary, P. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life Resource Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, E.; Fernández, L.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Nueno-Palop, C.; Narbad, A.; Olivares, M.; Xaus, J.; Rodríguez, J.M. Isolation of Commensal Bacteria from Umbilical Cord Blood of Healthy Neonates Born by Cesarean Section. Curr. Microbiol. 2005, 51, 270–274. [Google Scholar] [CrossRef]
- Jiménez, E.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Olivares, M.; Xaus, J.; Fernández, L.; Rodríguez, J.M. Is Meconium from Healthy Newborns Actually Sterile? Res. Microbiol. 2008, 159, 187–193. [Google Scholar] [CrossRef]
- Moles, L.; Gómez, M.; Heilig, H.; Bustos, G.; Fuentes, S.; de Vos, W.; Fernández, L.; Rodríguez, J.M.; Jiménez, E. Bacterial Diversity in Meconium of Preterm Neonates and Evolution of Their Fecal Microbiota during the First Month of Life. PLoS ONE 2013, 8, e66986. [Google Scholar] [CrossRef] [Green Version]
- Nishino, R.; Mikami, K.; Takahashi, H.; Tomonaga, S.; Furuse, M.; Hiramoto, T.; Aiba, Y.; Koga, Y.; Sudo, N. Commensal Microbiota Modulate Murine Behaviors in a Strictly Contamination-Free Environment Confirmed by Culture-Based Methods. Neurogastroenterol. Motil. 2013, 25, 521-e371. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Harris, M.A.; Reddy, C.A.; Carter, G.R. Anaerobic Bacteria from the Large Intestine of Mice. Appl. Environ. Microbiol. 1976, 31, 907–912. [Google Scholar] [CrossRef] [Green Version]
- Jovel, J.; Dieleman, L.A.; Kao, D.; Mason, A.L.; Wine, E. The Human Gut Microbiome in Health and Disease. Metagenomics Perspect. Methods Appl. 2018, 13, 197–213. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, Stability and Resilience of the Human Gut Microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Vaga, S.; Lee, S.; Ji, B.; Andreasson, A.; Talley, N.J.; Agréus, L.; Bidkhori, G.; Kovatcheva-Datchary, P.; Park, J.; Lee, D.; et al. Compositional and Functional Differences of the Mucosal Microbiota along the Intestine of Healthy Individuals. Sci. Rep. 2020, 10, 14977. [Google Scholar] [CrossRef]
- D’Amelio, P.D. Gut Microbiota, Immune System, and Bone. Calcif. Tissue Int. 2017, 102, 415–425. [Google Scholar] [CrossRef]
- Logsdon, A.F.; Erickson, M.A.; Rhea, E.M.; Salameh, T.S.; Banks, W.A. Gut Reactions: How the Blood-Brain Barrier Connects the Microbiome and the Brain. Exp. Biol. Med. 2018, 243, 159–165. [Google Scholar] [CrossRef]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut Microbiota-Derived Short Chain Fatty Acids Are Potential Mediators in Gut Inflammation. Anim. Nutr. (Zhongguo Xu Mu Shou Yi Xue Hui) 2021, 8, 350–360. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, SCFAs, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The Gut Microbiota Influences Blood-Brain Barrier Permeability in Mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.N.; Knausenberger, T.B.; Connell, E.; Le Gall, G.; Hardy, T.A.; Randall, D.W.; McCafferty, K.; Yaqoob, M.M.; Solito, E.; Müller, M.; et al. Cerebrovascular Damage Caused by the Gut Microbe-Derived Uraemic Toxin p-Cresol Sulfate Is Prevented by Blockade of the Epidermal Growth Factor Receptor. Brain Behav. Immun. 2022. [Google Scholar] [CrossRef]
- Brittany, D.; Needham, M.F.; Adame, M.D.; Wang, Z.; Boktor, J.C.; Haney, J.; Wu, W.-L.; Rabut, C.; Ladinsky, M.S.; Hwang, S.-J.; et al. A Gut-Derived Metabolite Alters Brain Activity and Anxiety Behaviour in Mice. Nature 2022, 602, 647–653. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal Barrier Function: Molecular Regulation and Disease Pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Yue, J.; Yao, Y.; Hou, P.; Zhang, T.; Zhang, Q.; Yi, L.; Mi, M. Dihydromyricetin Protects Intestinal Barrier Integrity by Promoting IL-22 Expression in ILC3s through the AMPK/SIRT3/STAT3 Signaling Pathway. Nutrients 2023, 15, 355. [Google Scholar] [CrossRef]
- Condette, C.J.; Khorsi-Cauet, H.; Morlière, P.; Zabijak, L.; Reygner, J.; Bach, V.; Gay-Quéheillard, J. Increased Gut Permeability and Bacterial Translocation after Chronic Chlorpyrifos Exposure in Rats. PLoS ONE 2014, 9, e102217. [Google Scholar] [CrossRef]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Herath, M.; Hosie, S.; Bornstein, J.C.; Franks, A.E.; Hill-Yardin, E.L. The Role of the Gastrointestinal Mucus System in Intestinal Homeostasis: Implications for Neurological Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 248. [Google Scholar] [CrossRef]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.Y.; Ko, H.J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Young, R.L.; Leong, L.; Rogers, G.B.; Spencer, N.J.; Jessup, C.F.; Keating, D.J. Cellular Regulation of Peripheral Serotonin. In Serotonin; Academic Press: Cambridge, MA, USA, 2019; pp. 137–153. [Google Scholar] [CrossRef]
- Putignani, L.; Del Chierico, F.; Vernocchi, P.; Cicala, M.; Cucchiara, S.; Dallapiccola, B. Gut Microbiota Dysbiosis as Risk and Premorbid Factors of IBD and IBS Along the Childhood-Adulthood Transition. Inflamm. Bowel Dis. 2016, 22, 487–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.; Moon, K.M.; Kim, C.Y. Review Article Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J. Immunol. Res. 2018, 2018, 2645465. [Google Scholar] [CrossRef] [Green Version]
- Holmes, J.L.; Van Itallie, C.M.; Rasmussen, J.E.; Anderson, J.M. Claudin Profiling in the Mouse during Postnatal Intestinal Development and along the Gastrointestinal Tract Reveals Complex Expression Patterns. Gene Expr. Patterns 2006, 6, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Scalise, A.A.; Kakogiannos, N.; Zanardi, F.; Iannelli, F.; Giannotta, M. The Blood–Brain and Gut–Vascular Barriers: From the Perspective of Claudins. Tissue Barriers 2021, 9, 1926190. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.; Hanley, N.; Campbell, M. Claudin-5: Gatekeeper of Neurological Function. Fluids Barriers CNS 2019, 16, 3. [Google Scholar] [CrossRef] [Green Version]
- Otani, T. Tight Junction Structure and Function Revisited. Trends Cell Biol. 2020, 30, 805–817. [Google Scholar] [CrossRef]
- Kuo, W.T.; Zuo, L.; Odenwald, M.A.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C.; Turner, J.R. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology 2021, 161, 1924–1939. [Google Scholar] [CrossRef]
- Bieczynski, F.; Painefilú, J.C.; Venturino, A.; Luquet, C.M.; Lionetto, M.G. Expression and Function of ABC Proteins in Fish Intestine. Front. Physiol. 2021, 12, 2230. [Google Scholar] [CrossRef]
- Cecchelli, R.; Berezowski, V.; Lundquist, S.; Culot, M.; Renftel, M.; Dehouck, M.P.; Fenart, L. Modelling of the Blood-Brain Barrier in Drug Discovery and Development. Nat. Rev. Drug Discov. 2007, 6, 650–661. [Google Scholar] [CrossRef]
- Gosselet, F.; Candela, P.; Cecchelli, R.; Fenart, L. La Barrière Hématoencéphalique Une Nouvelle Cible Thérapeutique Dans La Maladie d’Alzheimer? Med./Sci. 2011, 27, 987–992. [Google Scholar] [CrossRef] [Green Version]
- Mou, Y.; Du, Y.; Zhou, L.; Yue, J.; Hu, X.; Liu, Y.; Chen, S.; Lin, X.; Zhang, G.; Xiao, H.; et al. Gut Microbiota Interact with the Brain through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 1046. [Google Scholar] [CrossRef] [PubMed]
- De Aquino, C.C.; Leitão, R.A.; Alves, L.A.O.; Coelho-Santos, V.; Guerrant, R.L.; Ribeiro, C.F.; Malva, J.O.; Silva, A.P.; Oriá, R.B. Effect of Hypoproteic and High-Fat Diets on Hippocampal Blood-Brain Barrier Permeability and Oxidative Stress. Front. Nutr. 2019, 5, 131. [Google Scholar] [CrossRef] [PubMed]
- Oldendorf, W.H.; Cornford, M.E.; Brown, W.J. The Large Apparent Work of the Blood-Brain Barrier: The Mitochondria1 Content of Capillary Endothelial Cells in Brain and Other Tissues of the Rat. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1977, 1, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.A. Endothelial Vesicles in the Blood-Brain Barrier: Are They Related to Permeability? Cell. Mol. Neurobiol. 2000, 20, 149–163. [Google Scholar] [CrossRef]
- Gosselet, F.; Saint-Pol, J.; Fenart, L. Effects of Oxysterols on the Blood-Brain Barrier: Implications for Alzheimer’s Disease. Biochem. Biophys. Res. Commun. 2014, 446, 687–691. [Google Scholar] [CrossRef]
- Al-asmakh, M.; Hedin, L.; Al-asmakh, M.; Hedin, L. Microbiota and the Control of Blood-Tissue Barriers Microbiota and the Control of Blood-Tissue Barriers. Tissue Barriers 2015, 3, e1039691. [Google Scholar] [CrossRef] [Green Version]
- Haseloff, R.F.; Dithmer, S.; Winkler, L.; Wolburg, H.; Blasig, I.E. Transmembrane Proteins of the Tight Junctions at the Blood-Brain Barrier: Structural and Functional Aspects. Semin. Cell Dev. Biol. 2015, 38, 16–25. [Google Scholar] [CrossRef]
- Versele, R.; Sevin, E.; Gosselet, F.; Fenart, L.; Candela, P. TNF-α and IL-1 β Modulate Blood-Brain Barrier Permeability and Decrease Amyloid- β Peptide Efflux in a Human Blood-Brain Barrier Model. Int. J. Mol. Sci. 2022, 23, 10235. [Google Scholar] [CrossRef]
- Gosselet, F.; Azevedo, R.; Roig, A.; Rosell, A.; Culot, M. Neurochemistry International Central Nervous System Delivery of Molecules across the Blood-Brain Barrier. Neurochem. Int. 2021, 144, 104952. [Google Scholar] [CrossRef]
- Dib, S.; Pahnke, J.; Gosselet, F. Role of ABCA7 in Human Health and in Alzheimer’s Disease. Int. J. Mol. Sci. Rev. 2021, 7, 4603. [Google Scholar] [CrossRef] [PubMed]
- Parran, D.K.; Magnin, G.; Li, W.; Jortner, B.S.; Ehrich, M. Chlorpyrifos Alters Functional Integrity and Structure of an in Vitro BBB Model: Co-Cultures of Bovine Endothelial Cells and Neonatal Rat Astrocytes. Neurotoxicology 2005, 26, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ehrich, M. Transient Alterations of the Blood-Brain Barrier Tight Junction and Receptor Potential Channel Gene Expression by Chlorpyrifos. J. Appl. Toxicol. 2013, 33, 1187–1191. [Google Scholar] [CrossRef]
- Chedik, L.; Bruyere, A.; Bacle, A.; Potin, S.; Le Vée, M.; Fardel, O. Interactions of Pesticides with Membrane Drug Transporters: Implications for Toxicokinetics and Toxicity. Expert Opin. Drug Metab. Toxicol. 2018, 14, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Balaguer-Trias, J.; Deepika, D.; Schuhmacher, M.; Kumar, V. Impact of Contaminants on Microbiota: Linking the Gut–Brain Axis with Neurotoxicity. Int. J. Environ. Res. Public Health 2022, 19, 1368. [Google Scholar] [CrossRef]
- Collins, S.M.; Surette, M.; Bercik, P. The Interplay between the Intestinal Microbiota and the Brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. 2018, 17, 28. [Google Scholar]
- Spadoni, I.; Zagato, E.; Bertocchi, A.; Paolinelli, R.; Hot, E.; Di Sabatino, A.; Caprioli, F.; Bottiglieri, L.; Oldani, A.; Viale, G.; et al. A Gut-Vascular Barrier Controls the Systemic Dissemination of Bacteria. Science 2015, 350, 830–834. [Google Scholar] [CrossRef]
- Spadoni, I.; Pietrelli, A.; Pesole, G.; Rescigno, M. Gene Expression Profile of Endothelial Cells during Perturbation of the Gut Vascular Barrier. Gut Microbes 2016, 7, 540–548. [Google Scholar] [CrossRef] [Green Version]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [Green Version]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut Microbes and Metabolites as Modulators of Blood-Brain Barrier Integrity and Brain Health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, D.; Liu, M.; Li, H.; Song, J.; Jiang, X.; Wang, G.; Yang, X. Dysbacteriosis Induces Abnormal Neurogenesis via LPS in a Pathway Requiring NF-ΚB/IL-6. Pharmacol. Res. 2021, 167, 105543. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The Short-Chain Fatty Acid Acetate Reduces Appetite via a Central Homeostatic Mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erny, D.; Dokalis, N.; Mezö, C.; Castoldi, A.; Mossad, O.; Staszewski, O.; Frosch, M.; Villa, M.; Fuchs, V.; Mayer, A.; et al. Microbiota-Derived Acetate Enables the Metabolic Fitness of the Brain Innate Immune System during Health and Disease. Cell Metab. 2021, 33, 2260–2276.e7. [Google Scholar] [CrossRef] [PubMed]
- Duscha, A.; Gisevius, B.; Hirschberg, S.; Linker, R.A.; Gold, R.; Haghikia, A.; Duscha, A.; Gisevius, B.; Hirschberg, S.; Yissachar, N.; et al. Propionic Acid Shapes the Multiple Sclerosis Disease Course by an Immunomodulatory Article Propionic Acid Shapes the Multiple Sclerosis Disease Course by an Immunomodulatory Mechanism. Cell 2020, 180, 1067–1080. [Google Scholar] [CrossRef]
- Sarkar, S.R.; Banerjee, S. Gut Microbiota in Neurodegenerative Disorders; Elsevier B.V.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Yang, X.; Yu, D.; Xue, L.; Li, H.; Du, J. Probiotics Modulate the Microbiota–Gut–Brain Axis and Improve Memory Deficits in Aged SAMP8 Mice. Acta Pharm. Sin. B 2020, 10, 475–487. [Google Scholar] [CrossRef]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A Guiding Map for Inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef] [Green Version]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Santocchi, E.; Guiducci, L.; Fulceri, F.; Billeci, L.; Buzzigoli, E.; Apicella, F.; Calderoni, S.; Grossi, E.; Morales, M.A.; Muratori, F. Gut to Brain Interaction in Autism Spectrum Disorders: A Randomized Controlled Trial on the Role of Probiotics on Clinical, Biochemical and Neurophysiological Parameters. BMC Psychiatry 2016, 16, 183. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Song, X.; Huang, Y.; Yao, J.; Zhou, M.; Li, Z.; You, Q.; Guo, Q.; Lu, N. Wogonin Inhibits LPS-Induced Tumor Angiogenesis via Suppressing PI3K/Akt/NF-ΚB Signaling. Eur. J. Pharmacol. 2014, 737, 57–69. [Google Scholar] [CrossRef]
- Sui, Y.T.; Bullock, K.M.; Erickson, M.A.; Zhang, J.; Banks, W.A. Alpha Synuclein Is Transported into and out of the Brain by the Blood–Brain Barrier. Peptides 2014, 62, 197–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clairembault, T.; Leclair-Visonneau, L.; Coron, E.; Bourreille, A.; Le Dily, S.; Vavasseur, F.; Heymann, M.F.; Neunlist, M.; Derkinderen, P. Structural Alterations of the Intestinal Epithelial Barrier in Parkinson’s Disease. Acta Neuropathol. Commun. 2015, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Zhang, Y.; Wang, G.; Han, R.; Xie, X. Effects of Chlorpyrifos on the Gut Microbiome and Urine Metabolome in Mouse (Mus musculus). Chemosphere 2016, 153, 287–293. [Google Scholar] [CrossRef]
- Tang, Q.; Tang, J.; Ren, X.; Li, C. Glyphosate Exposure Induces Inflammatory Responses in the Small Intestine and Alters Gut Microbial Composition in Rats. Environ. Pollut. 2020, 261, 114129. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. International Code of Conduct on the Distribution and Use of Pesticides Guidelines on Developing a Reporting System for Health and Environmental Incidents Resulting from Exposure to Pesticide; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2009; ISBN 9789251068311.
- Kaur, R.; Mavi, G.K.; Raghav, S.; Khan, I. Pesticides Classification and Its Impact on Environment. Int. J. Curr. Microbiol. Appl. Sci. 2017, 8, 1889–1897. [Google Scholar] [CrossRef]
- Aroonvilairat, S.; Tangjarukij, C.; Sornprachum, T.; Chaisuriya, P.; Siwadune, T.; Ratanabanangkoon, K. Effects of Topical Exposure to a Mixture of Chlorpyrifos, Cypermethrin and Captan on the Hematological and Immunological Systems in Male Wistar Rats. Environ. Toxicol. Pharmacol. 2018, 59, 53–60. [Google Scholar] [CrossRef]
- Lukowicz, C.; Ellero-simatos, S.; Régnier, M.; Polizzi, A.; Lasserre, F.; Montagner, A.; Lippi, Y.; Jamin, E.L.; Martin, J.; Naylies, C.; et al. Metabolic Effects of a Chronic Dietary Exposure to a Low-Dose Pesticide Cocktail in Mice: Sexual Dimorphism and Role of the Constitutive Androstane Receptor. Environ. Health Perspect. 2018, 126, 067007. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.; Klément, W.; Dopavogui, L.; De Bock, F.; Lasserre, F.; Barretto, S.; Lukowicz, C.; Fougerat, A.; Polizzi, A.; Schaal, B.; et al. Perinatal Exposure to a Dietary Pesticide Cocktail Does Not Increase Susceptibility to High-Fat Diet-Induced Metabolic Perturbations at Adulthood but Modi Fi Es Urinary and Fecal Metabolic Fi Ngerprints in C57Bl6/J Mice. Environ. Int. 2020, 144, 106010. [Google Scholar] [CrossRef]
- Kaur, R. Metabolism of Pesticides by Human Cytocrome P450 (CYPs). Int. J. Creat. Res. Thoughts 2018, 6, 1293–1300. [Google Scholar] [CrossRef]
- Parathion|ToxFAQsTM|ATSDR. Available online: https://wwwn.cdc.gov/TSP/ToxFAQs/ToxFAQsDetails.aspx?faqid=1426&toxid=246 (accessed on 15 March 2023).
- Carrasco Cabrera, L.; Medina Pastor, P. The 2019 European Union Report on Pesticide Residues in Food. EFSA J. 2021, 19, e06491. [Google Scholar] [CrossRef]
- Tirelli, V.; Catone, T.; Turco, L.; Di Consiglio, E.; Testai, E.; De Angelis, I. Effects of the Pesticide Clorpyrifos on an in Vitro Model of Intestinal Barrier. Toxicol. Vitr. 2007, 21, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Reygner, J.; Lichtenberger, L.; Elmhiri, G.; Dou, S.; Bahi-Jaber, N.; Rhazi, L.; Depeint, F.; Bach, V.; Khorsi-Cauet, H.; Abdennebi-Najar, L. Inulin Supplementation Lowered the Metabolic Defects of Prolonged Exposure to Chlorpyrifos from Gestation to Young Adult Stage in Offspring Rats. PLoS ONE 2016, 11, e0164614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Réquilé, M.; Gonzàlez Alvarez, D.O.; Delanaud, S.; Rhazi, L.; Bach, V.; Depeint, F.; Khorsi-Cauet, H. Use of a Combination of in Vitro Models to Investigate the Impact of Chlorpyrifos and Inulin on the Intestinal Microbiota and the Permeability of the Intestinal Mucosa. Environ. Sci. Pollut. Res. 2018, 25, 22529–22540. [Google Scholar] [CrossRef] [PubMed]
- Djekkoun, N.; Depeint, F.; Guibourdenche, M.; El, H.; Et, K.; Aurélie, S.; Rhazi, L.; Gay, J.; Leila, Q.; Maurice, R.; et al. Perigestational Exposure of a Combination of a High-Fat Diet and Pesticide Impacts the Metabolic and Microbiotic Status of Dams and Pups; a Preventive Strategy Based on Prebiotics. Eur. J. Nutr. 2022, 62, 1253–1265. [Google Scholar] [CrossRef]
- Betancourt, A.M.; Carr, R.L. The Effect of Chlorpyrifos and Chlorpyrifos-Oxon on Brain Cholinesterase, Muscarinic Receptor Binding, and Neurotrophin Levels in Rats Following Early Postnatal Exposure. Toxicol. Sci. 2004, 71, 63–71. [Google Scholar] [CrossRef] [Green Version]
- El, H.; El, K.; Darwiche, W. Impact of Chronic Exposure to the Pesticide Chlorpyrifos on Respiratory Parameters and Sleep Apnea in Juvenile and Adult Rats. PLoS ONE 2018, 13, e0191237. [Google Scholar]
- Gao, A.B.; Chi, L.; Tu, P.; Bian, X.; Thomas, J.; Ru, H.; Lu, K. The Organophosphate Malathion Disturbs Gut Microbiome Development and the Quorum-Sensing System. Toxicol. Lett. 2017, 283, 52–57. [Google Scholar] [CrossRef]
- Gao, B.; Bian, X.; Chi, L.; Tu, P.; Ru, H.; Lu, K. Organophosphate Diazinon Altered Quorum Sensing, Cell Motility, Stress Response, and Carbohydrate Metabolism of Gut Microbiome. Toxicol. Sci. 2017, 157, 354–364. [Google Scholar] [CrossRef] [Green Version]
- Meng, Z.; Liu, L.; Yan, S.; Sun, W.; Jia, M.; Tian, S.; Huang, S.; Zhou, Z.; Zhu, W. Gut Microbiota: A Key Factor in the Host Health Effects Induced by Pesticide Exposure. J. Agric. Food Chem. 2020, 68, 10517–10531. [Google Scholar] [CrossRef]
- Timofeeva, O.A.; Roegge, C.S.; Seidler, F.J.; Slotkin, T.A.; Levin, E.D. Persistent Cognitive Alterations in Rats after Early Postnatal Exposure to Low Doses of the Organophosphate Pesticide, Diazinon. Neurotoxicol. Teratol. 2008, 30, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Balbuena, P.; Li, W.; Magnin-bissel, G.; Meldrum, J.B.; Ehrich, M. Comparison of Two Blood-Brain Barrier In Vitro Systems: Cytotoxicity and Transfer Assessments of Malathion/Oxon and Lead Acetate. Toxicol. Sci. 2010, 114, 260–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesan, R.; Park, Y.U.; Ji, E.; Yeo, E.; Kim, S.Y. Malathion Increases Apoptotic Cell Death by Inducing Lysosomal Membrane Permeabilization in N2a Neuroblastoma Cells: A Model for Neurodegeneration in Alzheimer’s Disease. Cell Death Discov. 2017, 11, 17007. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Al-ahmad, A.J. Effects of Glyphosate and Aminomethylphosphonic Acid on an Isogeneic Model of the Human Blood-Brain Barrier. Toxicol. Lett. 2018, 304, 39–49. [Google Scholar] [CrossRef]
- Heusinkveld, H.J.; Molendijk, J.; Van Den Berg, M.; Westerink, R.H.S. Azole Fungicides Disturb Intracellular Ca2+ in an Additive Manner in Dopaminergic PC12 Cells. Toxicol. Sci. 2013, 134, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Heusinkveld, H.J.; Westerink, R.H.S. Comparison of Different in Vitro Cell Models for the Assessment of Pesticide-Induced Dopaminergic Neurotoxicity Authors. Toxicol. Vitr. 2017, 45, 81–88. [Google Scholar] [CrossRef]
- Jin, C.; Xia, J.; Wu, S.; Tu, W.; Pan, Z.; Fu, Z.; Wang, Y.; Jin, Y. Insights into a Possible Influence on Gut Microbiota and Intestinal Barrier Function During Chronic Exposure of Mice to Imazalil. Toxicol. Sci. 2018, 162, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Martins-gomes, C.; Coutinho, T.E.; Silva, T.L.; Andreani, T.; Silva, M. In Vitro Enzymatic Inhibition Assays. Toxics 2022, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Supreeth, M.; Raju, N. Biotransformation of Chlorpyrifos and Endosulfan by Bacteria and Fungi. Appl. Microbiol. Biotechnol. 2017, 101, 5961–5971. [Google Scholar] [CrossRef]
- EPA. Frequent Questions about the Chlorpyrifos 2021 Final Rule|US EPA. Available online: https://www.epa.gov/ingredients-used-pesticide-products/frequent-questions-about-chlorpyrifos-2021-final-rule (accessed on 5 March 2023).
- Iowa State University. Updates on Chlorpyrifos Uses in 2022|Integrated Crop Management. Available online: https://crops.extension.iastate.edu/cropnews/2022/03/updates-chlorpyrifos-uses-2022 (accessed on 5 March 2023).
- Zhang, Q.; Zheng, S.; Wang, S.; Wang, W.; Xing, H.; Xu, S. Chlorpyrifos Induced Oxidative Stress to Promote Apoptosis and Autophagy through the Regulation of MiR-19a-AMPK Axis in Common Carp. Fish Shellfish Immunol. 2019, 93, 1093–1099. [Google Scholar] [CrossRef]
- Brahmand, M.B.; Yunesian, M.; Nabizadeh, R.; Nasseri, S.; Alimohammadi, M. Evaluation of Chlorpyrifos Residue in Breast Milk and Its Metabolite in Urine of Mothers and Their Infants Feeding Exclusively by Breast Milk in North of Iran. J. Environ. Heal. Sci. Eng. 2019, 17, 817–825. [Google Scholar] [CrossRef]
- Prasad, J.; Abraham, V.J.; Minz, S.; Abraham, S.; Joseph, A.; Muliyil, J.P.; George, K.; Jacob, K.S. Rates and Factors Associated with Suicide in Kaniyambadi Block, Tamil Nadu, South India, 2000−2002. Int. J. Soc. Psychiatry 2006, 52, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Nolan, R.J.; Rick, D.L.; Freshour, N.L.; Saunders, J.H. Chlorpyrifos: Pharmacokinetics in human volunteers. Toxicol. Appl. Pharmacol. 1984, 15, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Sancho, J.V.; Pozo, O.J. Direct Determination of Chlorpyrifos and Its Main Serum and Urine by Coupled-Column Liquid Chromatography/Electrospray-Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2000, 1490, 1485–1490. [Google Scholar] [CrossRef]
- Yang, F.; Li, J.; Pang, G.; Ren, F.; Fang, B. Effects of Diethyl Phosphate, a Non-Specific Metabolite of Organophosphorus Pesticides, on Serum Lipid, Hormones, Inflammation, and Gut Microbiota. Molecules 2019, 24, 2003. [Google Scholar] [CrossRef] [Green Version]
- NPIC. Chlorpyrifos Technical Fact Sheet by the National Pesticide Information Center. Available online: http://npic.orst.edu/factsheets/archive/chlorptech.html (accessed on 5 March 2023).
- Harishankar, M.K.; Sasikala, C.; Ramya, M. Efficiency of the Intestinal Bacteria in the Degradation of the Toxic Pesticide, Chlorpyrifos. 3 Biotech 2013, 3, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, W.; Lu, Y. Biodegradation of Insecticides by Gut Bacteria Isolated from Stored Grain Beetles and Its Implication in Host Insecticide Resistance. J. Stored Prod. Res. 2022, 96, 101943. [Google Scholar] [CrossRef]
- Ibrahim, S.; Gupta, R.K.; War, A.R.; Hussain, B.; Kumar, A.; Sofi, T.; Noureldeen, A.; Darwish, H. Degradation of Chlorpyriphos and Polyethylene by Endosymbiotic Bacteria from Citrus Mealybug. Saudi J. Biol. Sci. 2021, 28, 3214–3224. [Google Scholar] [CrossRef]
- Liu, J.; Tan, L.; Wang, J.; Wang, Z.; Ni, H.; Li, L. Complete Biodegradation of Chlorpyrifos by Engineered Pseudomonas Putida Cells Expressing Surface-Immobilized Laccases. Chemosphere 2016, 157, 200–207. [Google Scholar] [CrossRef]
- Gilani, R.A.; Rafique, M.; Rehman, A.; Munis, M.F.H.; Rehman, S.U.; Chaudhary, H.J. Biodegradation of Chlorpyrifos by Bacterial Genus Pseudomonas. J. Basic Microbiol. 2016, 56, 105–119. [Google Scholar] [CrossRef]
- Vidya Lakshmi, C.; Kumar, M.; Khanna, S. Biotransformation of Chlorpyrifos and Bioremediation of Contaminated Soil. Int. Biodeterior. Biodegrad. 2008, 62, 204–209. [Google Scholar] [CrossRef]
- Li, X.; Jiang, J.; Gu, L.; Ali, S.W.; He, J.; Li, S. Diversity of Chlorpyrifos-Degrading Bacteria Isolated from Chlorpyrifos-Contaminated Samples. Int. Biodeterior. Biodegrad. 2008, 62, 331–335. [Google Scholar] [CrossRef]
- Vidya Lakshmi, C.; Kumar, M.; Khanna, S. Biodegradation of Chlorpyrifos in Soil by Enriched Cultures. Curr. Microbiol. 2009, 58, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Ishag, A.E.S.A.; Abdelbagi, A.O.; Hammad, A.M.A.; Elsheikh, E.A.E.; Elsaid, O.E.; Hur, J.H.; Laing, M.D. Biodegradation of Chlorpyrifos, Malathion, and Dimethoate by Three Strains of Bacteria Isolated from Pesticide-Polluted Soils in Sudan. J. Agric. Food Chem. 2016, 64, 8491–8498. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, C.; Jiwal, S.; Rout, P.; Ramya, M. Biodegradation of Chlorpyrifos by Bacterial Consortium Isolated from Agriculture Soil. World J. Microbiol. Biotechnol. 2012, 28, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Shen, W.; Zhao, X.; Hou, Y.; Cao, H.; Cui, Z. Isolation and Characterization of 3,5,6-Trichloro-2-Pyridinol-Degrading Ralstonia sp. Strain T6. Bioresour. Technol. 2010, 101, 7479–7483. [Google Scholar] [CrossRef]
- Ostrea, E.M.; Morales, V.; Ngoumgna, E.; Prescilla, R.; Tan, E.; Hernandez, E.; Ramirez, G.B.; Cifra, H.L.; Manlapaz, M.L. Prevalence of Fetal Exposure to Environmental Toxins as Determined by Meconium Analysis. Neurotoxicology 2002, 23, 329–339. [Google Scholar] [CrossRef]
- Berton, T.; Mayhoub, F.; Chardon, K.; Duca, R.C.; Lestremau, F.; Bach, V.; Tack, K. Development of an Analytical Strategy Based on LC-MS/MS for the Measurement of Different Classes of Pesticides and Theirs Metabolites in Meconium: Application and Characterisation of Foetal Exposure in France. Environ. Res. 2014, 132, 311–320. [Google Scholar] [CrossRef]
- El-Baz, M.A.; El-Deek, S.E.; Nsar, A.Y.; El-Maali, N.A.; AbdelHafez, F.F.; Amin, A.F. Prenatal Pesticide Exposure: Meconium as a Biomarker and Impact on Fetal Weight. J. Environ. Anal. Toxicol. 2015, 5, 1000268. [Google Scholar] [CrossRef] [Green Version]
- Onchoi, C.; Kongtip, P.; Nankongnab, N.; Chantanakul, S.; Sujirarat, D.; Woskie, S. Organophosphates in Meconium of Newborn Babies Whose Mothers Resided in Agricultural Areas of Thailand. Southeast Asian J. Trop. Med. Public Health 2020, 51, 77–87. [Google Scholar]
- Bruckner, J.V. Differences in Sensitivity of Children and Adults to Chemical Toxicity: The NAS Panel Report. Regul. Toxicol. Pharmacol. 2000, 31, 280–285. [Google Scholar] [CrossRef]
- Richardson, J.R.; Fitsanakis, V.; Westerink, R.H.S.; Kanthasamy, A.G. Neurotoxicity of Pesticides. Acta Neuropathol. 2019, 138, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Fang, B.; Pang, G.F.; Zhang, M.; Ren, F.Z. Age- and Diet-Specific Effects of Chronic Exposure to Chlorpyrifos on Hormones, Inflammation and Gut Microbiota in Rats. Pestic. Biochem. Physiol. 2019, 159, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and Function of the Blood-Brain Barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Profaci, C.P.; Munji, R.N.; Pulido, R.S.; Daneman, R. The Blood–Brain Barrier in Health and Disease: Important Unanswered Questions. J. Exp. Med. 2020, 217, e20190062. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.Y. Pro-Life Role for c-Jun N-Terminal Kinase and P38 Mitogen-Activated Protein Kinase at Rostral Ventrolateral Medulla in Experimental Brain Stem Death. J. Biomed. Sci. 2012, 19, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farkhondeh, T.; Mehrpour, O.; Buhrmann, C.; Pourbagher-Shahri, A.M.; Shakibaei, M.; Samarghandian, S. Organophosphorus Compounds and MAPK Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 4258. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [Green Version]
- Ki, Y.W.; Park, J.H.; Lee, J.E.; Shin, I.C.; Koh, H.C. JNK and P38 MAPK Regulate Oxidative Stress and the Inflammatory Response in Chlorpyrifos-Induced Apoptosis. Toxicol. Lett. 2013, 218, 235–245. [Google Scholar] [CrossRef]
- Vandooren, J.; Van Den Steen, P.E.; Opdenakker, G. Biochemistry and Molecular Biology of Gelatinase B or Matrix Metalloproteinase-9 (MMP-9): The next Decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Youssef, M.; Rawat, M.; Guo, S.; Dokladny, K.; Haque, M.; Watterson, M.D.; Ma, T.Y. MMP-9-Induced Increase in Intestinal Epithelial Tight Permeability Is Mediated by P38 Kinase Signaling Pathway Activation of MLCK Gene. Am. J. Physiol.-Gastrointest. Liver Physiol. 2019, 316, G278–G290. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.T.; Bürgers, H.F.; Rabie, T.; Marti, H.H. Matrix Metalloproteinase-9 Mediates Hypoxia-Induced Vascular Leakage in the Brain via Tight Junction Rearrangement. J. Cereb. Blood Flow Metab. 2010, 30, 837–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, R.J.; Sharp, F.R. Implications of MMP9 for Blood Brain Barrier Disruption and Hemorrhagic Transformation Following Ischemic Stroke. Front. Cell. Neurosci. 2016, 10, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Shao, B.; Wang, J.; Shen, Z.; Liu, H.; Li, S. Chlorpyrifos Caused Necroptosis via MAPK/NF-ΚB/TNF-α Pathway in Common Carp (Cyprinus carpio L.) Gills. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 249, 109126. [Google Scholar] [CrossRef]
- Sule, R.O.; Condon, L.; Gomes, A.V. A Common Feature of Pesticides: Oxidative Stress—The Role of Oxidative Stress in Pesticide-Induced Toxicity. Oxid. Med. Cell. Longev. 2022, 2022, 5563759. [Google Scholar] [CrossRef]
- Ribeiro, G.; Ferri, A.; Clarke, G.; Cryan, J.F. Diet and the Microbiota–Gut–Brain-Axis: A Primer for Clinical Nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 443–450. [Google Scholar] [CrossRef]
- Idrees, M.; Imran, M.; Atiq, N.; Zahra, R.; Abid, R.; Alreshidi, M.; Roberts, T.; Abdelgadir, A.; Tipu, M.K.; Farid, A.; et al. Probiotics, Their Action Modality and the Use of Multi-Omics in Metamorphosis of Commensal Microbiota into Target-Based Probiotics. Front. Nutr. 2022, 9, 1–21. [Google Scholar] [CrossRef]
- Akimowicz, M.; Srednicka, P.; Juszczuk-kubiak, E.; Micha, W.; Roszko, M.Ł. Probiotics as a Biological Detoxification Tool of Food Chemical Contamination: A Review. Food Chem. Toxicol. 2021, 153, 112306. [Google Scholar] [CrossRef]
- Daisley, B.A.; Monachese, M.; Trinder, M.; Bisanz, J.E.; John, A.; Burton, J.P.; Reid, G.; Daisley, B.A.; Monachese, M.; Trinder, M.; et al. Immobilization of Cadmium and Lead by Lactobacillus rhamnosus GR-1 Mitigates Apical-to-Basolateral Heavy Metal Translocation in a Caco-2 Model of the Intestinal Epithelium Intestinal Epithelium. Gut Microbes 2019, 10, 321–333. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Long-Smith, C.; O’Riordan, K.J.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 477–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Den Abbeele, P.; Taminiau, B.; Pinheiro, I.; Duysburgh, C.; Jacobs, H.; Pijls, L.; Marzorati, M. Arabinoxylo-Oligosaccharides and Inulin Impact Inter-Individual Variation on Microbial Metabolism and Composition, Which Immunomodulates Human Cells. J. Agric. Food Chem. 2018, 66, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Perdijk, O.; Van Baarlen, P.; Fernandez-gutierrez, M.M.; Van Den Brink, E. Sialyllactose and Galactooligosaccharides Promote Epithelial Barrier Functioning and Distinctly Modulate Microbiota Composition and Short Chain Fatty Acid Production In Vitro. Front. Immunol. 2019, 10, 94. [Google Scholar] [CrossRef] [Green Version]
- Daguet, D.; Pinheiro, I.; Verhelst, A.; Possemiers, S. Arabinogalactan and Fructooligosaccharides Improve the Gut Barrier Function in Distinct Areas of the Colon in the Simulator of the Human Intestinal Microbial Ecosystem. J. Funct. Foods 2016, 20, 369–379. [Google Scholar] [CrossRef]
- Rose, E.C.; Odle, J.; Blikslager, A.T.; Ziegler, A.L. Probiotics, Prebiotics and Epithelial Tight Junctions: A Promising Approach to Modulate Intestinal Barrier Function. Int. J. Mol. Sci. 2021, 22, 6729. [Google Scholar] [CrossRef] [PubMed]
- Uerlings, J.; Schroyen, M.; Willems, E.; Tanghe, S.; Bruggeman, G. Differential Effects of Inulin or Its Fermentation Metabolites on Gut Barrier and Immune Function of Porcine Intestinal Epithelial Cells. J. Funct. Foods 2020, 67, 103855. [Google Scholar] [CrossRef]
- Wongkrasant, P.; Pongkorpsakol, P.; Ariyadamrongkwan, J.; Muanprasat, C. Biomedicine & Pharmacotherapy Original Article A Prebiotic Fructo-Oligosaccharide Promotes Tight Junction Assembly in Intestinal Epithelial Cells via an AMPK-Dependent Pathway. Biomed. Pharmacother. 2020, 129, 110415. [Google Scholar] [CrossRef]
- Wang, G.; Sun, W.; Pei, X.; Jin, Y.; Wang, H.; Tao, W.; Xiao, Z.; Liu, L.; Wang, M. Function Galactooligosaccharide Pretreatment Alleviates Damage of the Intestinal Barrier and inflammatory Responses in LPS-Challenged Mice. Food Funct. 2021, 12, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.M.; Parr-Brownlie, L.C. A Neuroscience Perspective of the Gut Theory of Parkinson’s Disease. Eur. J. Neurosci. 2019, 49, 817–823. [Google Scholar] [CrossRef]

| Pesticides | Effect on Gut | Effect on Microbiota | Effect on BBB | Neurotoxic Effect | References |
|---|---|---|---|---|---|
| Organophosaphates | |||||
| Chlorpyrifos (CPF) | a. ↓ in epithelial thickness of ileum and colon of rats after exposure to 1 mg/kg/day of CPF ↓ of tight junction gene expression of the intestinal barrier and ↑ of proinflammatory cytokines | c. Exposure to 1 mg/kg/day of CPF-induced (1) microbial dysbiosis in pregnant rats and offspring, a ↓ in potentially beneficial flora and an ↑ of the potentially pathogen one, and (2) bacterial translocation to sterile organs | d. Altered gene expression levels of claudin 5, ZO-1, and TRPC4 genes disrupting the barrier integrity in an in vitro BBB model | e. Exposure to 1.5 (low dose) and 3 (high dose) mg/kg/day of CPF-induced inhibition of cholinesterase in rats in a dose-related manner f. Inhibition of carboxylesterase (CaE) and cholinesterase (ChE) activities by 43–100% in an vitro BBB model treated with (0.1 to 10 µM) of CPF | a. [31,34,35] b. [119,120,121] c. [26,30,34,122] d and f. [87] e. [123] |
| b. ↓ of TJs gene expression of the IB (Caco-2/TC-7 model treated with SHIME supernatant exposed to 3.5 mg of CPF for 30 days) | g. Exposure of pregnant rats to 1 and 5 mg/kg/day of CPF-induced inhibition of AChE of juvenile and adult offspring leading to high sleep apnea index | g. [124] | |||
| Diazinon | h. Exposure to 4 ppm for 13 weeks in drinking water had an impact on bacterial populations and composition of Lachnospiraceae, Ruminococcaceae, Clostridiaceae, and Erysipelotrichaceae in male mice | i. Exposure to 0.5 and 2 mg/kg/day induced long-lasting alterations in cognitive function in adolescence and extending into adulthood of rats | h. [125,126,127] i. [128] | ||
| Malathion | j. ↓ in bacterial populations (depletion of 4 genera) in male mice exposed to 2 mg/mL in drinking water for 13 weeks | k. ↓ in the TEER in two BBB in vitro models treated with malathion (10−3 to 10−8 M) | l. Induces neurotoxicity through ChE inhibition and non-cholinergic mode: apoptotic cell death by targeting mitochondria in N2a neuroblastoma mouse cells (at 0.25, 0.5, or 1 mM for 8 h) | j. [125,127] k. [129] l. [130] | |
| Herbicides | |||||
| Glyphosate | m. ↑ in proinflammatory cytokines transcriptomic expression (IL-1β, IL-6, and TNF-α) after exposure to 5, 50, and 500 mg/kg for 35 days in male rats | n. ↓ in the relative abundance of Firmicutes and Lactobacillus but increased Fusobacteria in male rats exposed to 500 mg/kg for 35 days | o. ↑ in barrier permeability to fluorescein (at 1 and 10 µM of glyphosate) and ↓ in claudin-5 fluorescence intensity (at 100 and 1000 µM) after 24 h treatment of the BBB model | p. 24 h of treatment with high doses of glyphosate (100 µM) can affect neurons metabolic activity | m and n. Q. [110] o and p. [131] |
| Fungicides | |||||
| Imazalil | q. ↑ in a colonic inflammation biomarker (Lcn-2) ↑ in mRNA levels of TNF-α, IL-1β, IL-22, and IFN-ɣ in the colon after exposure to 100 mg/kg bw/day IMZ for 28 days in mice | r. ↓ in Bacteroidetes, Firmicutes, and Actinobacteria in the colon after exposure to 100 mg/kg bw/day IMZ for 28 days in mice | s. ↑ in oxidative stress ↑basal calcium ions Ca2+ (indicating inhibition of depolarization-evoked calcium influx) in an in vitro model of rat dopaminergic PC12 cells treated with 100 µM of IMZ | q and r. [25] s. [132,133] | |
| t. 15 weeks administration of IMZ at doses of 0.1, 0.5, and 2.5 mg/kg/day to C57BL/6 mice led to: | u. 48% inhibition of AChE by IMZ (at 500 µM, in vitro enzymatic inhibition assays) | t. [134] u. [135] | |||
| IB dysfunction | gut-microbiota dysbiosis | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou Diwan, M.; Lahimer, M.; Bach, V.; Gosselet, F.; Khorsi-Cauet, H.; Candela, P. Impact of Pesticide Residues on the Gut-Microbiota–Blood–Brain Barrier Axis: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 6147. https://doi.org/10.3390/ijms24076147
Abou Diwan M, Lahimer M, Bach V, Gosselet F, Khorsi-Cauet H, Candela P. Impact of Pesticide Residues on the Gut-Microbiota–Blood–Brain Barrier Axis: A Narrative Review. International Journal of Molecular Sciences. 2023; 24(7):6147. https://doi.org/10.3390/ijms24076147
Chicago/Turabian StyleAbou Diwan, Maria, Marwa Lahimer, Véronique Bach, Fabien Gosselet, Hafida Khorsi-Cauet, and Pietra Candela. 2023. "Impact of Pesticide Residues on the Gut-Microbiota–Blood–Brain Barrier Axis: A Narrative Review" International Journal of Molecular Sciences 24, no. 7: 6147. https://doi.org/10.3390/ijms24076147








