Phospholipids, the Masters in the Shadows during Healing after Acute Myocardial Infarction
Abstract
:1. Cardiovascular Diseases: The Unanswered Problem of the Century
2. Phospholipids: Old Knowledge Rediscovered
3. Healing after Myocardial Infarction: The Unsolved Puzzle of the Heart
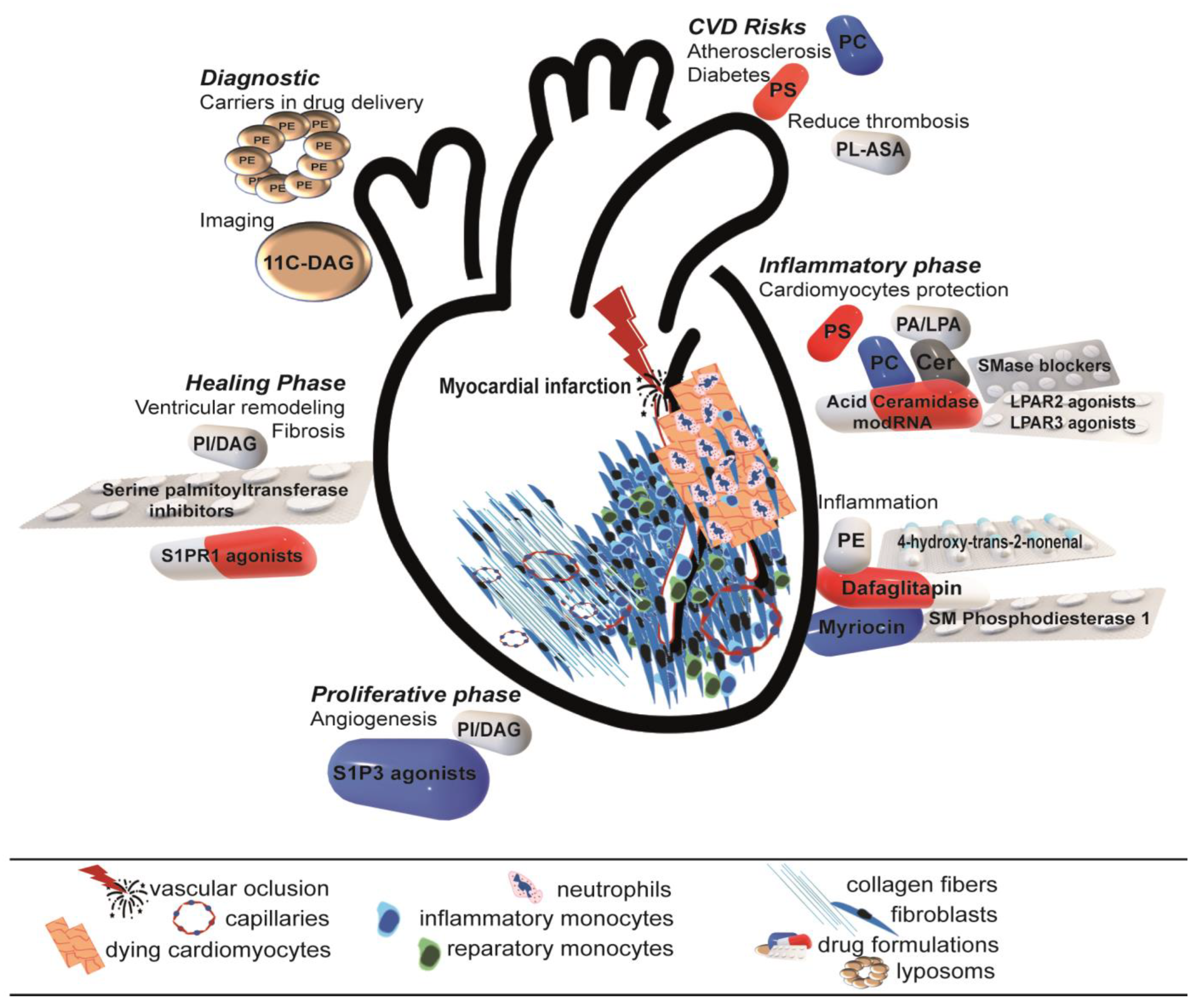
3.1. Phospholipids—The Quiet Leader behind the Doors
3.2. Therapeutical Strategies: Are They Really Novel?
3.3. Future Perspective: Simple Is Complicated
4. Conclusions: Too Simple to Be True
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaziano, T.A.; Bitton, A.; Anand, S.; Abrahams-Gessel, S.; Murphy, A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr. Probl. Cardiol. 2010, 35, 72–115. [Google Scholar] [CrossRef] [PubMed]
- Mechanic, O.J.; Gavin, M.; Grossman, S.A. Acute Myocardial Infarction. In StatPearls; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Scheen, A.J. From atherosclerosis to atherothrombosis: From a silent chronic pathology to an acute critical event. Rev. Med. De Liege 2018, 73, 224–228. [Google Scholar]
- Writing Committee Members; Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 16, 54–122. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthelemy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, A.P.; Rodrigues, A.; Risman, M.; McCoy, M.; Trindade, K.; Qu, L.; Cuchel, M.; Billheimer, J.; Rader, D.J. High-Density Lipoprotein (HDL) Phospholipid Content and Cholesterol Efflux Capacity Are Reduced in Patients With Very High HDL Cholesterol and Coronary Disease. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- McGranaghan, P.; Kirwan, J.A.; Garcia-Rivera, M.A.; Pieske, B.; Edelmann, F.; Blaschke, F.; Appunni, S.; Saxena, A.; Rubens, M.; Veledar, E.; et al. Lipid Metabolite Biomarkers in Cardiovascular Disease: Discovery and Biomechanism Translation from Human Studies. Metabolites 2021, 11, 621. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Hatch, G.M.; Wang, Y.; Yu, F.; Wang, M. The relationship between phospholipids and insulin resistance: From clinical to experimental studies. J. Cell. Mol. Med. 2019, 23, 702–710. [Google Scholar] [CrossRef]
- Maulik, N.; Kagan, V.E.; Tyurin, V.A.; Das, D.K. Redistribution of phosphatidylethanolamine and phosphatidylserine precedes reperfusion-induced apoptosis. Am. J. Physiol. 1998, 274, H242–H248. [Google Scholar] [CrossRef]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef]
- Cole, L.K.; Vance, J.E.; Vance, D.E. Phosphatidylcholine biosynthesis and lipoprotein metabolism. Biochim. Biophys. Acta 2012, 1821, 754–761. [Google Scholar] [CrossRef]
- Agudelo, C.W.; Samaha, G.; Garcia-Arcos, I. Alveolar lipids in pulmonary disease. A review. Lipids Health Dis. 2020, 19, 122. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, W.; Hoffmann, S.; Dombrowsky, H.; Rau, G.A.; Kamlage, A.; Kappler, M.; Haitsma, J.J.; Freihorst, J.; von der Hardt, H.; Poets, C.F. Phosphatidylcholine molecular species in lung surfactant: Composition in relation to respiratory rate and lung development. Am. J. Respir. Cell Mol. Biol. 2001, 25, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Stremmel, W.; Ehehalt, R.; Staffer, S.; Stoffels, S.; Mohr, A.; Karner, M.; Braun, A. Mucosal protection by phosphatidylcholine. Dig. Dis. 2012, 30 (Suppl. S3), 85–91. [Google Scholar] [CrossRef] [PubMed]
- Korytowski, A.; Abuillan, W.; Amadei, F.; Makky, A.; Gumiero, A.; Sinning, I.; Gauss, A.; Stremmel, W.; Tanaka, M. Accumulation of phosphatidylcholine on gut mucosal surface is not dominated by electrostatic interactions. Biochim. Biophys. Acta Biomembr. 2017, 1859, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Y.; Rimm, E.B.; Hu, F.B.; Albert, C.M.; Rexrode, K.M.; Manson, J.E.; Qi, L. Dietary phosphatidylcholine and risk of all-cause and cardiovascular-specific mortality among US women and men. Am. J. Clin. Nutr. 2016, 104, 173–180. [Google Scholar] [CrossRef]
- Pages, C.; Simon, M.F.; Valet, P.; Saulnier-Blache, J.S. Lysophosphatidic acid synthesis and release. Prostaglandins Other Lipid Mediat. 2001, 64, 1–10. [Google Scholar] [CrossRef]
- Pei, J.; Cai, L.; Wang, F.; Xu, C.; Pei, S.; Guo, H.; Sun, X.; Chun, J.; Cong, X.; Zhu, W.; et al. LPA(2) Contributes to Vascular Endothelium Homeostasis and Cardiac Remodeling After Myocardial Infarction. Circ. Res. 2022, 131, 388–403. [Google Scholar] [CrossRef]
- Wang, F.; Liu, S.; Pei, J.; Cai, L.; Liu, N.; Liang, T.; Dong, X.; Cong, X.; Chun, J.; Chen, J.; et al. LPA(3)-mediated lysophosphatidic acid signaling promotes postnatal heart regeneration in mice. Theranostics 2020, 10, 10892–10907. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, S.; Liou, L.C.; Ren, Q.; Zhang, Z.; Caldwell, G.A.; Caldwell, K.A.; Witt, S.N. Phosphatidylethanolamine deficiency disrupts alpha-synuclein homeostasis in yeast and worm models of Parkinson disease. Proc. Natl. Acad. Sci. USA 2014, 111, E3976–E3985. [Google Scholar] [CrossRef]
- Xu, F.Y.; Kardami, E.; Nemer, M.; Choy, P.C.; Hatch, G.M. Elevation in phosphatidylethanolamine is an early but not essential event for cardiac cell differentiation. Exp. Cell Res. 2000, 256, 358–364. [Google Scholar] [CrossRef]
- Leventis, P.A.; Grinstein, S. The distribution and function of phosphatidylserine in cellular membranes. Annu. Rev. Biophys. 2010, 39, 407–427. [Google Scholar] [CrossRef] [PubMed]
- Eichberg, J. Protein kinase C changes in diabetes: Is the concept relevant to neuropathy? Int. Rev. Neurobiol. 2002, 50, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.E.; Steenbergen, R. Metabolism and functions of phosphatidylserine. Prog. Lipid Res. 2005, 44, 207–234. [Google Scholar] [CrossRef] [PubMed]
- Jager, R.; Purpura, M.; Kingsley, M. Phospholipids and sports performance. J. Int. Soc. Sport. Nutr. 2007, 4, 5. [Google Scholar] [CrossRef]
- Mott, B.; Packwood, W.; Xie, A.; Belcik, J.T.; Taylor, R.P.; Zhao, Y.; Davidson, B.P.; Lindner, J.R. Echocardiographic Ischemic Memory Imaging Through Complement-Mediated Vascular Adhesion of Phosphatidylserine-Containing Microbubbles. JACC Cardiovasc. Imaging 2016, 9, 937–946. [Google Scholar] [CrossRef]
- Zhou, J.; Singh, N.; Monnier, C.; Marszalec, W.; Gao, L.; Jin, J.; Frisk, M.; Louch, W.E.; Verma, S.; Krishnamurthy, P.; et al. Phosphatidylinositol-4,5-Bisphosphate Binding to Amphiphysin-II Modulates T-Tubule Remodeling: Implications for Heart Failure. Front. Physiol. 2021, 12, 782767. [Google Scholar] [CrossRef] [PubMed]
- Shoki, M.; Kawaguchi, H.; Okamoto, H.; Sano, H.; Sawa, H.; Kudo, T.; Hirao, N.; Sakata, Y.; Yasuda, H. Phosphatidylinositol and inositolphosphatide metabolism in hypertrophied rat heart. Jpn. Circ. J. 1992, 56, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Prakoso, D.; De Blasio, M.J.; Tate, M.; Kiriazis, H.; Donner, D.G.; Qian, H.; Nash, D.; Deo, M.; Weeks, K.L.; Parry, L.J.; et al. Gene therapy targeting cardiac phosphoinositide 3-kinase (p110alpha) attenuates cardiac remodeling in type 2 diabetes. Am. J. Physiol.-Heart Circ. Physiol. 2020, 318, H840–H852. [Google Scholar] [CrossRef]
- Goto, K.; Nakano, T.; Hozumi, Y. Diacylglycerol kinase and animal models: The pathophysiological roles in the brain and heart. Adv. Enzym. Regul. 2006, 46, 192–202. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Role of Cardiolipin in Mitochondrial Function and Dynamics in Health and Disease: Molecular and Pharmacological Aspects. Cells 2019, 8, 728. [Google Scholar] [CrossRef]
- Haghighi, A.; Haack, T.B.; Atiq, M.; Mottaghi, H.; Haghighi-Kakhki, H.; Bashir, R.A.; Ahting, U.; Feichtinger, R.G.; Mayr, J.A.; Rotig, A.; et al. Sengers syndrome: Six novel AGK mutations in seven new families and review of the phenotypic and mutational spectrum of 29 patients. Orphanet J. Rare Dis. 2014, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Scorza, F.A.; Fiorini, A.C.; Scorza, C.A. MEGDEL Syndrome. Pediatr. Neurol. 2020, 110, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Soong, Y.; Seshan, S.V.; Szeto, H.H. Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis. Am. J. Physiol.-Ren. Physiol. 2014, 306, F970–F980. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 14205–14218. [Google Scholar] [CrossRef]
- Dudek, J.; Hartmann, M.; Rehling, P. The role of mitochondrial cardiolipin in heart function and its implication in cardiac disease. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 810–821. [Google Scholar] [CrossRef]
- Falabella, M.; Vernon, H.J.; Hanna, M.G.; Claypool, S.M.; Pitceathly, R.D.S. Cardiolipin, Mitochondria, and Neurological Disease. Trends Endocrinol. Metab. 2021, 32, 224–237. [Google Scholar] [CrossRef]
- Signorelli, P.; Conte, C.; Albi, E. The Multiple Roles of Sphingomyelin in Parkinson’s Disease. Biomolecules 2021, 11, 1311. [Google Scholar] [CrossRef]
- Pongrac Barlovic, D.; Harjutsalo, V.; Sandholm, N.; Forsblom, C.; Groop, P.H.; FinnDiane Study, G. Sphingomyelin and progression of renal and coronary heart disease in individuals with type 1 diabetes. Diabetologia 2020, 63, 1847–1856. [Google Scholar] [CrossRef]
- Stenemo, M.; Ganna, A.; Salihovic, S.; Nowak, C.; Sundstrom, J.; Giedraitis, V.; Broeckling, C.D.; Prenni, J.E.; Svensson, P.; Magnusson, P.K.E.; et al. The metabolites urobilin and sphingomyelin (30:1) are associated with incident heart failure in the general population. ESC Heart Fail. 2019, 6, 764–773. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Kitatani, K.; Idkowiak-Baldys, J.; Hannun, Y.A. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008, 20, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Ren, Z.; Pae, M.; Guo, W.; Cui, X.; Merrill, A.H.; Meydani, S.N. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J. Immunol. 2007, 179, 4829–4839. [Google Scholar] [CrossRef] [PubMed]
- Zietzer, A.; Dusing, P.; Reese, L.; Nickenig, G.; Jansen, F. Ceramide Metabolism in Cardiovascular Disease: A Network With High Therapeutic Potential. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Sun, M.; Wu, J.; Dong, H.; Liu, J.; Gao, R.; Fang, S.; Xing, L.; Hu, S.; Yu, B. Relationship between elevated plasma ceramides and plaque rupture in patients with ST-segment elevation myocardial infarction. Atherosclerosis 2020, 302, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Obinata, H.; Hla, T. Sphingosine 1-phosphate and inflammation. Int. Immunol. 2019, 31, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ma, S.; Xie, W.Q.; Zhang, Y.M.; Tao, L.; Li, Y.S.; Xiao, W.F. Sphingosine 1-phosphate and osteoporosis: Pathophysiology and therapeutic aspects-a narrative review. Ann. Palliat. Med. 2021, 10, 4799–4805. [Google Scholar] [CrossRef] [PubMed]
- Kurano, M.; Yatomi, Y. Sphingosine 1-Phosphate and Atherosclerosis. J. Atheroscler. Thromb. 2018, 25, 16–26. [Google Scholar] [CrossRef]
- Liu, Y.; Saiyan, S.; Men, T.Y.; Gao, H.Y.; Wen, C.; Liu, Y.; Zhou, X.; Wu, C.T.; Wang, L.S.; Cui, C.P. Hepatopoietin Cn reduces ethanol-induced hepatoxicity via sphingosine kinase 1 and sphingosine 1-phosphate receptors. J. Pathol. 2013, 230, 365–376. [Google Scholar] [CrossRef]
- Tsaousi, G.; Stavrou, G.; Fotiadis, K.; Kotzampassi, K.; Kolios, G. Implementation of Phospholipids as Pharmacological Modalities for Postoperative Adhesions Prevention. Eur. J. Pharmacol. 2019, 842, 189–196. [Google Scholar] [CrossRef]
- Magkrioti, C.; Galaris, A.; Kanellopoulou, P.; Stylianaki, E.A.; Kaffe, E.; Aidinis, V. Autotaxin and chronic inflammatory diseases. J. Autoimmun. 2019, 104, 102327. [Google Scholar] [CrossRef]
- Tabuchi, S. The autotaxin-lysophosphatidic acid-lysophosphatidic acid receptor cascade: Proposal of a novel potential therapeutic target for treating glioblastoma multiforme. Lipids Health Dis. 2015, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.E. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: Two metabolically related aminophospholipids. J. Lipid Res. 2008, 49, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Calzada, E.; Onguka, O.; Claypool, S.M. Phosphatidylethanolamine Metabolism in Health and Disease. Int. Rev. Cell Mol. Biol. 2016, 321, 29–88. [Google Scholar] [CrossRef] [PubMed]
- Stefanis, L. alpha-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef]
- Wang, J.; Yu, C.; Zhuang, J.; Qi, W.; Jiang, J.; Liu, X.; Zhao, W.; Cao, Y.; Wu, H.; Qi, J.; et al. The role of phosphatidylserine on the membrane in immunity and blood coagulation. Biomark. Res. 2022, 10, 4. [Google Scholar] [CrossRef]
- Kim, H.Y.; Huang, B.X.; Spector, A.A. Phosphatidylserine in the brain: Metabolism and function. Prog. Lipid Res. 2014, 56, 1–18. [Google Scholar] [CrossRef]
- Callahan, M.K.; Popernack, P.M.; Tsutsui, S.; Truong, L.; Schlegel, R.A.; Henderson, A.J. Phosphatidylserine on HIV envelope is a cofactor for infection of monocytic cells. J Immunol 2003, 170, 4840–4845. [Google Scholar] [CrossRef]
- Kingsley, M. Effects of phosphatidylserine supplementation on exercising humans. Sport. Med. 2006, 36, 657–669. [Google Scholar] [CrossRef]
- Jing, X.X.; Wang, Z.G.; Ran, H.T.; Li, L.; Wu, X.; Li, X.D.; Peng, X.Q.; Yang, C.J.; Li, X.S.; Zhang, Q.X. Evaluation of renal ischemia-reperfusion injury in rabbits using microbubbles targeted to activated neutrophils. Clin. Imaging 2008, 32, 178–182. [Google Scholar] [CrossRef]
- Lindner, J.R.; Song, J.; Xu, F.; Klibanov, A.L.; Singbartl, K.; Ley, K.; Kaul, S. Noninvasive ultrasound imaging of inflammation using microbubbles targeted to activated leukocytes. Circulation 2000, 102, 2745–2750. [Google Scholar] [CrossRef]
- Porter, T.R. Detection of Myocarditis With Molecular Echo Imaging: Another Potential Application for the Phosphatidyl Serine Microbubble. Circ. Cardiovasc. Imaging 2016, 9, e005249. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; Bucki, R.; Radhakrishnan, R. Regulation of actin assembly by PI(4,5)P2 and other inositol phospholipids: An update on possible mechanisms. Biochem. Biophys. Res. Commun. 2018, 506, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Le, O.T.; Nguyen, T.T.; Lee, S.Y. Phosphoinositide turnover in Toll-like receptor signaling and trafficking. BMB Rep. 2014, 47, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Gabelli, S.B.; Raben, D.M. Diacylglycerol kinases: Relationship to other lipid kinases. Adv. Biol. Regul. 2019, 71, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, S.; Merida, I. Diacylglycerol, when simplicity becomes complex. Trends Biochem. Sci. 2007, 32, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Opreanu, M.; Tikhonenko, M.; Bozack, S.; Lydic, T.A.; Reid, G.E.; McSorley, K.M.; Sochacki, A.; Perez, G.I.; Esselman, W.J.; Kern, T.; et al. The unconventional role of acid sphingomyelinase in regulation of retinal microangiopathy in diabetic human and animal models. Diabetes 2011, 60, 2370–2378. [Google Scholar] [CrossRef]
- Huang, W.C.; Chen, C.L.; Lin, Y.S.; Lin, C.F. Apoptotic sphingolipid ceramide in cancer therapy. J. Lipids 2011, 2011, 565316. [Google Scholar] [CrossRef]
- Zhao, L.; Spassieva, S.D.; Jucius, T.J.; Shultz, L.D.; Shick, H.E.; Macklin, W.B.; Hannun, Y.A.; Obeid, L.M.; Ackerman, S.L. A deficiency of ceramide biosynthesis causes cerebellar purkinje cell neurodegeneration and lipofuscin accumulation. PLOS Genet. 2011, 7, e1002063. [Google Scholar] [CrossRef]
- Pfeilschifter, J.; Huwiler, A. Identification of ceramide targets in interleukin-1 and tumor necrosis factor-alpha signaling in mesangial cells. Kidney Int. Suppl. 1998, 67, S34–S39. [Google Scholar] [CrossRef]
- Raine, C.S. Biology of disease. Analysis of autoimmune demyelination: Its impact upon multiple sclerosis. Lab. Investig. 1984, 50, 608–635. [Google Scholar]
- Holland, W.L.; Bikman, B.T.; Wang, L.P.; Yuguang, G.; Sargent, K.M.; Bulchand, S.; Knotts, T.A.; Shui, G.; Clegg, D.J.; Wenk, M.R.; et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Investig. 2011, 121, 1858–1870. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Basterr, M.J.; Hailemariam, T.K.; Hojjati, M.R.; Lu, S.; Liu, J.; Liu, R.; Zhou, H.; Jiang, X.C. The effect of dietary sphingolipids on plasma sphingomyelin metabolism and atherosclerosis. Biochim. Biophys. Acta 2005, 1735, 130–134. [Google Scholar] [CrossRef]
- Bismuth, J.; Lin, P.; Yao, Q.; Chen, C. Ceramide: A common pathway for atherosclerosis? Atherosclerosis 2008, 196, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Peng, Y.; Hang, W.; Li, N.; Zhou, N.; Wang, D.W. Emerging Roles of Ceramide in Cardiovascular Diseases. Aging Dis. 2022, 13, 232–245. [Google Scholar] [CrossRef]
- Tommasino, C.; Marconi, M.; Ciarlo, L.; Matarrese, P.; Malorni, W. Autophagic flux and autophagosome morphogenesis require the participation of sphingolipids. Apoptosis 2015, 20, 645–657. [Google Scholar] [CrossRef]
- Schwalm, S.; Pfeilschifter, J.; Huwiler, A. Targeting the sphingosine kinase/sphingosine 1-phosphate pathway to treat chronic inflammatory kidney diseases. Basic Clin. Pharmacol. Toxicol. 2014, 114, 44–49. [Google Scholar] [CrossRef]
- Kullenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Drescher, S.; van Hoogevest, P. The Phospholipid Research Center: Current Research in Phospholipids and Their Use in Drug Delivery. Pharmaceutics 2020, 12, 1235. [Google Scholar] [CrossRef]
- Liehn, E.A.; Postea, O.; Curaj, A.; Marx, N. Repair after myocardial infarction, between fantasy and reality: The role of chemokines. J. Am. Coll. Cardiol. 2011, 58, 2357–2362. [Google Scholar] [CrossRef]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- Huang, S.; Frangogiannis, N.G. Anti-inflammatory therapies in myocardial infarction: Failures, hopes and challenges. Br. J. Pharmacol. 2018, 175, 1377–1400. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, M.L.; Brunt, K.R.; Kirk, J.A.; Kleinbongard, P.; Calvert, J.W.; de Castro Bras, L.E.; DeLeon-Pennell, K.Y.; Del Re, D.P.; Frangogiannis, N.G.; Frantz, S.; et al. Guidelines for in vivo mouse models of myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H1056–H1073. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Pathophysiology of Myocardial Infarction. Compr. Physiol. 2015, 5, 1841–1875. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Inflammation in cardiac injury, repair and regeneration. Curr. Opin. Cardiol. 2015, 30, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Lyck, R.; Enzmann, G. The physiological roles of ICAM-1 and ICAM-2 in neutrophil migration into tissues. Curr. Opin. Hematol. 2015, 22, 53–59. [Google Scholar] [CrossRef]
- Ong, S.B.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Mukhametshina, R.T.; Kwek, X.Y.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol. Ther. 2018, 186, 73–87. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef]
- Curaj, A.; Schumacher, D.; Rusu, M.; Staudt, M.; Li, X.; Simsekyilmaz, S.; Jankowski, V.; Jankowski, J.; Dumitrascu, A.R.; Hausenloy, D.J.; et al. Neutrophils Modulate Fibroblast Function and Promote Healing and Scar Formation after Murine Myocardial Infarction. Int. J. Mol. Sci. 2020, 21, 3685. [Google Scholar] [CrossRef]
- Andreadou, I.; Cabrera-Fuentes, H.A.; Devaux, Y.; Frangogiannis, N.G.; Frantz, S.; Guzik, T.; Liehn, E.A.; Gomes, C.P.C.; Schulz, R.; Hausenloy, D.J. Immune cells as targets for cardioprotection: New players and novel therapeutic opportunities. Cardiovasc. Res. 2019, 115, 1117–1130. [Google Scholar] [CrossRef]
- Saxena, A.; Russo, I.; Frangogiannis, N.G. Inflammation as a therapeutic target in myocardial infarction: Learning from past failures to meet future challenges. Transl. Res. 2016, 167, 152–166. [Google Scholar] [CrossRef]
- Christia, P.; Frangogiannis, N.G. Targeting inflammatory pathways in myocardial infarction. Eur. J. Clin. Investig. 2013, 43, 986–995. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Chen, P.; Chen, Q.; Cai, Z.; Zhang, P. Cytokine storm: Behind the scenes of the collateral circulation after acute myocardial infarction. Inflamm. Res. 2022, 71, 1143–1158. [Google Scholar] [CrossRef] [PubMed]
- Bujak, M.; Frangogiannis, N.G. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc. Res. 2007, 74, 184–195. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Bovet, P.; Darioli, R.; Essinger, A.; Golay, A.; Sigwart, U.; Kappenberger, L. Phospholipids and other lipids in angiographically assessed coronary artery disease. Atherosclerosis 1989, 80, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Das, D.K.; Engelman, R.M.; Rousou, J.A.; Breyer, R.H.; Otani, H.; Lemeshow, S. Role of membrane phospholipids in myocardial injury induced by ischemia and reperfusion. Am. J. Physiol. 1986, 251, H71–H79. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Han, Y.; Zhang, Y.; Zhang, S.; Wei, C.; Cong, Z.; Du, W. Metabolomics Study of the Biochemical Changes in the Plasma of Myocardial Infarction Patients. Front. Physiol. 2018, 9, 1017. [Google Scholar] [CrossRef]
- Montero-Bullon, J.F.; Aveiro, S.S.; Melo, T.; Martins-Marques, T.; Lopes, D.; Neves, B.; Girao, H.; Rosario, M.D.M.; Domingues, P. Cardiac phospholipidome is altered during ischemia and reperfusion in an ex vivo rat model. Biochem. Biophys. Rep. 2021, 27, 101037. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Zhang, X.; Xu, W.; Jin, K.; Pang, Y.; Wu, Y.; Luo, J.; Xu, R.; Jiao, L.; et al. Mechanism of oxidized phospholipid-related inflammatory response in vascular ageing. Ageing Res. Rev. 2023, 86, 101888. [Google Scholar] [CrossRef]
- Scanavachi, G.; Coutinho, A.; Fedorov, A.A.; Prieto, M.; Melo, A.M.; Itri, R. Lipid Hydroperoxide Compromises the Membrane Structure Organization and Softens Bending Rigidity. Langmuir 2021, 37, 9952–9963. [Google Scholar] [CrossRef]
- Koleini, N.; Nickel, B.E.; Edel, A.L.; Fandrich, R.R.; Ravandi, A.; Kardami, E. Oxidized phospholipids in Doxorubicin-induced cardiotoxicity. Chem. Biol. Interact. 2019, 303, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, V.N.; Oskolkova, O.V.; Birukov, K.G.; Levonen, A.L.; Binder, C.J.; Stockl, J. Generation and biological activities of oxidized phospholipids. Antioxid. Redox Signal. 2010, 12, 1009–1059. [Google Scholar] [CrossRef] [PubMed]
- Rivara, M.B.; Ikizler, T.A.; Ellis, C.D.; Mehrotra, R.; Himmelfarb, J. Association of plasma F2-isoprostanes and isofurans concentrations with erythropoiesis-stimulating agent resistance in maintenance hemodialysis patients. BMC Nephrol. 2015, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.S.; Lee, J.H.; Arsenault, B.J.; Yang, X.; Bao, W.; DeMicco, D.; Laskey, R.; Witztum, J.L.; Tsimikas, S.; Investigators, T.N.T.T. Relationship of oxidized phospholipids on apolipoprotein B-100 to cardiovascular outcomes in patients treated with intensive versus moderate atorvastatin therapy: The TNT trial. J. Am. Coll. Cardiol. 2015, 65, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Adibhatla, R.M.; Hatcher, J.F. Phospholipase A(2), reactive oxygen species, and lipid peroxidation in CNS pathologies. BMB Rep. 2008, 41, 560–567. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Q.; Wang, D.; Kong, J.; Dai, W.; Wang, X.; Yu, X. Common lipid features of lethal ventricular tarchyarrhythmias (LVTAs) induced by myocardial infarction and myocardial ion channel diseases. Sci. Rep. 2017, 7, 4220. [Google Scholar] [CrossRef]
- Deng, J.; Jiang, Y.; Chen, Z.B.; Rhee, J.W.; Deng, Y.; Wang, Z.V. Mitochondrial Dysfunction in Cardiac Arrhythmias. Cells 2023, 12, 679. [Google Scholar] [CrossRef]
- Yang, K.C.; Bonini, M.G.; Dudley, S.C., Jr. Mitochondria and arrhythmias. Free. Radic. Biol. Med. 2014, 71, 351–361. [Google Scholar] [CrossRef]
- Gizurarson, S.; Shao, Y.; Miljanovic, A.; Ramunddal, T.; Boren, J.; Bergfeldt, L.; Omerovic, E. Electrophysiological effects of lysophosphatidylcholine on HL-1 cardiomyocytes assessed with a microelectrode array system. Cell. Physiol. Biochem. 2012, 30, 477–488. [Google Scholar] [CrossRef]
- Man, R.Y. Lysophosphatidylcholine-induced arrhythmias and its accumulation in the rat perfused heart. Br. J. Pharmacol. 1988, 93, 412–416. [Google Scholar] [CrossRef]
- Giffin, M.; Arthur, G.; Choy, P.C.; Man, R.Y. Lysophosphatidylcholine metabolism and cardiac arrhythmias. Can. J. Physiol. Pharmacol. 1988, 66, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.; Haupt, R.; Alms, S.; Holzmann, G.; Konig, T.; Kern, H.; Kox, W.; Rustow, B.; Schlame, M. Increase in fragmented phosphatidylcholine in blood plasma by oxidative stress. J. Lipid Res. 2000, 41, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Solati, Z.; Surendran, A.; Edel, A.; Roznik, M.; Allen, D.; Ravandi, A. Increase in Plasma Oxidized Phosphatidylcholines (OxPCs) in Patients Presenting With ST-Elevation Myocardial Infarction (STEMI). Front. Med. 2021, 8, 716944. [Google Scholar] [CrossRef] [PubMed]
- Yeang, C.; Hasanally, D.; Que, X.; Hung, M.Y.; Stamenkovic, A.; Chan, D.; Chaudhary, R.; Margulets, V.; Edel, A.L.; Hoshijima, M.; et al. Reduction of myocardial ischaemia-reperfusion injury by inactivating oxidized phospholipids. Cardiovasc. Res. 2019, 115, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Smiley, P.L.; Stremler, K.E.; Prescott, S.M.; Zimmerman, G.A.; McIntyre, T.M. Oxidatively fragmented phosphatidylcholines activate human neutrophils through the receptor for platelet-activating factor. J. Biol. Chem. 1991, 266, 11104–11110. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, A.; O’Hara, K.A.; Nelson, D.C.; Maddaford, T.G.; Edel, A.L.; Maddaford, G.; Dibrov, E.; Aghanoori, M.; Kirshenbaum, L.A.; Fernyhough, P.; et al. Oxidized phosphatidylcholines trigger ferroptosis in cardiomyocytes during ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1170–H1184. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Law, S.H.; Chan, M.L.; Marathe, G.K.; Parveen, F.; Chen, C.H.; Ke, L.Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef]
- Soehnlein, O.; Lindbom, L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010, 10, 427–439. [Google Scholar] [CrossRef]
- Lieber, C.S. Prevention and treatment of liver fibrosis based on pathogenesis. Alcohol Clin. Exp. Res. 1999, 23, 944–949. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Xu, Y.J.; Sheu, S.S.; Tappia, P.S.; Panagia, V. Phosphatidic acid: A potential signal transducer for cardiac hypertrophy. J. Mol. Cell. Cardiol. 1997, 29, 2865–2871. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.J.; Panagia, V.; Shao, Q.; Wang, X.; Dhalla, N.S. Phosphatidic acid increases intracellular free Ca2+ and cardiac contractile force. Am. J. Physiol. 1996, 271, H651–H659. [Google Scholar] [CrossRef]
- Plo, I.; Lautier, D.; Levade, T.; Sekouri, H.; Jaffrezou, J.P.; Laurent, G.; Bettaieb, A. Phosphatidylcholine-specific phospholipase C and phospholipase D are respectively implicated in mitogen-activated protein kinase and nuclear factor kappaB activation in tumour-necrosis-factor-alpha-treated immature acute-myeloid-leukaemia cells. Biochem. J. 2000, 351 Pt 2, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Dent, M.R.; Singal, T.; Dhalla, N.S.; Tappia, P.S. Expression of phospholipase D isozymes in scar and viable tissue in congestive heart failure due to myocardial infarction. J. Cell. Mol. Med. 2004, 8, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Panagia, V.; Tappia, P.S.; Liu, S.Y.; Takeda, N.; Dhalla, N.S. Alterations of sarcolemmal phospholipase D and phosphatidate phosphohydrolase in congestive heart failure. Biochim. Biophys. Acta 2002, 1584, 65–72. [Google Scholar] [CrossRef]
- Hakuno, D.; Kimura, M.; Ito, S.; Satoh, J.; Nakashima, Y.; Horie, T.; Kuwabara, Y.; Nishiga, M.; Ide, Y.; Baba, O.; et al. Hepatokine alpha1-Microglobulin Signaling Exacerbates Inflammation and Disturbs Fibrotic Repair in Mouse Myocardial Infarction. Sci. Rep. 2018, 8, 16749. [Google Scholar] [CrossRef]
- Patel, D.; Witt, S.N. Ethanolamine and Phosphatidylethanolamine: Partners in Health and Disease. Oxidative Med. Cell. Longev. 2017, 2017, 4829180. [Google Scholar] [CrossRef]
- Park, S.; Kim, B.K.; Park, S.K. Supplementation with phosphatidylethanolamine confers anti-oxidant and anti-aging effects via hormesis and reduced insulin/IGF-1-like signaling in C. elegans. Mech. Ageing Dev. 2021, 197, 111498. [Google Scholar] [CrossRef]
- Johnson, J.M.; Peterlin, A.D.; Balderas, E.; Sustarsic, E.G.; Maschek, J.A.; Lang, M.J.; Jara-Ramos, A.; Panic, V.; Morgan, J.T.; Villanueva, C.J.; et al. Mitochondrial phosphatidylethanolamine modulates UCP1 to promote brown adipose thermogenesis. Sci. Adv. 2023, 9, eade7864. [Google Scholar] [CrossRef]
- Schumacher, D.; Curaj, A.; Staudt, M.; Cordes, F.; Dumitrascu, A.R.; Rolles, B.; Beckers, C.; Soppert, J.; Rusu, M.; Simsekyilmaz, S.; et al. Phosphatidylserine Supplementation as a Novel Strategy for Reducing Myocardial Infarct Size and Preventing Adverse Left Ventricular Remodeling. Int. J. Mol. Sci. 2021, 22, 4401. [Google Scholar] [CrossRef] [PubMed]
- Liehn, E.A.; Tuchscheerer, N.; Kanzler, I.; Drechsler, M.; Fraemohs, L.; Schuh, A.; Koenen, R.R.; Zander, S.; Soehnlein, O.; Hristov, M.; et al. Double-edged role of the CXCL12/CXCR4 axis in experimental myocardial infarction. J. Am. Coll. Cardiol. 2011, 58, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Chang, W. Phosphatidylserine in Diabetes Research. Mol. Pharm. 2023, 20, 82–89. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Wang, M.; Yang, Z.; Yang, J.; Ren, Y.; Cao, L.; Han, X.; Huang, L.; Sun, Z.; et al. Phosphatidylserine-Specific Phospholipase A1 Alleviates Lipopolysaccharide-Induced Macrophage Inflammation by Inhibiting MAPKs Activation. Biol. Pharm. Bull. 2022, 45, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tang, J.; Cui, X.; Qin, B.; Zhang, J.; Zhang, L.; Zhang, H.; Liu, G.; Wang, W.; Zhang, J. New Insights and Novel Therapeutic Potentials for Macrophages in Myocardial Infarction. Inflammation 2021, 44, 1696–1712. [Google Scholar] [CrossRef] [PubMed]
- Maciel, E.; Neves, B.M.; Martins, J.; Colombo, S.; Cruz, M.T.; Domingues, P.; Domingues, M.R.M. Oxidized phosphatidylserine mitigates LPS-triggered macrophage inflammatory status through modulation of JNK and NF-kB signaling cascades. Cell Signal. 2019, 61, 30–38. [Google Scholar] [CrossRef]
- Ghigo, A.; Morello, F.; Perino, A.; Hirsch, E. Phosphoinositide 3-kinases in health and disease. Subcell. Biochem. 2012, 58, 183–213. [Google Scholar] [CrossRef]
- Jiang, B.H.; Liu, L.Z. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv. Cancer Res. 2009, 102, 19–65. [Google Scholar] [CrossRef]
- Niizeki, T.; Takeishi, Y.; Arimoto, T.; Takahashi, H.; Shishido, T.; Koyama, Y.; Goto, K.; Walsh, R.A.; Kubota, I. Cardiac-specific overexpression of diacylglycerol kinase zeta attenuates left ventricular remodeling and improves survival after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1105–H1112. [Google Scholar] [CrossRef]
- Cohen, M.V.; Downey, J.M. Myocardial preconditioning promises to be a novel approach to the treatment of ischemic heart disease. Annu. Rev. Med. 1996, 47, 21–29. [Google Scholar] [CrossRef]
- Sasaki, T.; Shishido, T.; Kadowaki, S.; Kitahara, T.; Suzuki, S.; Katoh, S.; Funayama, A.; Netsu, S.; Watanabe, T.; Goto, K.; et al. Diacylglycerol kinase alpha exacerbates cardiac injury after ischemia/reperfusion. Heart Vessel. 2014, 29, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Pizzuto, M.; Pelegrin, P. Cardiolipin in Immune Signaling and Cell Death. Trends Cell Biol. 2020, 30, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Mitochondrial bioenergetics and cardiolipin alterations in myocardial ischemia-reperfusion injury: Implications for pharmacological cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1341–H1352. [Google Scholar] [CrossRef]
- Jia, D.; Zhang, J.; Nie, J.; Andersen, J.P.; Rendon, S.; Zheng, Y.; Liu, X.; Tian, Z.; Shi, Y. Cardiolipin remodeling by ALCAT1 links hypoxia to coronary artery disease by promoting mitochondrial dysfunction. Mol. Ther. 2021, 29, 3498–3511. [Google Scholar] [CrossRef]
- Kuang, Y.; Li, X.; Liu, X.; Wei, L.; Chen, X.; Liu, J.; Zhuang, T.; Pi, J.; Wang, Y.; Zhu, C.; et al. Vascular endothelial S1pr1 ameliorates adverse cardiac remodelling via stimulating reparative macrophage proliferation after myocardial infarction. Cardiovasc. Res. 2021, 117, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Novgorodov, S.A.; Gudz, T.I. Ceramide and mitochondria in ischemia/reperfusion. J. Cardiovasc. Pharmacol. 2009, 53, 198–208. [Google Scholar] [CrossRef]
- He, X.; Schuchman, E.H. Ceramide and Ischemia/Reperfusion Injury. J. Lipids 2018, 2018, 3646725. [Google Scholar] [CrossRef]
- Argaud, L.; Prigent, A.F.; Chalabreysse, L.; Loufouat, J.; Lagarde, M.; Ovize, M. Ceramide in the antiapoptotic effect of ischemic preconditioning. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H246–H251. [Google Scholar] [CrossRef]
- Hadas, Y.; Vincek, A.S.; Youssef, E.; Zak, M.M.; Chepurko, E.; Sultana, N.; Sharkar, M.T.K.; Guo, N.; Komargodski, R.; Kurian, A.A.; et al. Altering Sphingolipid Metabolism Attenuates Cell Death and Inflammatory Response After Myocardial Infarction. Circulation 2020, 141, 916–930. [Google Scholar] [CrossRef]
- Borodzicz, S.; Czarzasta, K.; Kuch, M.; Cudnoch-Jedrzejewska, A. Sphingolipids in cardiovascular diseases and metabolic disorders. Lipids Health Dis. 2015, 14, 55. [Google Scholar] [CrossRef]
- Ji, R.; Akashi, H.; Drosatos, K.; Liao, X.; Jiang, H.; Kennel, P.J.; Brunjes, D.L.; Castillero, E.; Zhang, X.; Deng, L.Y.; et al. Increased de novo ceramide synthesis and accumulation in failing myocardium. JCI Insight 2017, 2, e82922. [Google Scholar] [CrossRef] [PubMed]
- Sposito, A.C.; de Lima-Junior, J.C.; Moura, F.A.; Barreto, J.; Bonilha, I.; Santana, M.; Virginio, V.W.; Sun, L.; Carvalho, L.S.F.; Soares, A.A.S.; et al. Reciprocal Multifaceted Interaction Between HDL (High-Density Lipoprotein) and Myocardial Infarction. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1550–1564. [Google Scholar] [CrossRef]
- Szczepanek, K.; Chen, Q.; Larner, A.C.; Lesnefsky, E.J. Cytoprotection by the modulation of mitochondrial electron transport chain: The emerging role of mitochondrial STAT3. Mitochondrion 2012, 12, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Theilmeier, G.; Schmidt, C.; Herrmann, J.; Keul, P.; Schafers, M.; Herrgott, I.; Mersmann, J.; Larmann, J.; Hermann, S.; Stypmann, J.; et al. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation 2006, 114, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Waeber, C.; Walther, T. Sphingosine-1-phosphate as a potential target for the treatment of myocardial infarction. Circ. J. 2014, 78, 795–802. [Google Scholar] [CrossRef]
- Walter, D.H.; Rochwalsky, U.; Reinhold, J.; Seeger, F.; Aicher, A.; Urbich, C.; Spyridopoulos, I.; Chun, J.; Brinkmann, V.; Keul, P.; et al. Sphingosine-1-phosphate stimulates the functional capacity of progenitor cells by activation of the CXCR4-dependent signaling pathway via the S1P3 receptor. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 275–282. [Google Scholar] [CrossRef]
- Zheng, Z.; Lei, C.; Liu, H.; Jiang, M.; Zhou, Z.; Zhao, Y.; Yu, C.Y.; Wei, H. A ROS-Responsive Liposomal Composite Hydrogel Integrating Improved Mitochondrial Function and Pro-Angiogenesis for Efficient Treatment of Myocardial Infarction. Adv. Healthc. Mater. 2022, 11, e2200990. [Google Scholar] [CrossRef]
- Napolitano, G.; Karin, M. Sphingolipids: The oil on the TRAFire that promotes inflammation. Sci. Signal. 2010, 3, pe34. [Google Scholar] [CrossRef]
- Klyachkin, Y.M.; Nagareddy, P.R.; Ye, S.; Wysoczynski, M.; Asfour, A.; Gao, E.; Sunkara, M.; Brandon, J.A.; Annabathula, R.; Ponnapureddy, R.; et al. Pharmacological Elevation of Circulating Bioactive Phosphosphingolipids Enhances Myocardial Recovery After Acute Infarction. Stem Cells Transl. Med. 2015, 4, 1333–1343. [Google Scholar] [CrossRef]
- Yamada, Y.; Wakao, S.; Kushida, Y.; Minatoguchi, S.; Mikami, A.; Higashi, K.; Baba, S.; Shigemoto, T.; Kuroda, Y.; Kanamori, H.; et al. S1P-S1PR2 Axis Mediates Homing of Muse Cells Into Damaged Heart for Long-Lasting Tissue Repair and Functional Recovery After Acute Myocardial Infarction. Circ. Res. 2018, 122, 1069–1083. [Google Scholar] [CrossRef]
- Wu, X.; Xu, J.; Li, X.; Dai, J.; Wang, L. Inhibition of SphK1/S1P Signaling Pathway Alleviates Fibrosis and Inflammation of Rat Myocardium after Myocardial Infarction. Comput. Math. Methods Med. 2022, 2022, 5985375. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.G.M.; Gomes, A.C.; Naves, M.M.V.; Mota, J.F. Nuts and Legume Seeds for Cardiovascular Risk Reduction: Scientific Evidence and Mechanisms of Action. Nutr. Rev. 2015, 73, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Wattanapenpaiboon, N.; Wahlqvist, M.W. Phytonutrient Deficiency: The Place of Palm Fruit. Asia Pac. J. Clin. Nutr. 2003, 12, 363–368. [Google Scholar] [PubMed]
- Ebong, P.E.; Owu, D.U.; Isong, E.U. Influence of Palm Oil (Elaesis Guineensis) on Health. Plant Foods Hum. Nutr. 1999, 53, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-X.; Peng, C.; Zhang, H.; Qin, L.-P. Sinomenium Acutum: A Review of Chemistry, Pharmacology, Pharmacokinetics, and Clinical Use. Pharm. Biol. 2012, 50, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, Q.; Liu, C.; Liu, J.; Luo, G.; He, L.; Yuan, T.; He, R.-R.; Yao, Z. Regulation of Phospholipid Peroxidation Signaling by a Traditional Chinese Medicine Formula for Coronary Heart Disease. Phytomedicine 2023, 114, 154749. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Ogawa, M.; Suzuki, J.-I.; Hirata, Y.; Nagai, R.; Isobe, M. A Comparison between Imidapril and Ramipril on Attenuation of Ventricular Remodeling after Myocardial Infarction. J. Cardiovasc. Pharmacol. 2012, 59, 323–330. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Prats, J.; Deliargyris, E.N.; Schneider, D.J.; Scheiman, J.; Kimmelstiel, C.; Steg, P.G.; Alberts, M.; Rosengart, T.; Mehran, R.; et al. Pharmacokinetic and Pharmacodynamic Profile of a Novel Phospholipid Aspirin Formulation. Clin. Pharmacokinet. 2022, 61, 465–479. [Google Scholar] [CrossRef]
- Hermansen, K.; Dinesen, B.; Hoie, L.H.; Morgenstern, E.; Gruenwald, J. Effects of Soy and Other Natural Products on LDL:HDL Ratio and Other Lipid Parameters: A Literature Review. Adv. Ther. 2003, 20, 50–78. [Google Scholar] [CrossRef]
- Marchesi, M.; Booth, E.A.; Davis, T.; Bisgaier, C.L.; Lucchesi, B.R. Apolipoprotein A-IMilano and 1-Palmitoyl-2-Oleoyl Phosphatidylcholine Complex (ETC-216) Protects the in Vivo Rabbit Heart from Regional Ischemia-Reperfusion Injury. J. Pharmacol. Exp. Ther. 2004, 311, 1023–1031. [Google Scholar] [CrossRef]
- Bigeh, A.; Sanchez, A.; Maestas, C.; Gulati, M. Inflammatory bowel disease and the risk for cardiovascular disease: Does all inflammation lead to heart disease? Trends Cardiovasc. Med. 2020, 30, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Collen, L.V.; Mowat, C.; Isaacs, K.L.; Singh, S.; Kane, S.V.; Farraye, F.A.; Snapper, S.; Jneid, H.; Lavie, C.J.; et al. Inflammatory Bowel Disease and Cardiovascular Diseases. Am. J. Med. 2022, 135, 1453–1460. [Google Scholar] [CrossRef]
- Stremmel, W.; Vural, H.; Evliyaoglu, O.; Weiskirchen, R. Wirksamkeit von darmlöslichem Lecithin (Phosphatidylcholin) zur Behandlung der Colitis ulcerosa: Eine Metaanalyse [Efficacy of enteric lecithin (phosphatidylcholine) in the treatment of ulcerative colitis: A meta-analysis]. MMW Fortschr. Med. 2022, 164 (Suppl. S7), 3–11. [Google Scholar] [CrossRef] [PubMed]
- Stremmel, W.; Ehehalt, R.; Autschbach, F.; Karner, M. Phosphatidylcholine for steroid-refractory chronic ulcerative colitis: A randomized trial. Ann. Intern. Med. 2007, 147, 603–610. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, B.-M.; Lee, S.H.; Sohn, J.-T.; Choi, J.W.; Cho, C.-W.; Hong, H.-D.; Rhee, Y.K.; Kim, H.-J. Ginseng-Induced Changes to Blood Vessel Dilation and the Metabolome of Rats. Nutrients 2020, 12, 2238. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-J.; Choi, S.-H.; Kim, H.-J.; Lee, B.-H.; Rhim, H.; Kim, H.-C.; Hwang, S.-H.; Nah, S.-Y. Bioactive Lipids in Gintonin-Enriched Fraction from Ginseng. J. Ginseng Res. 2019, 43, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, H.; Al-Darraji, A.; Abo-Aly, M.; Peng, H.; Shokri, E.; Chelvarajan, L.; Donahue, R.R.; Levitan, B.M.; Gao, E.; Hernandez, G.; et al. Autotaxin Inhibition Reduces Cardiac Inflammation and Mitigates Adverse Cardiac Remodeling after Myocardial Infarction. J. Mol. Cell. Cardiol. 2020, 149, 95–114. [Google Scholar] [CrossRef]
- Kawai, H.; Chaudhry, F.; Shekhar, A.; Petrov, A.; Nakahara, T.; Tanimoto, T.; Kim, D.; Chen, J.; Lebeche, D.; Blankenberg, F.G.; et al. Molecular Imaging of Apoptosis in Ischemia Reperfusion Injury With Radiolabeled Duramycin Targeting Phosphatidylethanolamine: Effective Target Uptake and Reduced Nontarget Organ Radiation Burden. JACC Cardiovasc. Imaging 2018, 11, 1823–1833. [Google Scholar] [CrossRef]
- Lukyanov, A.N.; Hartner, W.C.; Torchilin, V.P. Increased Accumulation of PEG-PE Micelles in the Area of Experimental Myocardial Infarction in Rabbits. J. Control. Release 2004, 94, 187–193. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, J.; Tuazon, J.P.; Borlongan, C.V.; Yu, G. MicroRNA-133a and Myocardial Infarction. Cell Transplant. 2019, 28, 831–838. [Google Scholar] [CrossRef]
- Galiñanes, M.; Goss, M.W.; McGill, C.J.; Hearse, D.J.; Brooks, G. Diacylglycerol-Induced Protection against Injury during Ischemia and Reperfusion in the Rat Heart: Comparative Studies with Ischemic Preconditioning. Int. J. Cardiol. 1998, 65, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Otani, H.; Kagaya, Y.; Imahori, Y.; Yasuda, S.; Fujii, R.; Chida, M.; Namiuchi, S.; Takeda, M.; Sakuma, M.; Watanabe, J.; et al. Myocardial 11C-Diacylglycerol Accumulation and Left Ventricular Remodeling in Patients after Myocardial Infarction. J. Nucl. Med. 2005, 46, 553–559. [Google Scholar] [PubMed]
- Paradies, G.; Petrosillo, G.; Paradies, V.; Reiter, R.J.; Ruggiero, F.M. Melatonin, Cardiolipin and Mitochondrial Bioenergetics in Health and Disease. J. Pineal Res. 2010, 48, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, N.; Sharma, D.; Gulbins, E.; Becker, K.A.; Edelmann, B. Inhibition of acid sphingomyelinase by tricyclic antidepressants and analogons. Front. Physiol. 2014, 5, 331. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lin, P.; Wang, X.; Zou, G.; Li, K. Sphingomyelin Phosphodiesterase 1 (SMPD1) Mediates the Attenuation of Myocardial Infarction-Induced Cardiac Fibrosis by Astaxanthin. Biochem. Biophys. Res. Commun. 2018, 503, 637–643. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.-Z.; Zhao, S.-H.; Ji, X.; Qiu, J.; Wang, J.; Zhou, Y.; Cai, Q.; Zhang, J.; Gao, H.-Q. Astaxanthin Attenuated Pressure Overload-Induced Cardiac Dysfunction and Myocardial Fibrosis: Partially by Activating SIRT1. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1715–1728. [Google Scholar] [CrossRef]
- Reforgiato, M.R.; Milano, G.; Fabriàs, G.; Casas, J.; Gasco, P.; Paroni, R.; Samaja, M.; Ghidoni, R.; Caretti, A.; Signorelli, P. Inhibition of Ceramide de Novo Synthesis as a Postischemic Strategy to Reduce Myocardial Reperfusion Injury. Basic Res. Cardiol. 2016, 111, 12. [Google Scholar] [CrossRef]
- Palmer, M.; Curran, J.; Bowler, P. Clinical experience and safety using phosphatidylcholine injections for the localized reduction of subcutaneous fat: A multicentre, retrospective UK study. J. Cosmet. Dermatol. 2006, 5, 218–226. [Google Scholar] [CrossRef]
- Knittelfelder, O.L.; Kohlwein, S.D. Quantitative Analysis of Yeast Phospholipids and Sterols by High-Performance Liquid Chromatography-Evaporative Light-Scattering Detection. Cold Spring Harb. Protoc. 2017, 5, 5472. [Google Scholar] [CrossRef]
- Bielawski, J.; Pierce, J.S.; Snider, J.; Rembiesa, B.; Szulc, Z.M.; Bielawska, A. Sphingolipid analysis by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Adv. Exp. Med. Biol. 2010, 688, 46–59. [Google Scholar] [CrossRef]
- Aldana, J.; Romero-Otero, A.; Cala, M.P. Exploring the Lipidome: Current Lipid Extraction Techniques for Mass Spectrometry Analysis. Metabolites 2020, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Engel, K.M.; Prabutzki, P.; Leopold, J.; Nimptsch, A.; Lemmnitzer, K.; Vos, D.R.N.; Hopf, C.; Schiller, J. A new update of MALDI-TOF mass spectrometry in lipid research. Prog. Lipid Res. 2022, 86, 101145. [Google Scholar] [CrossRef] [PubMed]
- Hyötyläinen, T.; Orešič, M. Optimizing the lipidomics workflow for clinical studies--practical considerations. Anal. Bioanal. Chem. 2015, 407, 4973–4993. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Levin, S.M.; Barnard, N.D. Association between plant-based diets and plasma lipids: A systematic review and meta-analysis. Nutr. Rev. 2017, 75, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Desnoyers, M.; Gilbert, K.; Rousseau, G. Cardioprotective Effects of Omega-3 Polyunsaturated Fatty Acids: Dichotomy between Experimental and Clinical Studies. Mar. Drugs 2018, 16, 234. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L.; Rosenblatt, S.; de Mattos, G.F.; Settineri, R.; Breeding, P.C.; Ellithorpe, R.R.; Ash, M.E. Clinical Uses of Membrane Lipid Replacement Supplements in Restoring Membrane Function and Reducing Fatigue in Chronic Diseases and Cancer. Discoveries 2016, 4, e54. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; Eckel, R.H. Lipids, Lipoproteins, and Cardiovascular Disease: Clinical Pharmacology Now and in the Future. J. Clin. Endocrinol. Metab. 2016, 101, 804–814. [Google Scholar] [CrossRef]
| Phospholipid | Localization | Function | Experimental Design |
|---|---|---|---|
| PC | The outer surface of cell membrane | Membrane integrity | Human study [10] |
| Liver | Generation of HDL, VLDL | Human study [11] | |
| DPPC; PC | Lungs | Pulmonary surfactant component [12] | Ztm:MF1 mice [13]; Male Sprague Dawley rats [13]; Mixed-breed York-Pyatrain-Landrace pigs [13]; Human study [13] |
| PC | Colon | Protection against bacterial infections | Male rats [14] |
| Colon | Possible cause of ulcerative colitis | Porcine lipid model [15] | |
| Cardiovascular system | Increases all-cause and cardiovascular mortality | Human study [16] | |
| PA/LPA | Serum Plasma Aqueous humor | Intracellular signaling | Human and animal studies [17] |
| LPA | Heart | Suppression of fibrogenesis | (Lpar2-KO) MI mice model [18] |
| Heart | Stimulation of myocardial cell proliferation | LPA3 and LPA1 knockout mice [19]; Neonatal Sprague Dawley rats [19] | |
| PE | Skeletal muscle | Influences insulin sensitivity | Animal models [10] |
| Brain | Reduces α-synuclein accumulation and the formation of Lewy bodies in Parkinson’s disease | Yeast; Caenorhabditis elegans worm models [20] | |
| Heart | Differentiation of P19 teratocarcinoma cells into cardiomyocytes | Cell culture [21] | |
| PS | The inner layer of the plasma membrane | Apoptosis signaling | Cell culture [22] |
| Cellular level | Activates protein kinases C | Cell culture [23,24] | |
| Skeletal muscle | Sports performance enhancement | Human study [25] | |
| Inner layer of plasma membrane | Inflammation assessment through ultrasound techniques | Wild-type C57Bl/6 mice [26] | |
| PI | Cardiomyocytes | Regulation of T-tubules and Ca2+ handling | Isolated Sprague Dawley, Wistar Kyoto and spontaneously hypertensive rat hearts [27]; Isolated human hearts [27] |
| Heart | Hypertrophy, heart failure and diabetic cardiomyopathy | Isolated Sprague Dawley, Wistar Kyoto and spontaneously hypertensive rat hearts [27]; Isolated human hearts [27]; Stroke-prone spontaneously hypertensive rats [28]; Wistar-Kyoto rats [28]; FVB/N male mice [29] | |
| DAG | Heart | Linked to cardiac hypertrophy | Animal model [30] |
| CL | Inner membrane of the mitochondria | Mitochondrial bioenergetic metabolism | Human study [31,32,33,34,35,36]; Animal model [37] |
| Heart | Development of inherited disorders | ||
| Involved in ischemia/reperfusion injury and heart failure | |||
| SM | Brain | Myelination Regulation of the chromatin function | Experimental and human studies [38] |
| Heart | Development of coronary heart disease | Human study [39] | |
| Development of heart failure | Human study [40] | ||
| Cer | Cellular level | Cell growth Cell differentiation Senescence Apoptosis | Experimental and human studies [41,42]; C57BL mice [43] |
| Heart | Involved in the development of atherosclerosis and valvular diseases | ApoE-KO mice; murine model [44] | |
| Correlates with plaque rupture and the severity of coronary artery stenosis | Human study [45] | ||
| S1P | In plasma, transported by HDL and albumin | Intracellular signaling | Animal, cellular and human studies [46,47] |
| Involved in atherosclerosis | Human study [48] | ||
| Liver | Fibrogenesis | HPPCnliver+/+ transgenic FVB mice [49] |
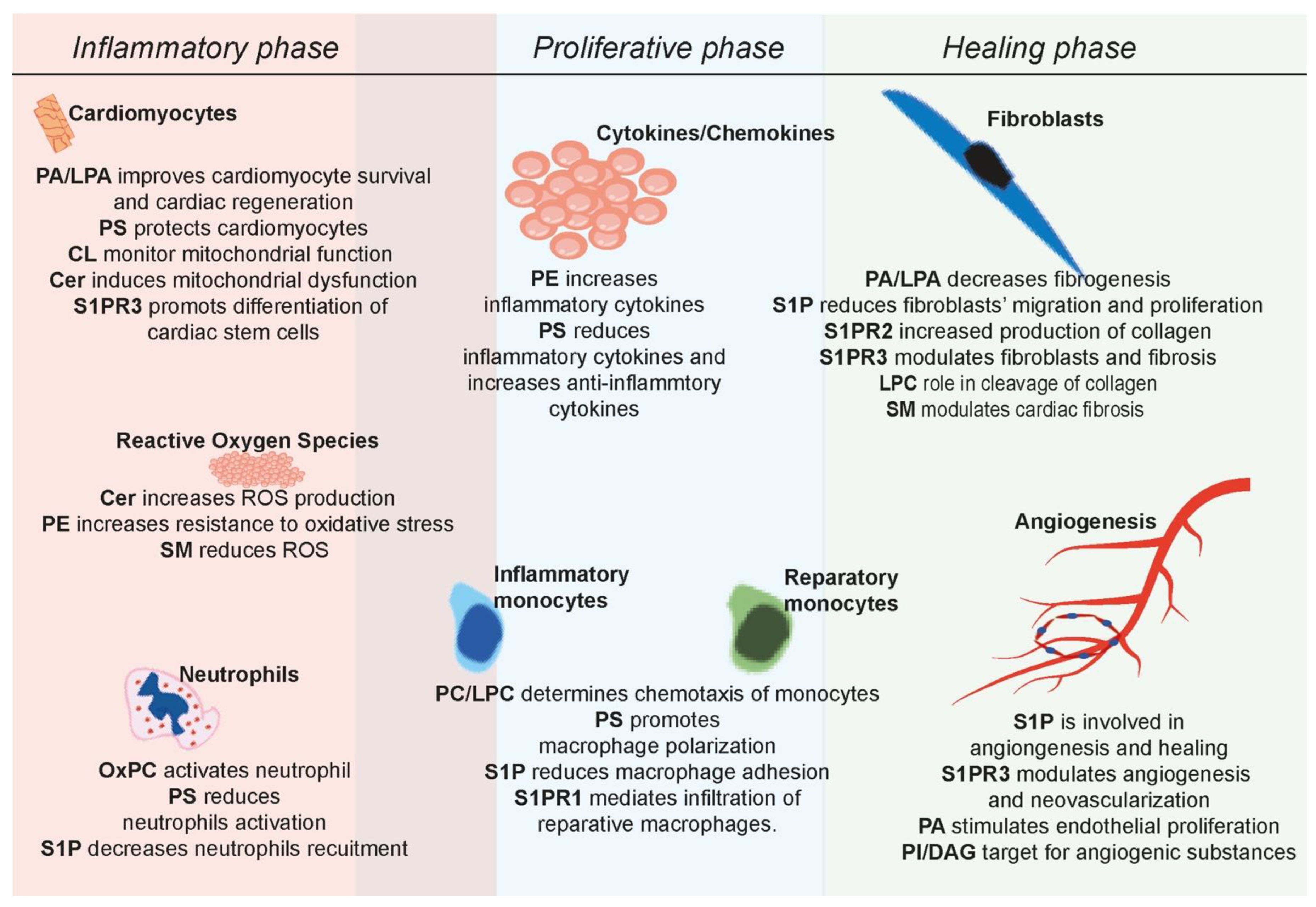
| Phospholipid | Function | Experimental Design |
|---|---|---|
| PC (OxPC) | Elevated in the plasma of STEMI patients | Human study [114] |
| Associated with increased scar size and ventricular remodeling | Adult rat ventricular cardiomyocytes [115] | |
| Activates neutrophils | Human neutrophils [116] | |
| Induces ferroptosis | Adult rat ventricular cardiomyocytes [117] | |
| LPC | Biomarker for CVDs (i.e., MI, atherosclerosis and diabetes) | Human study [120] |
| Chemotaxis of monocytes and macrophages | Human and mouse monocytes, mouse model [121] | |
| PA | Increases the intracellular concentration of free Ca2+ in adult cardiomyocytes; Auguments inotropism | Isolated rat cardiomyocytes, rat model [123]; Isolated Male Sprague Dawley rat cardiomyocytes [124] |
| Stimulates protein synthesis in cardiomyocytes through augmentation of PLC and protein kinase C activity | Cell culture [125]; Male Sprague Dawley rats MI model [126,127] | |
| PA-α1-microglobulin complex stimulates inflammation, macrophage migration and polarization and inhibits fibrogeneiss in the infarct border area | Mouse MI model [128] | |
| LPA | Encourages cardiac function | LPA3 and LPA1 knockout mice; neonatal Sprague Dawley rats [19] |
| Lessens fibrosis and ventricular remodeling after MI | (Lpar2-KO) MI mice model [18] | |
| Increases angiogenesis and endothelial cell proliferation and functionality | ||
| PE | PE-α1-microglobulin complex stimulates inflammationby increasing the mRNA expression of inflammatory cytokines and chemokines, decreasing α-smooth muscle actin and collagen 3a1 | Mouse MI model [128] |
| Induces ferroptosis | Cardiomyocyte cell culture [102] | |
| Protein synthesis as a lipid and chaperon | Cellular, plant and animal models and human studies [129] | |
| Triggers autophagy | ||
| Increases the resistance to oxidative stress | Caenorhabditis elegans worm models [130] | |
| Involved in uncoupling protein 1-dependent respiration without compromising electron transfer efficiency or ATP synthesis | Animal model [131] | |
| PS | Cardioprotection | Mouse MI model [132,133] |
| Reduces neutrophil activation | ||
| Protects against diabetes | Animal study [134] | |
| Anti-inflammatory activity by inhibiting phosphorylation of MAPKs | RAW264.7 macrophages culture [135] | |
| PS-containing liposomes | Protects against type 1 and type 2 diabetes | Animal study [134] |
| Modulate the monocyte phenotype | Mouse, rat and human cellular models, mouse, rat and pig myocardial I/R models, mouse and rat MI models, human studies [136] | |
| OxPS | Inhibits macrophage production of NO and IL-1β transcription | RAW264.7 macrophage culture [137] |
| PI | Ischemic preconditioning | Human study [105] |
| Cardioprotection | Transgenic mice [138] | |
| Development of different types of cardiomyopathies | Isolated Sprague Dawley, Wistar Kyoto and spontaneously hypertensive rat hearts [27]; Isolated human hearts [27]; Stroke-prone spontaneously hypertensive rats [28]; Wistar-Kyoto rats [28]; FVB/N male mice [29] | |
| Main promoter of angiogenesis in the infarcted heart | Various animal models (zebrafish, chicken embryos, mice) [139] | |
| PI turnover seems to correlate with myocyte hypertrophy and increased performance | Mouse MI model [140] | |
| DAG | Left ventricular remodeling | Mouse MI model [140] |
| Involved in post-myocardial infarction dysfunction and mortality | ||
| Preconditioning | Animal models; human myocytes [141] | |
| Enhances tolerance to ischemia/reperfusion injury | Transgenic mice ischemia model [142] | |
| CL | Its alteration increases mitochondrial dysfunction, ROS production and apoptosis | Rat MI model [144] ALCAT1-KO MI mice model [145] |
| SM | Lowers rate of neonatal lethality | Sphingomyelin synthase (SMS)-KO mice; animal studies [76] |
| Increases insulin secretion, inflammatory signals and atherosclerosis | ||
| Increases inflammatory signals | ||
| Protects against ROS accumulation and mitochondrial dysfunction | ||
| Cer | Apoptosis and autophagy | Animal studies [76] |
| Increases ROS production in myocardial ischemia/reperfusion injury | Mouse ischemia model [147,148] | |
| Lower levels following cardioprotection through ischemic preconditioning | Rabbit ischemia model [149] | |
| High levels of Cer in post-infarcted human myocardium | Mouse MI model [150] | |
| Increases cell death | ||
| Increases fibrosis and worsening of heart function post-MI | Human study [151,152] | |
| S1P | Cardioprotective effects | Cellular and animal models, human studies [154]; Cellular and animal models (mouse, rat) [153] |
| Anti-inflammatory effects during healing after myocardial infarction | In vivo mouse model of myocardial ischemi/reperfusion [155] | |
| Main modulator of angiogenesis during scar formation | Animal model [76]; Humans, human cells, mouse MI/reperfusion model, rat cardiomyocytes, isolated and perfused rat hearts [156]; patient-derived endothelial progenitor cells and mouse model of hind limb ischemia [157] | |
| Controls vascular tone, endothelial and smooth muscle cell proliferation | Animal model [76]; Humans, human cells, mouse MI/reperfusion model, rat cardiomyocytes, isolated and perfused rat hearts [156]; Patient-derived endothelial progenitor cells and mouse model of hind limb ischemia [157] | |
| Activates CXCR4 phosphorylation and Jak2 phosphorylation | Patient-derived endothelial progenitor cells and mouse model of hind limb ischemia [157] | |
| Improves endothelial homeostasis together with ApoM | Cellular and animal models, human studies [153] | |
| Enhances the recruitment of bone-marrow-derived progenitor cells to the infarcted myocardium | Mouse MI model [160] | |
| Reduces ventricular remodeling and infarction scar | ||
| Binding of S1P to S1PR1 affects reparative macrophage accumulation at later stages post-myocardial infarction | Mouse model of MI and parabiosis [146] | |
| Binding S1P to S1PR2 mediates recruitment of muse cells into the infarcted areas, reducing infarct size and improving heart function | Rabbit model of AMI and human and rabbit muse cells [161] | |
| Binding of S1P to S1PR3 in fibroblasts increases migration and proliferation | Humans, human cells, mouse MI/reperfusion model, rat cardiomyocytes, isolated and perfused rat hearts [162]; rat MI model [156,162] | |
| Modulates the production of collagen | Humans, human cells, mouse MI/reperfusion model, rat cardiomyocytes, isolated and perfused rat hearts [156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pistritu, D.-V.; Vasiliniuc, A.-C.; Vasiliu, A.; Visinescu, E.-F.; Visoiu, I.-E.; Vizdei, S.; Martínez Anghel, P.; Tanca, A.; Bucur, O.; Liehn, E.A. Phospholipids, the Masters in the Shadows during Healing after Acute Myocardial Infarction. Int. J. Mol. Sci. 2023, 24, 8360. https://doi.org/10.3390/ijms24098360
Pistritu D-V, Vasiliniuc A-C, Vasiliu A, Visinescu E-F, Visoiu I-E, Vizdei S, Martínez Anghel P, Tanca A, Bucur O, Liehn EA. Phospholipids, the Masters in the Shadows during Healing after Acute Myocardial Infarction. International Journal of Molecular Sciences. 2023; 24(9):8360. https://doi.org/10.3390/ijms24098360
Chicago/Turabian StylePistritu, Dan-Valentin, Anisia-Cristiana Vasiliniuc, Anda Vasiliu, Elena-Florentina Visinescu, Ioana-Elena Visoiu, Smaranda Vizdei, Paula Martínez Anghel, Antoanela Tanca, Octavian Bucur, and Elisa Anamaria Liehn. 2023. "Phospholipids, the Masters in the Shadows during Healing after Acute Myocardial Infarction" International Journal of Molecular Sciences 24, no. 9: 8360. https://doi.org/10.3390/ijms24098360






