Amelioration of Pulmonary Fibrosis by Matrix Metalloproteinase-2 Overexpression
Abstract
:1. Introduction
2. Results
2.1. No Difference in Body Weight between WT and hMMP-2 TG Mice with Lung Fibrosis
2.2. Decreased Inflammatory Cells in the Lungs from hMMP-2 TG Mice
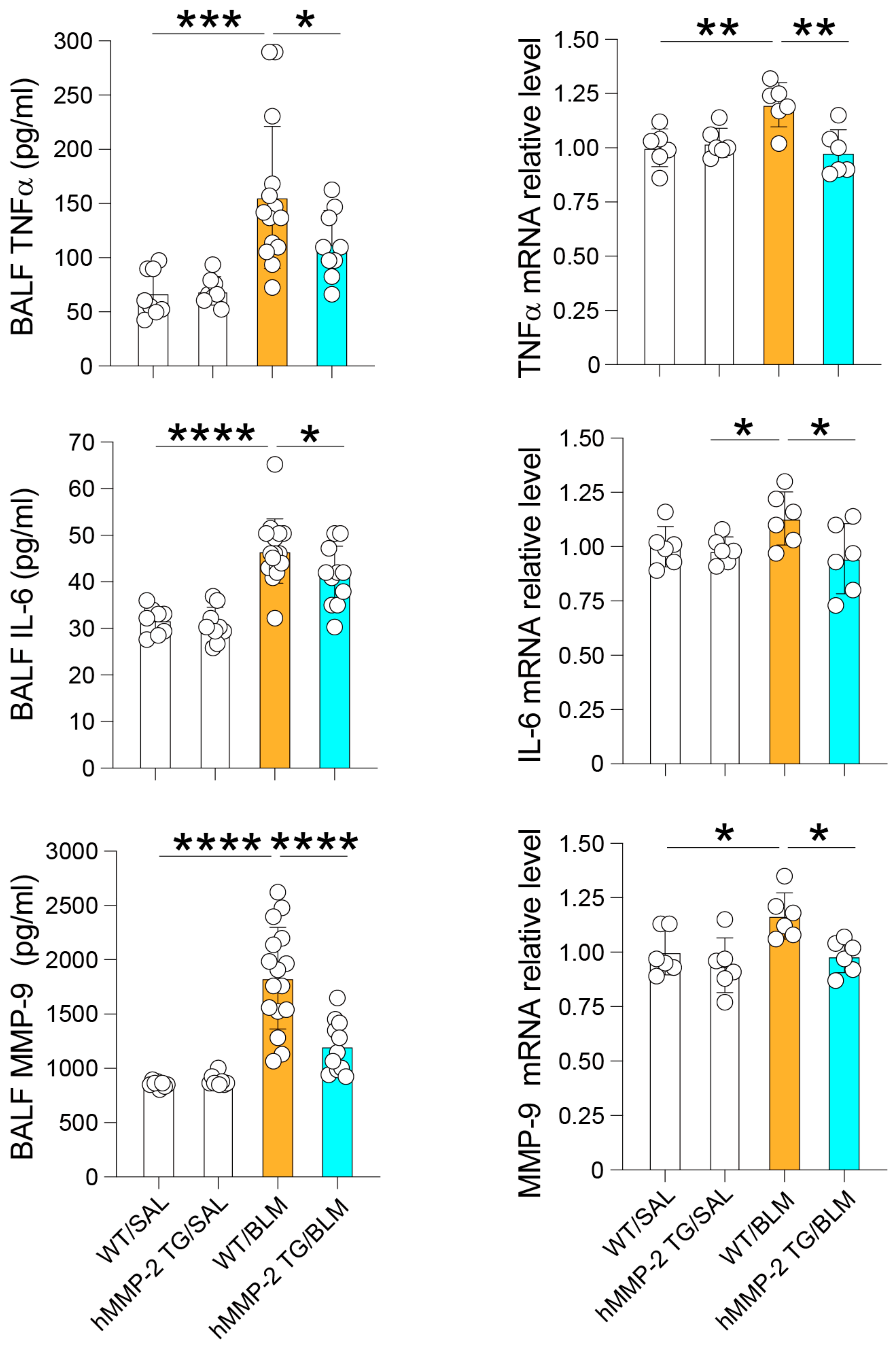
2.3. Reduced BALF Levels of Inflammatory Cytokines and Total Protein in hMMP-2 TG Mice
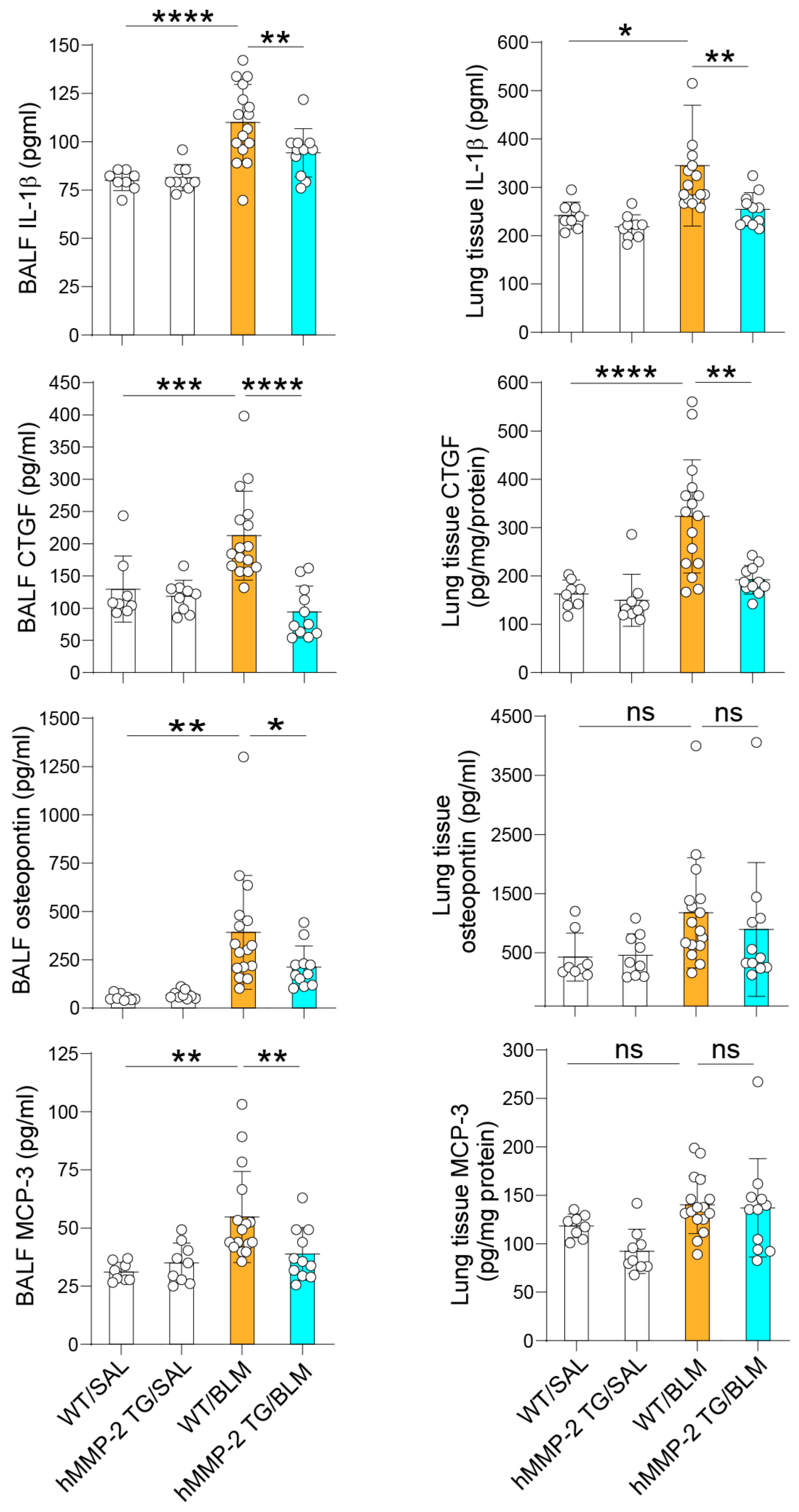
2.4. The Levels of the MMP-2 Substrates IL-1β, Osteopontin, MCP-3, and CTGF Are Decreased in the Lungs of hMMP-2 TG Mice
2.5. mRNA Expression, Protein Level, and MMP-2 Activity in the Lung of hMMP-2 TG Mice
2.6. Decreased Levels of Collagen, α-Smooth Musth Actin, and Fibronectin1 I in hMMP-2 TG Mice
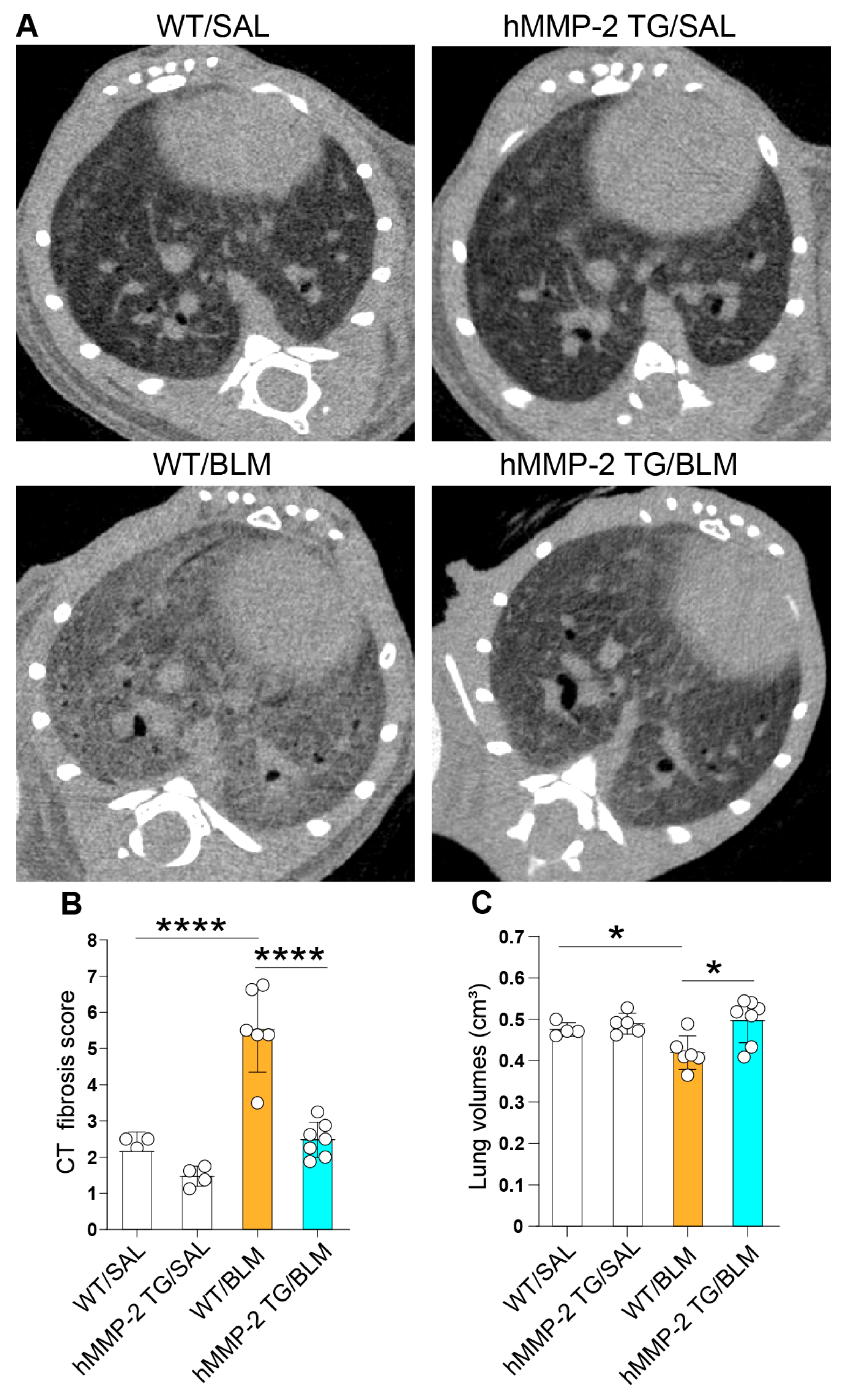
2.7. Amelioration of Pulmonary Fibrosis in hMMP-2 TG Mice
2.8. Correlation between Lung Fibrosis and Inflammatory Markers
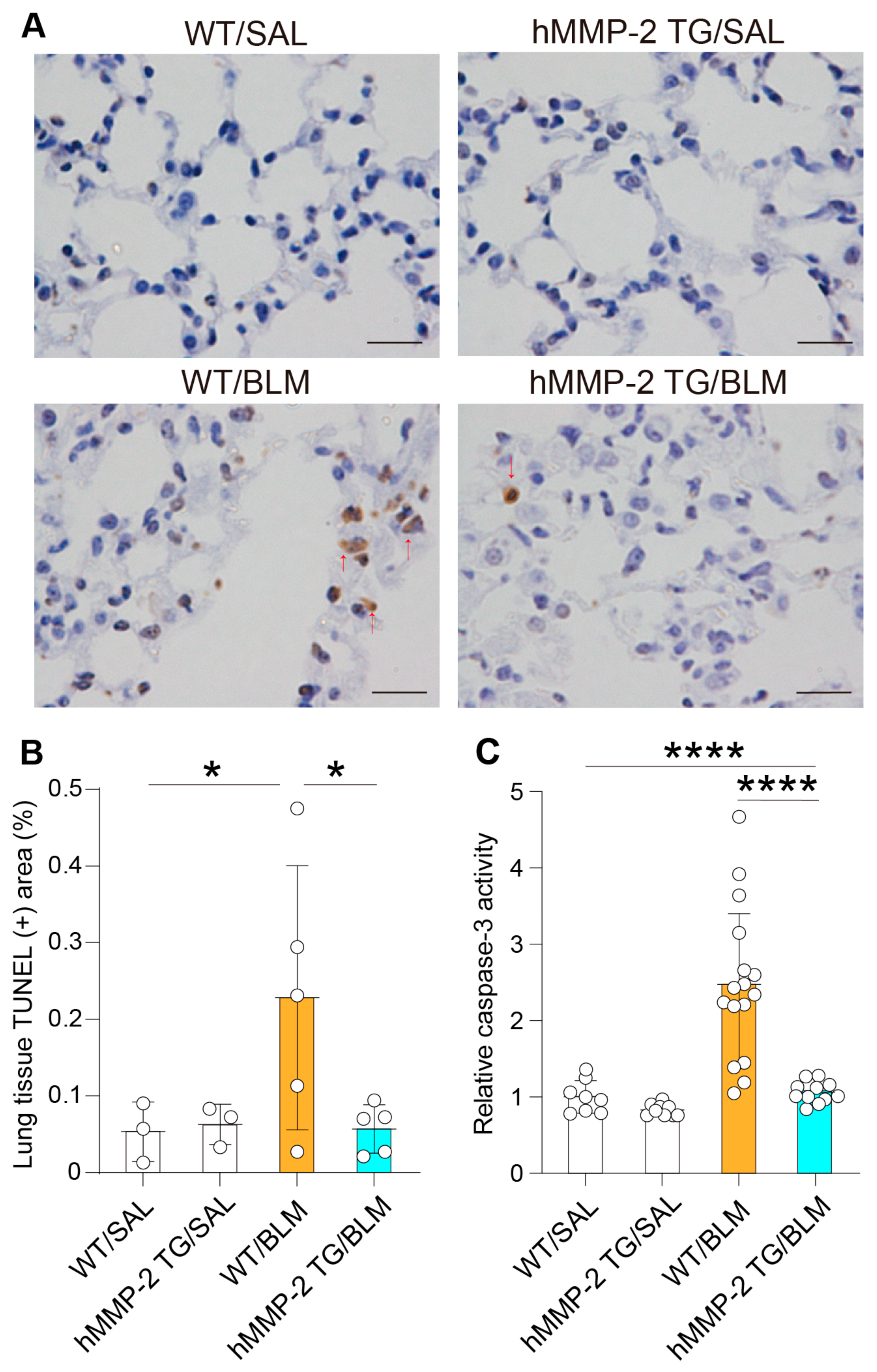
2.9. Inhibition of Lung Cell Apoptosis by hMMP-2 Overexpression
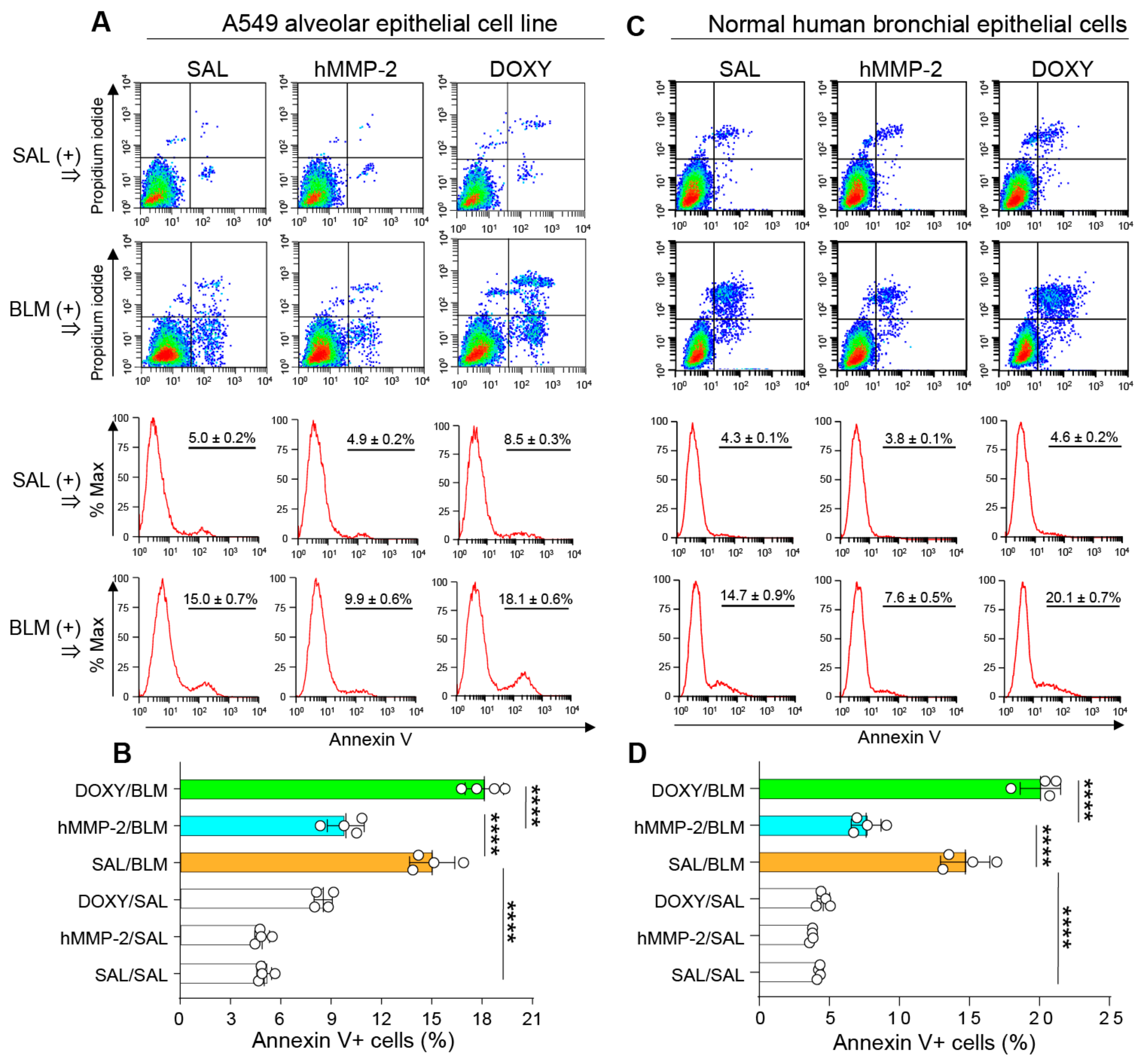
2.10. Active hMMP-2 Inhibits Apoptosis of Lung Epithelial Cells
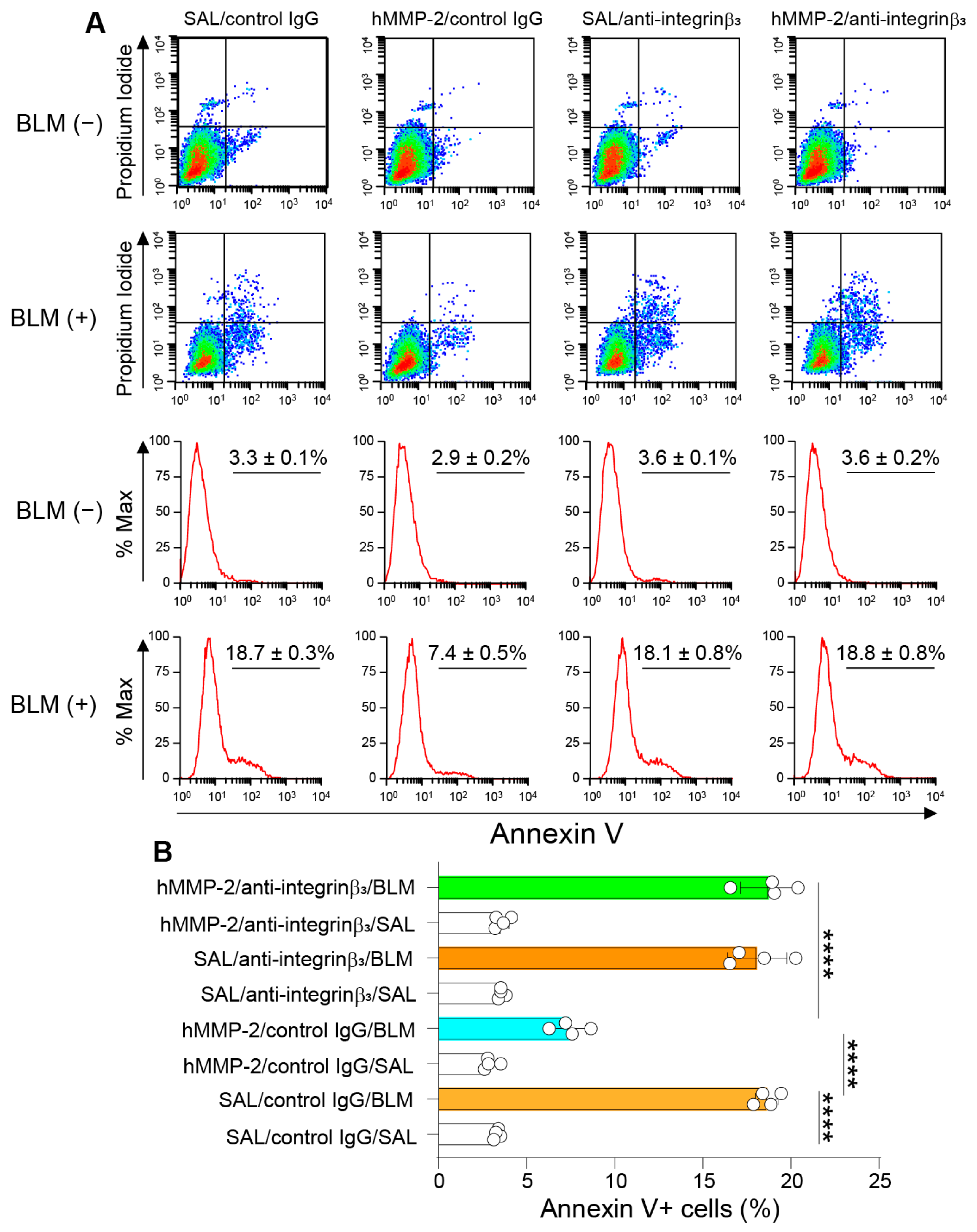
2.11. Integrin-β3 Mediates the Inhibitory Activity of MMP-2 on Apoptosis of Lung Epithelial Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Micro-Computed Tomography
4.4. Collection of Bronchoalveolar Lavage Fluid and Peripheral Blood
4.5. Collection of Lung Samples
4.6. Cell Culture
4.7. Evaluation of Apoptosis
4.8. Biochemical Analysis
4.9. Zymography and Western Blotting
4.10. Analysis of Gene Expression
4.11. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lederer, D.J.; Martinez, F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 378, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Collard, H.R.; Jones, M.G. Idiopathic pulmonary fibrosis. Lancet 2017, 389, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Chen, S.Y.; Yeh, W.S.; Maroni, B.; Li, Q.; Lee, Y.C.; Collard, H.R. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: Incidence, prevalence, and survival, 2001–2011. Lancet Respir. Med. 2014, 2, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Weycker, D.; Edelsberg, J.; Bradford, W.Z.; Oster, G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2006, 174, 810–816. [Google Scholar] [CrossRef] [Green Version]
- Hutchinson, J.; Fogarty, A.; Hubbard, R.; McKeever, T. Global incidence and mortality of idiopathic pulmonary fibrosis: A systematic review. Eur. Respir. J. 2015, 46, 795–806. [Google Scholar] [CrossRef] [Green Version]
- Du Bois, R.M. An earlier and more confident diagnosis of idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2012, 21, 141–146. [Google Scholar] [CrossRef]
- Baratella, E.; Ruaro, B.; Giudici, F.; Wade, B.; Santagiuliana, M.; Salton, F.; Confalonieri, P.; Simbolo, M.; Scarpa, A.; Tollot, S.; et al. Evaluation of correlations between genetic variants and high-resolution computed tomography patterns in idiopathic pulmonary fibrosis. Diagnostics 2021, 11, 762. [Google Scholar] [CrossRef]
- Calabrese, F.; Lunardi, F.; Tauro, V.; Pezzuto, F.; Fortarezza, F.; Vedovelli, L.; Faccioli, E.; Balestro, E.; Schiavon, M.; Esposito, G.; et al. RNA sequencing of epithelial cell/fibroblastic foci sandwich in idiopathic pulmonary fibrosis: New insights on the signaling pathway. Int. J. Mol. Sci. 2022, 23, 3323. [Google Scholar] [CrossRef]
- Veres-Székely, A.; Pap, D.; Szebeni, B.; Őrfi, L.; Szász, C.; Pajtók, C.; Lévai, E.; Szabó, A.J.; Vannay, Á. Transient agarose spot (TAS) assay: A new method to investigate cell migration. Int. J. Mol. Sci. 2022, 23, 2119. [Google Scholar]
- Shimizu, S.; Gabazza, E.C.; Ogawa, T.; Tojima, I.; Hoshi, E.; Kouzaki, H.; Shimizu, T. Role of thrombin in chronic rhinosinusitis-associated tissue remodeling. Am. J. Rhinol. Allergy 2011, 25, 7–11. [Google Scholar] [CrossRef]
- Liu, G.; Philp, A.M.; Corte, T.; Travis, M.A.; Schilter, H.; Hansbro, N.G.; Burns, C.J.; Eapen, M.S.; Sohal, S.S.; Burgess, J.K.; et al. Therapeutic targets in lung tissue remodelling and fibrosis. Pharmacol. Ther. 2021, 1078, 22539. [Google Scholar] [CrossRef]
- Gabazza, E.C.; Taguchi, O.; Kamada, H.; Hayashi, T.; Adachi, Y.; Suzuki, K. Progress in the understanding of protease-activated receptors. Int. J. Hematol. 2004, 79, 117–122. [Google Scholar] [CrossRef]
- Fukuda, Y.; Mochimaru, H.; Terasaki, Y.; Kawamoto, M.; Kudoh, S. Mechanism of structural remodeling in pulmonary fibrosis. Chest 2001, 120, 41S–43S. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Stetler-Stevenson, W.G.; Fleming, M.V.; Fishback, N.; Koss, M.N.; Liotta, L.A.; Ferrans, V.J.; Travis, W.D. Immunohistochemical study of metalloproteinases and their tissue inhibitors in the lungs of patients with diffuse alveolar damage and idiopathic pulmonary fibrosis. Am. J. Pathol. 1996, 149, 1241–1256. [Google Scholar] [PubMed]
- Maatta, M.; Laurila, H.P.; Holopainen, S.; Aaltonen, K.; Lilja-Maula, L.; Viitanen, S.; Rajamaki, M.M. Matrix metalloproteinase-2, -7, and -9 activities in dogs with idiopathic pulmonary fibrosis compared to healthy dogs and dogs with other respiratory diseases. J. Vet. Intern. Med. 2021, 35, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Suga, M.; Iyonaga, K.; Okamoto, T.; Gushima, Y.; Miyakawa, H.; Akaike, T.; Ando, M. Characteristic elevation of matrix metalloproteinase activity in idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2000, 162, 1949–1956. [Google Scholar] [CrossRef] [Green Version]
- Summer, R.; Krishna, R.; Schriner, D.; Cuevas-Mora, K.; Sales, D.; Para, R.; Roman, J.; Nieweld, C.; Gochuico, B.R.; Romero, F. Matrix metalloproteinase activity in the lung is increased in Hermansky-Pudlak syndrome. Orphanet J. Rare Dis. 2019, 14, 162. [Google Scholar] [CrossRef] [Green Version]
- Todd, J.L.; Vinisko, R.; Liu, Y.; Neely, M.L.; Overton, R.; Flaherty, K.R.; Noth, I.; Newby, L.K.; Lasky, J.A.; Olman, M.A.; et al. Circulating matrix metalloproteinases and tissue metalloproteinase inhibitors in patients with idiopathic pulmonary fibrosis in the multicenter IPF-PRO Registry cohort. BMC Pulm. Med. 2020, 20, 64. [Google Scholar] [CrossRef] [Green Version]
- Chulia-Peris, L.; Carreres-Rey, C.; Gabasa, M.; Alcaraz, J.; Carretero, J.; Pereda, J. Matrix Metalloproteinases and Their Inhibitors in Pulmonary Fibrosis: EMMPRIN/CD147 Comes into Play. Int. J. Mol. Sci. 2022, 23, 6894. [Google Scholar] [CrossRef]
- Mahalanobish, S.; Saha, S.; Dutta, S.; Sil, P.C. Matrix metalloproteinase: An upcoming therapeutic approach for idiopathic pulmonary fibrosis. Pharmacol. Res. 2020, 152, 104591. [Google Scholar] [CrossRef]
- Pardo, A.; Cabrera, S.; Maldonado, M.; Selman, M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir. Res. 2016, 17, 23. [Google Scholar] [CrossRef] [Green Version]
- Aoyagi-Ikeda, K.; Maeno, T.; Matsui, H.; Ueno, M.; Hara, K.; Aoki, Y.; Aoki, F.; Shimizu, T.; Doi, H.; Kawai-Kowase, K.; et al. Notch induces myofibroblast differentiation of alveolar epithelial cells via transforming growth factor-beta-Smad3 pathway. Am. J. Respir. Cell Mol. Biol. 2011, 45, 136–144. [Google Scholar] [PubMed]
- Craig, V.J.; Quintero, P.A.; Fyfe, S.E.; Patel, A.S.; Knolle, M.D.; Kobzik, L.; Owen, C.A. Profibrotic activities for matrix metalloproteinase-8 during bleomycin-mediated lung injury. J. Immunol. 2013, 190, 4283–4296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Prieto, E.; Gonzalez-Lopez, A.; Cabrera, S.; Astudillo, A.; Gutierrez-Fernandez, A.; Fanjul-Fernandez, M.; Batalla-Solis, E.; Puente, X.S.; Fueyo, A.; Lopez-Otin, C.; et al. Resistance to bleomycin-induced lung fibrosis in MMP-8 deficient mice is mediated by interleukin-10. PLoS ONE 2010, 5, e13242. [Google Scholar] [CrossRef] [PubMed]
- Gharib, S.A.; Johnston, L.K.; Huizar, I.; Birkland, T.P.; Hanson, J.; Wang, Y.; Parks, W.C.; Manicone, A.M. MMP28 promotes macrophage polarization toward M2 cells and augments pulmonary fibrosis. J. Leukoc. Biol. 2014, 95, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jara, P.; Calyeca, J.; Romero, Y.; Placido, L.; Yu, G.; Kaminski, N.; Maldonado, V.; Cisneros, J.; Selman, M.; Pardo, A. Matrix metalloproteinase (MMP)-19-deficient fibroblasts display a profibrotic phenotype. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L511–L522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohani, M.G.; McMahan, R.S.; Razumova, M.V.; Hertz, A.L.; Cieslewicz, M.; Pun, S.H.; Regnier, M.; Wang, Y.; Birkland, T.P.; Parks, W.C. MMP-10 Regulates Collagenolytic Activity of Alternatively Activated Resident Macrophages. J. Invest. Dermatol. 2015, 135, 2377–2384. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, C.M.; Dolgonos, L.; Zemans, R.L.; Young, S.K.; Robertson, J.; Briones, N.; Suzuki, T.; Campbell, M.N.; Gauldie, J.; Radisky, D.C.; et al. Matrix metalloproteinase 3 is a mediator of pulmonary fibrosis. Am. J. Pathol. 2011, 179, 1733–1745. [Google Scholar] [CrossRef]
- Yu, G.; Kovkarova-Naumovski, E.; Jara, P.; Parwani, A.; Kass, D.; Ruiz, V.; Lopez-Otin, C.; Rosas, I.O.; Gibson, K.F.; Cabrera, S.; et al. Matrix metalloproteinase-19 is a key regulator of lung fibrosis in mice and humans. Am. J. Respir. Crit. Care Med. 2012, 186, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, S.; Maciel, M.; Hernandez-Barrientos, D.; Barrientos, D.; Calyeca, J.; Gaxiola, M.; Selman, M.; Pardo, A. Delayed resolution of bleomycin-induced pulmonary fibrosis in absence of MMP13 (collagenase 3). Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L961–L976. [Google Scholar] [CrossRef]
- Checa, M.; Ruiz, V.; Montano, M.; Velazquez-Cruz, R.; Selman, M.; Pardo, A. MMP-1 polymorphisms and the risk of idiopathic pulmonary fibrosis. Hum. Genet. 2008, 124, 465–472. [Google Scholar] [CrossRef]
- Flechsig, P.; Hartenstein, B.; Teurich, S.; Dadrich, M.; Hauser, K.; Abdollahi, A.; Grone, H.J.; Angel, P.; Huber, P.E. Loss of matrix metalloproteinase-13 attenuates murine radiation-induced pulmonary fibrosis. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Gabasa, M.; Arshakyan, M.; Llorente, A.; Chulia-Peris, L.; Pavelescu, I.; Xaubet, A.; Pereda, J.; Alcaraz, J. Interleukin-1beta modulation of the mechanobiology of primary human pulmonary fibroblasts: Potential implications in lung repair. Int. J. Mol. Sci. 2020, 21, 8417. [Google Scholar] [CrossRef] [PubMed]
- Herrera, I.; Cisneros, J.; Maldonado, M.; Ramirez, R.; Ortiz-Quintero, B.; Anso, E.; Chandel, N.S.; Selman, M.; Pardo, A. Matrix metalloproteinase (MMP)-1 induces lung alveolar epithelial cell migration and proliferation, protects from apoptosis, and represses mitochondrial oxygen consumption. J. Biol. Chem. 2013, 288, 25964–25975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manicone, A.M.; Huizar, I.; McGuire, J.K. Matrilysin (Matrix Metalloproteinase-7) regulates anti-inflammatory and antifibrotic pulmonary dendritic cells that express CD103 (alpha(E)beta(7)-integrin). Am. J. Pathol. 2009, 175, 2319–2331. [Google Scholar] [CrossRef] [Green Version]
- Nkyimbeng, T.; Ruppert, C.; Shiomi, T.; Dahal, B.; Lang, G.; Seeger, W.; Okada, Y.; D’Armiento, J.; Gunther, A. Pivotal role of matrix metalloproteinase 13 in extracellular matrix turnover in idiopathic pulmonary fibrosis. PLoS ONE 2013, 8, e73279. [Google Scholar] [CrossRef]
- Sen, A.I.; Shiomi, T.; Okada, Y.; D’Armiento, J.M. Deficiency of matrix metalloproteinase-13 increases inflammation after acute lung injury. Exp. Lung Res. 2010, 36, 615–624. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Zhang, J.; Shi, L.; Ning, Y.; Zhu, Y.; Chen, S.; Yang, M.; Chen, J.; Zhou, G.W.; Li, Q. NOGO-B promotes EMT in lung fibrosis via MMP14 mediates free TGF-β1 formation. Oncotarget 2017, 8, 71024–71037. [Google Scholar] [CrossRef] [Green Version]
- Zigrino, P.; Brinckmann, J.; Niehoff, A.; Lu, Y.; Giebeler, N.; Eckes, B.; Kadler, K.E.; Mauch, C. Fibroblast-derived MMP-14 regulates collagen homeostasis in adult skin. J. Investig. Dermatol. 2016, 136, 1575–1583. [Google Scholar] [CrossRef] [Green Version]
- Zuo, F.; Kaminski, N.; Eugui, E.; Allard, J.; Yakhini, Z.; Ben-Dor, A.; Lollini, L.; Morris, D.; Kim, Y.; DeLustro, B.; et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc. Natl. Acad. Sci. USA 2002, 99, 6292–6297. [Google Scholar] [CrossRef] [Green Version]
- Betsuyaku, T.; Fukuda, Y.; Parks, W.C.; Shipley, J.M.; Senior, R.M. Gelatinase B is required for alveolar bronchiolization after intratracheal bleomycin. Am. J. Pathol. 2000, 157, 525–535. [Google Scholar] [CrossRef] [Green Version]
- Bormann, T.; Maus, R.; Stolper, J.; Tort Tarres, M.; Brandenberger, C.; Wedekind, D.; Jonigk, D.; Welte, T.; Gauldie, J.; Kolb, M.; et al. Role of matrix metalloprotease-2 and MMP-9 in experimental lung fibrosis in mice. Respir. Res. 2022, 23, 180. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, S.; Gaxiola, M.; Arreola, J.L.; Ramirez, R.; Jara, P.; D’Armiento, J.; Richards, T.; Selman, M.; Pardo, A. Overexpression of MMP9 in macrophages attenuates pulmonary fibrosis induced by bleomycin. Int. J. Biochem. Cell Biol. 2007, 39, 2324–2338. [Google Scholar] [CrossRef]
- Espindola, M.S.; Habiel, D.M.; Coelho, A.L.; Stripp, B.; Parks, W.C.; Oldham, J.; Martinez, F.J.; Noth, I.; Lopez, D.; Mikels-Vigdal, A.; et al. Differential Responses to Targeting Matrix Metalloproteinase 9 in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2021, 203, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wu, Z.; Bai, D.; Tang, R.; Phan, S. Matrix metalloproteinase-12 (MM12) inhibits myofibroblasts differentiation and lung fibrosis. FASEB J. 2012, 29. [Google Scholar] [CrossRef]
- Kang, H.R.; Cho, S.J.; Lee, C.G.; Homer, R.J.; Elias, J.A. Transforming growth factor (TGF)-beta1 stimulates pulmonary fibrosis and inflammation via a Bax-dependent, bid-activated pathway that involves matrix metalloproteinase-12. J. Biol. Chem. 2007, 282, 7723–7732. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Kim, S.H.; Seo, J.Y.; Chung, H.; Kwak, H.J.; Lee, S.K.; Yoon, H.J.; Shin, D.H.; Park, S.S.; Sohn, J.W. Blockade of the Wnt/beta-catenin pathway attenuates bleomycin-induced pulmonary fibrosis. Tohoku J. Exp. Med. 2011, 223, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Manoury, B.; Nenan, S.; Guenon, I.; Boichot, E.; Planquois, J.M.; Bertrand, C.P.; Lagente, V. Macrophage metalloelastase (MMP-12) deficiency does not alter bleomycin-induced pulmonary fibrosis in mice. J. Inflamm. 2006, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, G.; Hagood, J.S.; Sanders, Y.; Ramirez, R.; Becerril, C.; Segura, L.; Barrera, L.; Selman, M.; Pardo, A. Absence of Thy-1 results in TGF-beta induced MMP-9 expression and confers a profibrotic phenotype to human lung fibroblasts. Lab. Investig. 2011, 1206, 91–1218. [Google Scholar]
- Xu, L.; Bian, W.; Gu, X.H.; Shen, C. Genetic polymorphism in matrix metalloproteinase-9 and transforming growth factor-beta1 and susceptibility to combined pulmonary fibrosis and emphysema in a Chinese population. Kaohsiung J. Med. Sci. 2017, 33, 124–129. [Google Scholar] [CrossRef]
- Dean, R.A.; Overall, C.M. Proteomics discovery of metalloproteinase substrates in the cellular context by iTRAQ labeling reveals a diverse MMP-2 substrate degradome. Mol. Cell. Proteomics 2007, 6, 611–623. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Mukaiyama, A.; Itoh, Y.; Nagase, H.; Thogersen, I.B.; Enghild, J.J.; Sasaguri, Y.; Mori, Y. Degradation of interleukin 1beta by matrix metalloproteinases. J. Biol. Chem. 1996, 271, 14657–14660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overall, C.M.; Dean, R.A. Degradomics: Systems biology of the protease web. Pleiotropic roles of MMPs in cancer. Cancer Metastasis Rev. 2006, 25, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.A.; Butler, G.S.; Hamma-Kourbali, Y.; Delbe, J.; Brigstock, D.R.; Courty, J.; Overall, C.M. Identification of candidate angiogenic inhibitors processed by matrix metalloproteinase 2 (MMP-2) in cell-based proteomic screens: Disruption of vascular endothelial growth factor (VEGF)/heparin affin regulatory peptide (pleiotrophin) and VEGF/Connective tissue growth factor angiogenic inhibitory complexes by MMP-2 proteolysis. Mol. Cell. Biol. 2007, 27, 8454–8465. [Google Scholar] [PubMed] [Green Version]
- Madtes, D.K.; Elston, A.L.; Hackman, R.C.; Dunn, A.R.; Clark, J.G. Transforming growth factor-alpha deficiency reduces pulmonary fibrosis in transgenic mice. Am. J. Respir. Cell Mol. Biol. 1999, 20, 924–934. [Google Scholar] [CrossRef]
- Kim, S.J.; Cheresh, P.; Jablonski, R.P.; Williams, D.B.; Kamp, D.W. The Role of Mitochondrial DNA in Mediating Alveolar Epithelial Cell Apoptosis and Pulmonary Fibrosis. Int. J. Mol. Sci. 2015, 16, 21486–21519. [Google Scholar] [CrossRef]
- Nishihama, K.; Yasuma, T.; Yano, Y.; D’ Alessandro-Gabazza, C.N.; Toda, M.; Hinneh, J.A.; Baffour Tonto, P.; Takeshita, A.; Totoki, T.; Mifuji-Moroka, R.; et al. Anti-apoptotic activity of human matrix metalloproteinase-2 attenuates diabetes mellitus. Metabolism 2018, 82, 88–99. [Google Scholar] [CrossRef]
- Fukuda, Y.; Ishizaki, M.; Kudoh, S.; Kitaichi, M.; Yamanaka, N. Localization of matrix metalloproteinases-1, -2, and -9 and tissue inhibitor of metalloproteinase-2 in interstitial lung diseases. Lab. Investig. 1998, 78, 687–698. [Google Scholar]
- McKeown, S.; Richter, A.G.; O’Kane, C.; McAuley, D.F.; Thickett, D.R. MMP expression and abnormal lung permeability are important determinants of outcome in IPF. Eur. Respir. J. 2009, 33, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Pollock, A.S.; Mahimkar, R.; Olson, J.L.; Lovett, D.H. Matrix metalloproteinase 2 and basement membrane integrity: A unifying mechanism for progressive renal injury. FASEB J. 2006, 20, 1898–1900. [Google Scholar] [CrossRef] [Green Version]
- Keane, M.P.; Arenberg, D.A.; Lynch, J.P., 3rd; Whyte, R.I.; Iannettoni, M.D.; Burdick, M.D.; Wilke, C.A.; Morris, S.B.; Glass, M.C.; DiGiovine, B.; et al. The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J. Immunol. 1997, 159, 1437–1443. [Google Scholar] [CrossRef]
- Nguyen, M.; Arkell, J.; Jackson, C.J. Human endothelial gelatinases and angiogenesis. Int. J. Biochem. Cell Biol. 2001, 33, 960–970. [Google Scholar] [CrossRef]
- Ruiz, V.; Ordonez, R.M.; Berumen, J.; Ramirez, R.; Uhal, B.; Becerril, C.; Pardo, A.; Selman, M. Unbalanced collagenases/TIMP-1 expression and epithelial apoptosis in experimental lung fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 285, L1026–L1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seomun, Y.; Kim, J.; Lee, E.H.; Joo, C.K. Overexpression of matrix metalloproteinase-2 mediates phenotypic transformation of lens epithelial cells. Biochem. J. 2001, 358, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Jackson, K.; McGuire, P.G. Degradation of type IV collagen by matrix metalloproteinases is an important step in the epithelial-mesenchymal transformation of the endocardial cushions. Dev. Biol. 2000, 227, 606–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Velden, J.L.; Guala, A.S.; Leggett, S.E.; Sluimer, J.; Badura, E.C.; Janssen-Heininger, Y.M. Induction of a mesenchymal expression program in lung epithelial cells by wingless protein (Wnt)/beta-catenin requires the presence of c-Jun N-terminal kinase-1 (JNK1). Am. J. Respir. Cell Mol. Biol. 2012, 47, 306–314. [Google Scholar] [CrossRef] [Green Version]
- Tomaru, A.; Gabazza, E.; Kobayashi, T.; Kobayashi, H.; Taguchi, O.; Takagi, T.; Oonishi, M.; Fujiwara, K.; D’Alessandro Gabazza, C.; Takahashi, Y.; et al. Matrix metalloproteinase-2 is protective in bleomycin-induced pulmonary fibrosis. Eur. Respir. J. 2015, 46, PA1903. [Google Scholar]
- Moodley, Y.; Atienza, D.; Manuelpillai, U.; Samuel, C.S.; Tchongue, J.; Ilancheran, S.; Boyd, R.; Trounson, A. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am. J. Pathol. 2009, 175, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Mias, C.; Lairez, O.; Trouche, E.; Roncalli, J.; Calise, D.; Seguelas, M.H.; Ordener, C.; Piercecchi-Marti, M.D.; Auge, N.; Salvayre, A.N.; et al. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells 2009, 27, 2734–2743. [Google Scholar] [CrossRef]
- Takamiya, Y.; Fukami, K.; Yamagishi, S.; Kaida, Y.; Nakayama, Y.; Obara, N.; Iwatani, R.; Ando, R.; Koike, K.; Matsui, T.; et al. Experimental diabetic nephropathy is accelerated in matrix metalloproteinase-2 knockout mice. Nephrol. Dial. Transplant. 2013, 28, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Montano, M.; Ramos, C.; Gonzalez, G.; Vadillo, F.; Pardo, A.; Selman, M. Lung collagenase inhibitors and spontaneous and latent collagenase activity in idiopathic pulmonary fibrosis and hypersensitivity pneumonitis. Chest 1989, 96, 1115–1119. [Google Scholar] [CrossRef]
- Montfort, I.; Perez-Tamayo, R. Collagenase in experimental carbon tetrachloride cirrhosis of the liver. Am. J. Pathol. 1978, 92, 411–420. [Google Scholar]
- Selman, M.; Ruiz, V.; Cabrera, S.; Segura, L.; Ramirez, R.; Barrios, R.; Pardo, A. TIMP-1, -2, -3, and -4 in idiopathic pulmonary fibrosis. A prevailing nondegradative lung microenvironment? Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L562–L574. [Google Scholar] [CrossRef] [PubMed]
- Drakopanagiotakis, F.; Xifteri, A.; Polychronopoulos, V.; Bouros, D. Apoptosis in lung injury and fibrosis. Eur. Respir. J. 2008, 32, 1631–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chetty, C.; Bhoopathi, P.; Lakka, S.S.; Rao, J.S. MMP-2 siRNA induced Fas/CD95-mediated extrinsic II apoptotic pathway in the A549 lung adenocarcinoma cell line. Oncogene 2007, 26, 7675–7683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silletti, S.; Kessler, T.; Goldberg, J.; Boger, D.L.; Cheresh, D.A. Disruption of matrix metalloproteinase 2 binding to integrin alpha vbeta 3 by an organic molecule inhibits angiogenesis and tumor growth in vivo. Proc. Natl. Acad. Sci. USA 2001, 98, 119–124. [Google Scholar] [CrossRef]
- Manicone, A.M.; McGuire, J.K. Matrix metalloproteinases as modulators of inflammation. Semin. Cell. Dev. Biol. 2008, 19, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Parks, W.C.; Wilson, C.L.; Lopez-Boado, Y.S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 2004, 4, 617–629. [Google Scholar] [CrossRef]
- McQuibban, G.A.; Butler, G.S.; Gong, J.H.; Bendall, L.; Power, C.; Clark-Lewis, I.; Overall, C.M. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J. Biol. Chem. 2001, 4350, 2763–43508. [Google Scholar] [CrossRef] [Green Version]
- McQuibban, G.A.; Gong, J.H.; Tam, E.M.; McCulloch, C.A.; Clark-Lewis, I.; Overall, C.M. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science 2000, 1202, 289–1206. [Google Scholar] [CrossRef]
- Zhang, K.; McQuibban, G.A.; Silva, C.; Butler, G.S.; Johnston, J.B.; Holden, J.; Clark-Lewis, I.; Overall, C.M.; Power, C. HIV-induced metalloproteinase processing of the chemokine stromal cell derived factor-1 causes neurodegeneration. Nat. Neurosci. 2003, 6, 1064–1071. [Google Scholar] [CrossRef]
- McQuibban, G.A.; Gong, J.H.; Wong, J.P.; Wallace, J.L.; Clark-Lewis, I.; Overall, C.M. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood 2002, 100, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Corry, D.B.; Rishi, K.; Kanellis, J.; Kiss, A.; Song Lz, L.Z.; Xu, J.; Feng, L.; Werb, Z.; Kheradmand, F. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat. Immunol. 2002, 3, 347–353. [Google Scholar] [CrossRef]
- Greenlee, K.J.; Corry, D.B.; Engler, D.A.; Matsunami, R.K.; Tessier, P.; Cook, R.G.; Werb, Z.; Kheradmand, F. Proteomic identification of in vivo substrates for matrix metalloproteinases 2 and 9 reveals a mechanism for resolution of inflammation. J. Immunol. 2006, 177, 7312–7321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, Y.; Kobayashi, T.; D’Alessandro-Gabazza, C.N.; Gabazza, C.N.; Toda, M.; Fujiwara, K.; Okano, T.; Fujimoto, H.; Asayama, K.; Takeshita, A.; et al. Protective Role of Matrix Metalloproteinase-2 in Allergic Bronchial Asthma. Front. Immunol. 2019, 10, 1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanner, L.; Single, A.B.; Bhongir, R.K.V.; Heusel, M.; Mohanty, T.; Karlsson, C.A.Q.; Pan, L.; Clausson, C.M.; Bergwik, J.; Wang, K.; et al. Small-molecule-mediated OGG1 inhibition attenuates pulmonary inflammation and lung fibrosis in a murine lung fibrosis model. Nat. Commun. 2023, 14, 643. [Google Scholar] [CrossRef]
- Jolly, M.K.; Ward, C.; Eapen, M.S.; Myers, S.; Hallgren, O.; Levine, H.; Sohal, S.S. Epithelial-mesenchymal transition, a spectrum of states: Role in lung development, homeostasis, and disease. Dev. Dyn. 2018, 247, 346–358. [Google Scholar] [CrossRef] [Green Version]
- Craig, V.J.; Zhang, L.; Hagood, J.S.; Owen, C.A. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 53, 585–600. [Google Scholar] [CrossRef] [Green Version]
- Shafieian, M.; Chen, S.; Wu, S. Integrin-linked kinase mediates CTGF-induced epithelial to mesenchymal transition in alveolar type II epithelial cells. Pediatr. Res. 2015, 77, 520–527. [Google Scholar] [CrossRef] [Green Version]
- Sonnylal, S.; Xu, S.; Jones, H.; Tam, A.; Sreeram, V.R.; Ponticos, M.; Norman, J.; Agrawal, P.; Abraham, D.; de Crombrugghe, B. Connective tissue growth factor causes EMT-like cell fate changes in vivo and in vitro. J. Cell Sci. 2013, 126, 2164–2175. [Google Scholar] [CrossRef] [Green Version]
- Lipson, K.E.; Wong, C.; Teng, Y.; Spong, S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012, 5, S24. [Google Scholar] [CrossRef] [Green Version]
- Noskovičová, N.; Petřek, M.; Eickelberg, O.; Heinzelmann, K. Platelet-derived growth factor signaling in the lung. From lung development and disease to clinical studies. Am. J. Respir. Cell Mol. Biol. 2015, 52, 263–284. [Google Scholar] [CrossRef] [PubMed]
- Onishi, M.; Kobayashi, T.; D’Alessandro-Gabazza, C.N.; Fujimoto, H.; Chelakkot-Govindalayathil, A.L.; Takahashi, Y.; Yasuma, T.; Nishihama, K.; Toda, M.; Takei, Y.; et al. Mice overexpressing latent matrix metalloproteinase-2 develop lung emphysema after short-term exposure to cigarette smoke extract. Biochem. Biophys. Res. Commun. 2018, 497, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Redente, E.F.; Jacobsen, K.M.; Solomon, J.J.; Lara, A.R.; Faubel, S.; Keith, R.C.; Henson, P.M.; Downey, G.P.; Riches, D.W. Age and sex dimorphisms contribute to the severity of bleomycin-induced lung injury and fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L510–L518. [Google Scholar] [CrossRef] [PubMed]
- Boveda-Ruiz, D.; D’Alessandro-Gabazza, C.N.; Toda, M.; Toda, M.; Takagi, T.; Naito, M.; Matsushima, Y.; Matsumoto, T.; Kobayashi, T.; Gil-Bernabe, P.; et al. Differential role of regulatory T cells in early and late stages of pulmonary fibrosis. Immunobiology 2013, 218, 245–254. [Google Scholar] [CrossRef]
- Lemaire, R.; Burwell, T.; Sun, H.; Delaney, T.; Bakken, J.; Cheng, L.; Rebelatto, M.C.; Czapiga, M.; de-Mendez, I.; Coyle, A.J.; et al. Resolution of Skin Fibrosis by Neutralization of the Antifibrinolytic Function of Plasminogen Activator Inhibitor 1. Arthritis Rheumatol. 2016, 68, 473–483. [Google Scholar] [CrossRef]
- Watanabe, T.; Nishimoto, T.; Mlakar, L.; Heywood, J.; Malaab, M.; Hoffman, S.; Feghali-Bostwick, C. Optimization of a murine and human tissue model to recapitulate dermal and pulmonary features of systemic sclerosis. PLoS ONE 2017, 12, e0179917. [Google Scholar] [CrossRef] [Green Version]
- D’Alessandro-Gabazza, C.N.; Kobayashi, T.; Yasuma, T.; Toda, M.; Kim, H.; Fujimoto, H.; Hataji, O.; Takeshita, A.; Nishihama, K.; Okano, T.; et al. A Staphylococcus pro-apoptotic peptide induces acute exacerbation of pulmonary fibrosis. Nat. Commun. 2020, 11, 1539. [Google Scholar] [CrossRef] [Green Version]
- D’Alessandro-Gabazza, C.N.; Yasuma, T.; Kobayashi, T.; Toda, M.; Abdel-Hamid, A.M.; Fujimoto, H.; Hataji, O.; Nakahara, H.; Takeshita, A.; Nishihama, K.; et al. Inhibition of lung microbiota-derived proapoptotic peptides ameliorates acute exacerbation of pulmonary fibrosis. Nat. Commun. 2022, 13, 1558. [Google Scholar] [CrossRef]
- Ashcroft, T.; Simpson, J.M.; Timbrell, V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 1988, 41, 467–470. [Google Scholar] [CrossRef] [Green Version]
- Wallach-Dayan, S.B.; Izbicki, G.; Cohen, P.Y.; Gerstl-Golan, R.; Fine, A.; Breuer, R. Bleomycin initiates apoptosis of lung epithelial cells by ROS but not by Fas/FasL pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L790–L796. [Google Scholar] [CrossRef]
- Deguchi, H.; Takeya, H.; Wada, H.; Gabazza, E.C.; Hayashi, N.; Urano, H.; Suzuki, K. Dilazep, an antiplatelet agent, inhibits tissue factor expression in endothelial cells and monocytes. Blood 1997, 90, 2345–2356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, S.; Gabazza, E.C.; Hayashi, T.; Ido, M.; Adachi, Y.; Suzuki, K. Thrombin stimulates the expression of PDGF in lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L503–L510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birkedal-Hansen, H.; Taylor, R.E. Detergent-activation of latent collagenase and resolution of its component molecules. Biochem. Biophys. Res. Commun. 1982, 107, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3, a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, R.; Yasuma, T.; Fridman D’Alessandro, V.; Toda, M.; Ito, T.; Tomaru, A.; D’Alessandro-Gabazza, C.N.; Tsuruga, T.; Okano, T.; Takeshita, A.; et al. Amelioration of Pulmonary Fibrosis by Matrix Metalloproteinase-2 Overexpression. Int. J. Mol. Sci. 2023, 24, 6695. https://doi.org/10.3390/ijms24076695
Inoue R, Yasuma T, Fridman D’Alessandro V, Toda M, Ito T, Tomaru A, D’Alessandro-Gabazza CN, Tsuruga T, Okano T, Takeshita A, et al. Amelioration of Pulmonary Fibrosis by Matrix Metalloproteinase-2 Overexpression. International Journal of Molecular Sciences. 2023; 24(7):6695. https://doi.org/10.3390/ijms24076695
Chicago/Turabian StyleInoue, Ryo, Taro Yasuma, Valeria Fridman D’Alessandro, Masaaki Toda, Toshiyuki Ito, Atsushi Tomaru, Corina N. D’Alessandro-Gabazza, Tatsuki Tsuruga, Tomohito Okano, Atsuro Takeshita, and et al. 2023. "Amelioration of Pulmonary Fibrosis by Matrix Metalloproteinase-2 Overexpression" International Journal of Molecular Sciences 24, no. 7: 6695. https://doi.org/10.3390/ijms24076695





