Low-Temperature Plasma Techniques in Biomedical Applications and Therapeutics: An Overview
Abstract
:1. Introduction
2. Introduction Low-Temperature Plasma on Medicine
2.1. Low-Temperature Plasma Pathogen Inactivation and Antimicrobial Therapy
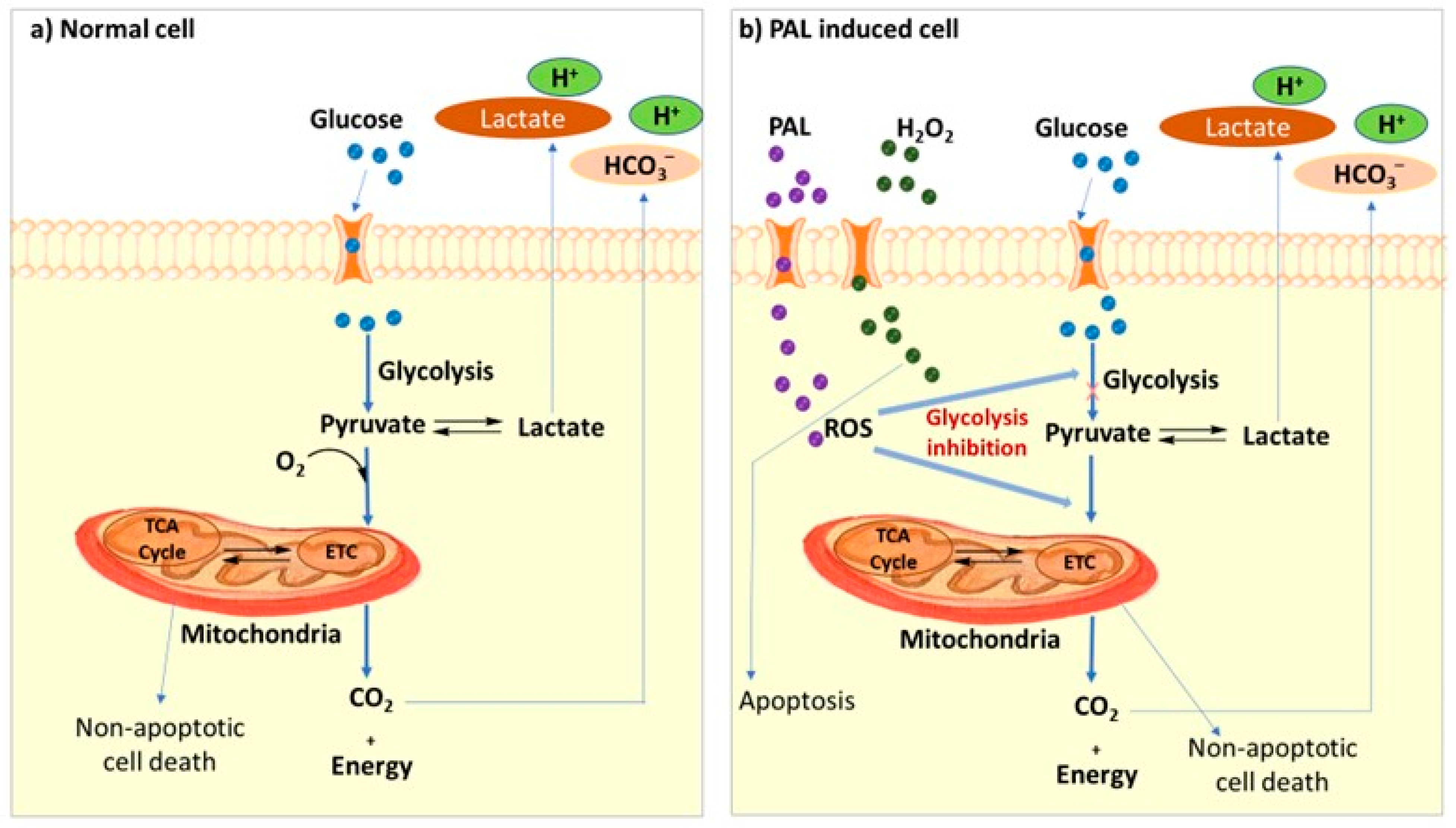
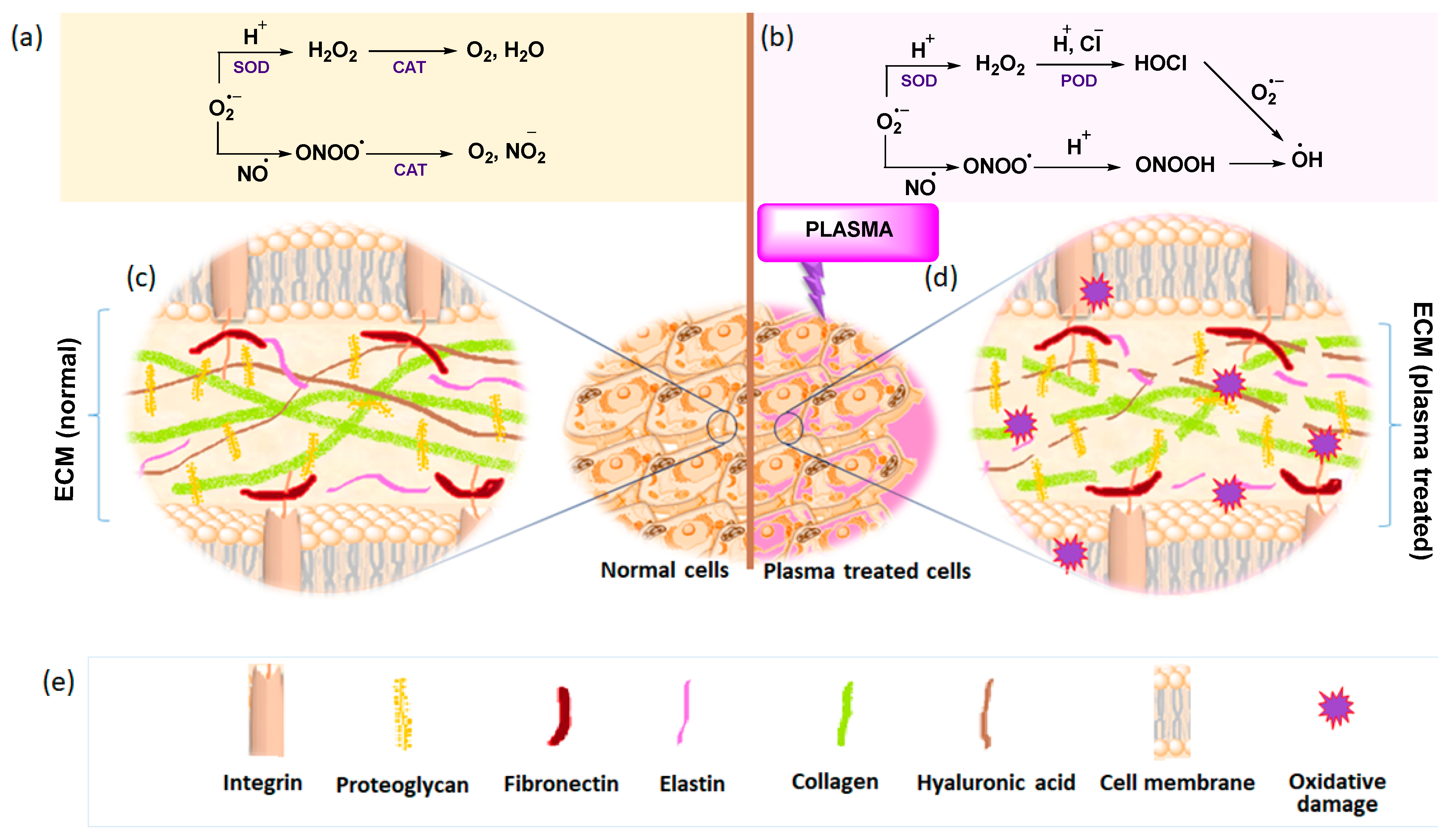
2.2. Cancer Treatment with Low-Temperature Plasma
2.3. Plasma-Induced Changes in the Chemical Structure of Biomolecules
2.4. Low-Temperature Plasma-Assisted Drug/Gene Delivery Systems and Tissue Engineering
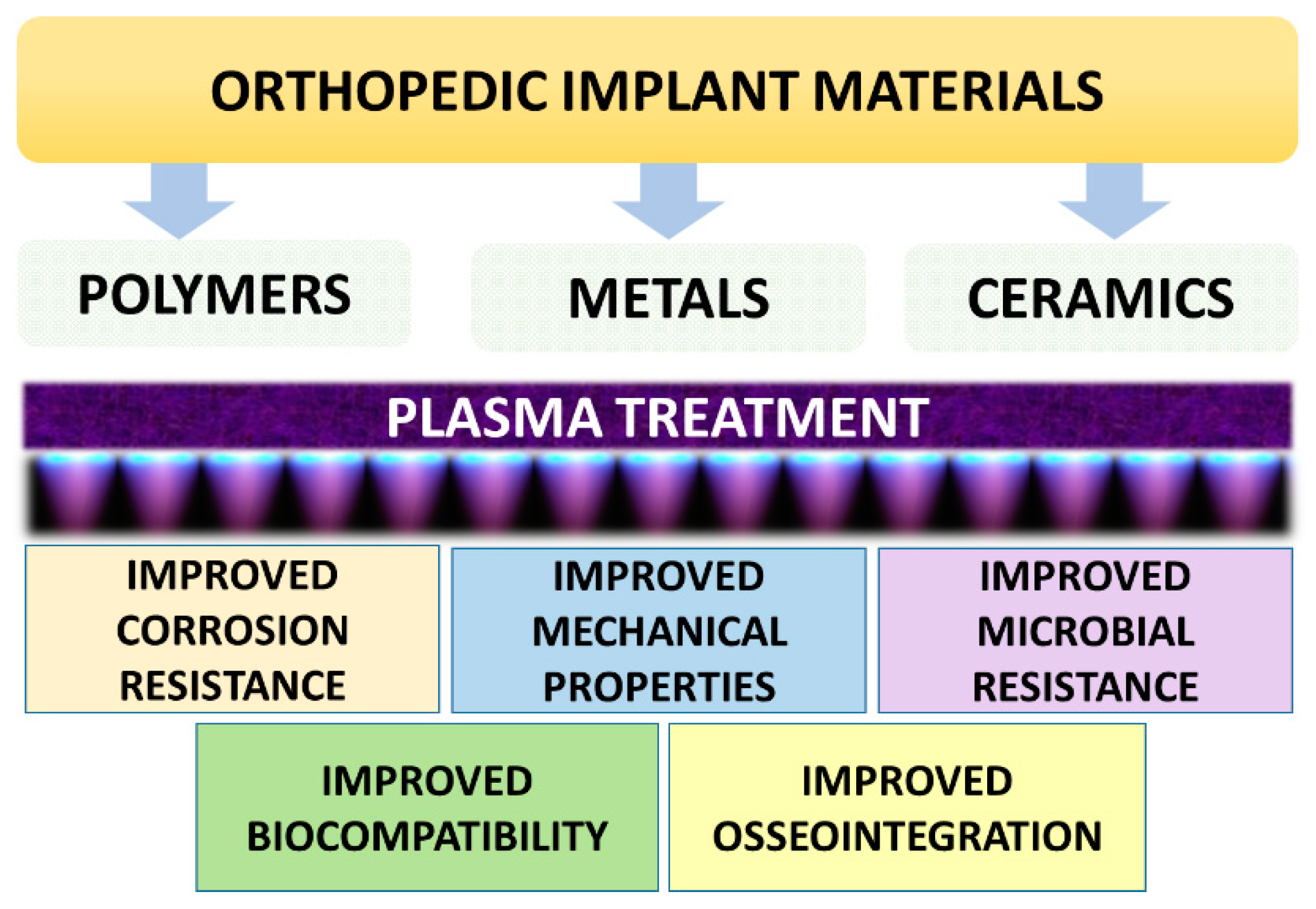
3. Low-Temperature Plasma on Medicinal Implants
3.1. Low-Temperature Plasma-Based Orthopedic Implants
3.2. Low-Temperature Plasma: Application in Dentistry
4. Low-Temperature Plasma-Assisted Biomaterials Process in Alabama
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernhardt, T.; Semmler, M.L.; Schäfer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. Plasma Medicine: Applications of Cold Atmospheric Pressure Plasma in Dermatology. Oxidative Med. Cell. Longev. 2019, 2019, 3873928. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M. Cold Plasma in Medicine and Healthcare: The New Frontier in Low Temperature Plasma Applications. Front. Phys. 2020, 8, 74. [Google Scholar] [CrossRef]
- Kashif, C.; Auwal Mustapha, I.; Syed Zuhaib Haider, R.; Jalil, A. Plasma Kinetic Theory. In Kinetic Theory; George, Z.K., Athanasios, C.M., Eds.; IntechOpen: Rijeka, Croatia, 2017; p. Ch. 7. [Google Scholar]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef] [PubMed]
- Szente, R.N. Industrial applications of thermal plasmas. AIP Conf. Proc. 1995, 345, 487–494. [Google Scholar]
- Samal, S. Thermal plasma technology: The prospective future in material processing. J. Clean. Prod. 2017, 142, 3131–3150. [Google Scholar] [CrossRef]
- Kortshagen, U.R.; Sankaran, R.M.; Pereira, R.N.; Girshick, S.L.; Wu, J.J.; Aydil, E.S. Nonthermal Plasma Synthesis of Nanocrystals: Fundamental Principles, Materials, and Applications. Chem. Rev. 2016, 116, 11061–11127. [Google Scholar] [CrossRef] [PubMed]
- Neyts, E.C.; Ostrikov, K.; Sunkara, M.K.; Bogaerts, A. Plasma Catalysis: Synergistic Effects at the Nanoscale. Chem. Rev. 2015, 115, 13408–13446. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, G.; Obenchain, R.; Zhang, R.; Bai, F.; Fang, T.; Wang, H.; Lu, Y.; Wirz, R.E.; Gu, Z. Cold atmospheric plasma delivery for biomedical applications. Mater. Today 2022, 54, 153–188. [Google Scholar] [CrossRef]
- Gao, L.; Shi, X.; Wu, X. Applications and challenges of low temperature plasma in pharmaceutical field. J. Pharm. Anal. 2021, 11, 28–36. [Google Scholar] [CrossRef]
- Naujokat, H.; Harder, S.; Schulz, L.Y.; Wiltfang, J.; Flörke, C.; Açil, Y. Surface conditioning with cold argon plasma and its effect on the osseointegration of dental implants in miniature pigs. J. Cranio-Maxillofac. Surg. 2019, 47, 484–490. [Google Scholar] [CrossRef]
- Pańka, D.; Jeske, M.; Łukanowski, A.; Baturo-Cieśniewska, A.; Prus, P.; Maitah, M.; Maitah, K.; Malec, K.; Rymarz, D.; Muhire, J.D.; et al. Can Cold Plasma Be Used for Boosting Plant Growth and Plant Protection in Sustainable Plant Production? Agronomy 2022, 12, 841. [Google Scholar] [CrossRef]
- Rao, W.; Li, Y.; Dhaliwal, H.; Feng, M.; Xiang, Q.; Roopesh, M.S.; Pan, D.; Du, L. The Application of Cold Plasma Technology in Low-Moisture Foods. Food Eng. Rev. 2023, 15, 86–112. [Google Scholar] [CrossRef]
- Makabe, T.; Petrovic, Z.L. Plasma Electronics: Applications in Microelectronic Device Fabrication, 2nd ed.; Routeledge Taylor & Francis Group: London, UK, 2016; p. 412. [Google Scholar]
- Huang, Y.-W.; Yu, Q.-F.; Li, M.; Sun, S.-N.; Zhao, H.; Jin, S.-X.; Fan, J.; Wang, J.-G. An overview of low-temperature plasma surface modification of carbon materials for removal of pollutants from liquid and gas phases. Plasma Process. Polym. 2021, 18, 2000171. [Google Scholar] [CrossRef]
- Mumtaz, S.; Khan, R.; Rana, J.N.; Javed, R.; Iqbal, M.; Choi, E.H.; Han, I. Review on the Biomedical and Environmental Applications of Nonthermal Plasma. Catalysts 2023, 13, 685. [Google Scholar] [CrossRef]
- von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Weltmann, K.D.; von Woedtke, T. Plasma medicine—Current state of research and medical application. Plasma Phys. Control. Fusion 2017, 59, 014031. [Google Scholar] [CrossRef]
- Takamatsu, T.; Uehara, K.; Sasaki, Y.; Miyahara, H.; Matsumura, Y.; Iwasawa, A.; Ito, N.; Azuma, T.; Kohno, M.; Okino, A. Investigation of reactive species using various gas plasmas. RSC Adv. 2014, 4, 39901–39905. [Google Scholar] [CrossRef]
- Wen, X.; Xin, Y.; Hamblin, M.R.; Jiang, X. Applications of cold atmospheric plasma for transdermal drug delivery: A review. Drug Deliv. Transl. Res. 2021, 11, 741–747. [Google Scholar] [CrossRef]
- Das, S.; Gajula, V.P.; Mohapatra, S.; Singh, G.; Kar, S. Role of cold atmospheric plasma in microbial inactivation and the factors affecting its efficacy. Health Sci. Rev. 2022, 4, 100037. [Google Scholar] [CrossRef]
- Ma, C.; Nikiforov, A.; De Geyter, N.; Morent, R.; Ostrikov, K. Plasma for biomedical decontamination: From plasma-engineered to plasma-active antimicrobial surfaces. Curr. Opin. Chem. Eng. 2022, 36, 100764. [Google Scholar] [CrossRef]
- Karthik, C.; Rajalakshmi, S.; Thomas, S.; Thomas, V. Intelligent polymeric biomaterials surface driven by plasma processing. Curr. Opin. Biomed. Eng. 2023, 26, 100440. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, D.; Qiu, R.; Tang, Y.; Du, C. Non-thermal plasma technology for organic contaminated soil remediation: A review. Chem. Eng. J. 2017, 313, 157–170. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Y.-S.; Zhang, S.-Q.; Ding, Y.-W.; Gao, M. Improving effects of low-temperature atmospheric plasma on abdominal surgical site infection induced by ESBL-E. coli in rats. AIP Adv. 2022, 12, 075115. [Google Scholar] [CrossRef]
- Li, Y.-F.; Shimizu, T.; Zimmermann, J.L.; Morfill, G.E. Cold Atmospheric Plasma for Surface Disinfection. Plasma Process. Polym. 2012, 9, 585–589. [Google Scholar] [CrossRef]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; van Dijk, J.; Zimmermann, J.L. Plasma medicine: An introductory review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Kogelschatz, U.; Eliasson, B.; Egli, W. Dielectric-Barrier Discharges. Principle and Applications. J. Phys. IV Proc. 1997, 07, C4–C47. [Google Scholar] [CrossRef]
- Laroussi, M.; Lu, X. Room-temperature atmospheric pressure plasma plume for biomedical applications. Appl. Phys. Lett. 2005, 87, 113902. [Google Scholar] [CrossRef]
- Maisch, T.; Shimizu, T.; Isbary, G.; Heinlin, J.; Karrer, S.; Klämpfl Tobias, G.; Li, Y.-F.; Morfill, G.; Zimmermann Julia, L. Contact-Free Inactivation of Candida albicans Biofilms by Cold Atmospheric Air Plasma. Appl. Environ. Microbiol. 2012, 78, 4242–4247. [Google Scholar] [CrossRef]
- von Woedtke, T.; Metelmann, H.R.; Weltmann, K.D. Clinical Plasma Medicine: State and Perspectives of in Vivo Application of Cold Atmospheric Plasma. Contrib. Plasma Phys. 2014, 54, 104–117. [Google Scholar] [CrossRef]
- Lu, X.; Ye, T.; Cao, Y.; Sun, Z.; Xiong, Q.; Tang, Z.; Xiong, Z.; Hu, J.; Jiang, Z.; Pan, Y. The roles of the various plasma agents in the inactivation of bacteria. J. Appl. Phys. 2008, 104, 053309. [Google Scholar] [CrossRef]
- Kostov, K.G.; Rocha, V.; Koga-Ito, C.Y.; Matos, B.M.; Algatti, M.A.; Honda, R.Y.; Kayama, M.E.; Mota, R.P. Bacterial sterilization by a dielectric barrier discharge (DBD) in air. Surf. Coat. Technol. 2010, 204, 2954–2959. [Google Scholar] [CrossRef]
- Kim, S.J.; Chung, T.H.; Bae, S.H.; Leem, S.H. Bacterial inactivation using atmospheric pressure single pin electrode microplasma jet with a ground ring. Appl. Phys. Lett. 2009, 94, 141502. [Google Scholar] [CrossRef]
- Sharma, A.; Pruden, A.; Stan, O.; Collins, G.J. Bacterial Inactivation Using an RF-Powered Atmospheric Pressure Plasma. IEEE Trans. Plasma Sci. 2006, 34, 1290–1296. [Google Scholar] [CrossRef]
- Wiegand, C.; Fink, S.; Hipler, U.C.; Beier, O.; Horn, K.; Pfuch, A.; Schimanski, A.; Grünler, B. Cold atmospheric pressure plasmas exhibit antimicrobial properties against critical bacteria and yeast species. J. Wound Care 2017, 26, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Miletić, M.; Vuković, D.; Živanović, I.; Dakić, I.; Soldatović, I.; Maletić, D.; Lazović, S.; Malović, G.; Petrović, Z.; Puač, N. Inhibition of methicillin resistant Staphylococcus aureus by a plasma needle. Cent. Eur. J. Phys. 2014, 12, 160–167. [Google Scholar] [CrossRef]
- Liu, X.; Feng, H.; Ying, G.; Jianjun, S. Sterilization of Staphylococcus Aureus by an Atmospheric Non-Thermal Plasma Jet. Plasma Sci. Technol. 2013, 15, 439. [Google Scholar] [CrossRef]
- Lim, J.-P.; Uhm, H.S.; Li, S.-Z. Influence of oxygen in atmospheric-pressure argon plasma jet on sterilization of Bacillus atrophaeous spores. Phys. Plasmas 2007, 14, 093504. [Google Scholar] [CrossRef]
- Li, S.; Lim, J. Comparison of Sterilizing Effect of Nonequilibrium Atmospheric-Pressure He/O2 and Ar/O2 Plasma Jets. Plasma Sci. Technol. 2008, 10, 61. [Google Scholar]
- Xu, G.; Zhang, G.; Shi, X.; Ma, Y.; Wang, N.; Li, Y. Bacteria Inactivation Using DBD Plasma Jet in Atmospheric Pressure Argon. Plasma Sci. Technol. 2009, 11, 83. [Google Scholar]
- Wang, L.; Xia, C.; Guo, Y.; Yang, C.; Cheng, C.; Zhao, J.; Yang, X.; Cao, Z. Bactericidal efficacy of cold atmospheric plasma treatment against multidrug-resistant Pseudomonas aeruginosa. Future Microbiol. 2020, 15, 115–125. [Google Scholar] [CrossRef]
- Martines, E.; Brun, P.; Brun, P.; Cavazzana, R.; Deligianni, V.; Leonardi, A.; Tarricone, E.; Zuin, M. Towards a plasma treatment of corneal infections. Clin. Plasma Med. 2013, 1, 17–24. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, S.H.; Ju, H.W.; Lee, H.; Lee, Y.; Park, S.; Yang, H.; Park, S.-J.; Eden, J.G.; Yang, J.; et al. Microplasma Jet Arrays as a Therapeutic Choice for Fungal Keratitis. Sci. Rep. 2018, 8, 2422. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, G.; Moser, D.; Müller, S.; Pfister, W.; Sculean, A.; Eick, S. The Antimicrobial Effect of Cold Atmospheric Plasma against Dental Pathogens—A Systematic Review of In-Vitro Studies. Antibiotics 2021, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Boopathy, B.; Radhakrishnan, M.; Rao, L.; Schlüter, O.K.; Tiwari, B.K. Plasma processing: A sustainable tech-nology in agri-food processing. Sustain. Food Technol. 2023, 1, 9–49. [Google Scholar] [CrossRef]
- Bhatt, K.; Mayo, J.T.; Xu, G.; Ramsey, D.M.; Sysoeva, T.A. Topical: Considerations for Use of Low-Temperature Gas Plasmas for Mitigation of Biofilms in Microgravity Environments; The University of Alabama in Huntsville: Huntsville, AL, USA, 2021. [Google Scholar]
- Panariello, B.H.D.; Mody, D.P.; Eckert, G.J.; Witek, L.; Coelho, P.G.; Duarte, S. Low-Temperature Plasma Short Exposure to Decontaminate Peri-Implantitis-Related Multispecies Biofilms on Titanium Surfaces In Vitro. BioMed Res. Int. 2022, 2022, 1549774. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, G.; Favaro, L.; Müller, S.; Sculean, A.; Eick, S. The In-Vitro Activity of a Cold Atmospheric Plasma Device Utilizing Ambient Air against Bacteria and Biofilms Associated with Periodontal or Peri-Implant Diseases. Antibiotics 2022, 11, 752. [Google Scholar] [CrossRef] [PubMed]
- Matthes, R.; Lührman, A.; Holtfreter, S.; Kolata, J.; Radke, D.; Hübner, N.O.; Assadian, O.; Kramer, A. Antibacterial activity of cold atmospheric pressure argon plasma against 78 genetically different (mecA, luk-P, agr or Capsular Polysaccharide Type) staphylococcus aureus strains. Ski. Pharmacol. Physiol. 2016, 29, 83–91. [Google Scholar] [CrossRef]
- Laroussi, M.; Bekeschus, S.; Keidar, M.; Bogaerts, A.; Fridman, A.; Lu, X.; Ostrikov, K.; Hori, M.; Stapelmann, K.; Miller, V.; et al. Low Temperature Plasma for Biology, Hygiene, and Medicine: Perspective and Roadmap. IEEE Trans. Radiat. Plasma Med. Sci. 2021, 6, 127–157. [Google Scholar] [CrossRef]
- Dahle, S.; Žigon, J.; Fink, R. Cold plasma for sustainable control of hygienically relevant biofilms. The interaction of plasma distance and exposure time. Int. J. Environ. Health Res. 2022, 1–15. [Google Scholar] [CrossRef]
- Shahidi, S.; Rashidi, A.; Wiener, J.; Ghoranneviss, M. Antibacterial activity on polyamide and natural fabrics using low temperature plasma. NSTI Nanotechnol. Conf. Expo NSTI-Nanotech 2009, 3, 210–214. [Google Scholar]
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.J.; Gilmore, B.F. Microbiological interactions with cold plasma. J. Appl. Microbiol. 2017, 123, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Theinkom, F.; Singer, L.; Cieplik, F.; Cantzler, S.; Weilemann, H.; Cantzler, M.; Hiller, K.A.; Maisch, T.; Zimmermann, J.L. Antibacterial efficacy of cold atmospheric plasma against Enterococcus faecalis planktonic cultures and biofilms in vitro. PLoS ONE 2019, 14, e0223925. [Google Scholar] [CrossRef] [PubMed]
- Małajowicz, J.; Khachatryan, K.; Kozłowska, M. Properties of Water Activated with Low-Temperature Plasma in the Context of Microbial Activity. Beverages 2022, 8, 63. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Shaji, M.; Rabinovich, A.; Surace, M.; Sales, C.; Fridman, A. Physical Properties of Plasma-Activated Water. Plasma 2023, 6, 45–57. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 38610. [Google Scholar] [CrossRef] [PubMed]
- Mai-Prochnow, A.; Zhou, R.; Zhang, T.; Ostrikov, K.; Mugunthan, S.; Rice, S.A.; Cullen, P.J. Interactions of plasma-activated water with biofilms: Inactivation, dispersal effects and mechanisms of action. NPJ Biofilms Microbiomes 2021, 7, 11. [Google Scholar] [CrossRef]
- Hong, Q.; Dong, X.; Yu, H.; Sun, H.; Chen, M.; Wang, Y.; Yu, Q. The Antimicrobial Property of Plasma Activated Liquids (PALs) against Oral Bacteria Streptococcus mutans. Dental 2021, 3, 1–7. [Google Scholar] [CrossRef]
- Machala, Z.; Graves, D.B. Frugal Biotech Applications of Low-Temperature Plasma. Trends Biotechnol. 2018, 36, 579–581. [Google Scholar] [CrossRef]
- Pavlovich, M.J.; Chang, H.-W.; Sakiyama, Y.; Clark, D.S.; Graves, D.B. Ozone correlates with antibacterial effects from indirect air dielectric barrier discharge treatment of water. J. Phys. D Appl. Phys. 2013, 46, 145202. [Google Scholar] [CrossRef]
- Nițescu, B.; Pițigoi, D.; Tălăpan, D.; Nițescu, M.; Aramă, S.Ș.; Pavel, B.; Streinu-Cercel, A.; Rafila, A.; Aramă, V. Etiology and Multi-Drug Resistant Profile of Bacterial Infections in Severe Burn Patients, Romania 2018–2022. Medicina 2023, 59, 1143. [Google Scholar] [PubMed]
- Dokter, J.; Brusselaers, N.; Hendriks, W.D.; Boxma, H. Bacteriological cultures on admission of the burn patient: To do or not to do, that’s the question. Burns 2016, 42, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Brehmer, F.; Haenssle, H.A.; Daeschlein, G.; Ahmed, R.; Pfeiffer, S.; Görlitz, A.; Simon, D.; Schön, M.P.; Wandke, D.; Emmert, S. Alleviation of chronic venous leg ulcers with a hand-held dielectric barrier discharge plasma generator (PlasmaDerm® VU-2010): Results of a monocentric, two-armed, open, prospective, randomized and controlled trial (NCT01415622). J. Eur. Acad. Dermatol. Venereol. 2015, 29, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhuang, J.; Woedtke, T.V.; Kolb, J.F.; Weltmann, K.D.; Zhang, J.; Fang, J. Synergistic antibacterial effects of low temperature plasma combined with pulsed electric fields. In Proceedings of the 2014 IEEE 41st International Conference on Plasma Sciences (ICOPS) Held with 2014 IEEE International Conference on High-Power Particle Beams (BEAMS), Washington, DC USA, 25–29 May 2014; p. 1. [Google Scholar]
- Harley, J.C.; Suchowerska, N.; McKenzie, D.R. Cancer treatment with gas plasma and with gas plasma–activated liquid: Positives, potentials and problems of clinical translation. Biophys. Rev. 2020, 12, 989–1006. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, E.; Ptasińska, S. Recent Advances in Plasma-Based Cancer Treatments: Approaching Clinical Translation through an Intracellular View. Biophysica 2021, 1, 48–72. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Seebauer, C.; Miller, V.; Fridman, A.; Bauer, G.; Graves, D.B.; Pouvesle, J.-M.; Rutkowski, R.; Schuster, M.; Bekeschus, S.; et al. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clin. Plasma Med. 2018, 9, 6–13. [Google Scholar] [CrossRef]
- Tanaka, H.; Nakamura, K.; Mizuno, M.; Ishikawa, K.; Takeda, K.; Kajiyama, H.; Utsumi, F.; Kikkawa, F.; Hori, M. Non-thermal atmospheric pressure plasma activates lactate in Ringer’s solution for anti-tumor effects. Sci. Rep. 2016, 6, 36282. [Google Scholar] [CrossRef]
- Freund, E.; Liedtke, K.R.; van der Linde, J.; Metelmann, H.-R.; Heidecke, C.-D.; Partecke, L.-I.; Bekeschus, S. Physical plasma-treated saline promotes an immunogenic phenotype in CT26 colon cancer cells in vitro and in vivo. Sci. Rep. 2019, 9, 634. [Google Scholar] [CrossRef]
- Yan, D.; Nourmohammadi, N.; Bian, K.; Murad, F.; Sherman, J.H.; Keidar, M. Stabilizing the cold plasma-stimulated medium by regulating medium’s composition. Sci. Rep. 2016, 6, 26016. [Google Scholar] [CrossRef]
- Tanaka, H.; Mizuno, M.; Ishikawa, K.; Nakamura, K.; Kajiyama, H.; Kano, H.; Kikkawa, F.; Hori, M. Plasma-Activated Medium Selectively Kills Glioblastoma Brain Tumor Cells by Down-Regulating a Survival Signaling Molecule, AKT Kinase. Plasma Med. 2011, 1, 265–277. [Google Scholar] [CrossRef]
- Mohades, S.; Barekzi, N.; Razavi, H.; Maruthamuthu, V.; Laroussi, M. Temporal evaluation of the anti-tumor efficiency of plasma-activated media. Plasma Process. Polym. 2016, 13, 1206–1211. [Google Scholar] [CrossRef]
- Tanaka, H.; Maeda, S.; Nakamura, K.; Hashizume, H.; Ishikawa, K.; Ito, M.; Ohno, K.; Mizuno, M.; Motooka, Y.; Okazaki, Y.; et al. Plasma-activated Ringer’s lactate solution inhibits the cellular respiratory system in HeLa cells. Plasma Process. Polym. 2021, 18, 2100056. [Google Scholar] [CrossRef]
- Takeda, S.; Yamada, S.; Hattori, N.; Nakamura, K.; Tanaka, H.; Kajiyama, H.; Kanda, M.; Kobayashi, D.; Tanaka, C.; Fujii, T.; et al. Intraperitoneal Administration of Plasma-Activated Medium: Proposal of a Novel Treatment Option for Peritoneal Metastasis From Gastric Cancer. Ann. Surg. Oncol. 2017, 24, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.J.; Kim, K.I.; Kim, G.; Moon, E.; Yang, S.S.; Lee, J.-S. Atmospheric-Pressure Plasma Jet Induces Apoptosis Involving Mitochondria via Generation of Free Radicals. PLoS ONE 2011, 6, e28154. [Google Scholar] [CrossRef] [PubMed]
- Min Joh, H.; Ja Kim, S.; Chung, T.H.; Leem, S.H. Reactive oxygen species-related plasma effects on the apoptosis of human bladder cancer cells in atmospheric pressure pulsed plasma jets. Appl. Phys. Lett. 2012, 101, 053703. [Google Scholar] [CrossRef]
- Keidar, M. Plasma for cancer treatment. Plasma Sources Sci. Technol. 2015, 24, 033001. [Google Scholar] [CrossRef]
- Kim, C.-H.; Bahn, J.H.; Lee, S.-H.; Kim, G.-Y.; Jun, S.-I.; Lee, K.; Baek, S.J. Induction of cell growth arrest by atmospheric non-thermal plasma in colorectal cancer cells. J. Biotechnol. 2010, 150, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Gelbrich, N.; Miebach, L.; Berner, J.; Freund, E.; Saadati, F.; Schmidt, A.; Stope, M.; Zimmermann, U.; Burchardt, M.; Bekeschus, S. Medical gas plasma augments bladder cancer cell toxicity in preclinical models and patient-derived tumor tissues. J. Adv. Res. 2023, 47, 209–223. [Google Scholar] [CrossRef]
- Yan, D.; Talbot, A.; Nourmohammadi, N.; Sherman, J.H.; Cheng, X.; Keidar, M. Toward understanding the selective anticancer capacity of cold atmospheric plasma—A model based on aquaporins (Review). Biointerphases 2015, 10, 040801. [Google Scholar] [CrossRef]
- Dobrynin, D.; Fridman, G.; Friedman, G.; Fridman, A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 2009, 11, 115020. [Google Scholar] [CrossRef]
- Ishikawa, K.; Takeda, K.; Yoshimura, S.; Kondo, T.; Tanaka, H.; Toyokuni, S.; Nakamura, K.; Kajiyama, H.; Mizuno, M.; Hori, M. Generation and measurement of low-temperature plasma for cancer therapy: A historical review. Free Radic. Res. 2023, 57, 239–270. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Privat-Maldonado, A.; Bengtson, C.; Razzokov, J.; Smits, E.; Bogaerts, A. Modifying the Tumour Microenvironment: Challenges and Future Perspectives for Anticancer Plasma Treatments. Cancers 2019, 11, 1920. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45, 263001. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Jiang, Y.-C.; Sun, C.-K.; Chen, Q.-M. Role of the tumor microenvironment in tumor progression and the clinical applications (Review). Oncol. Rep. 2016, 35, 2499–2515. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Gweon, B.; Kim, D.B.; Choe, W.; Shin, J.H. A Feasibility Study for the Cancer Therapy Using Cold Plasma. In Proceedings of the 13th International Conference on Biomedical Engineering, Singapore, 3–6 December 2008; pp. 355–357. [Google Scholar]
- Ko, J.-C.; Wang, Y.-T.; Yang, J.-L. Dual and opposing roles of ERK in regulating G1 and S-G2/M delays in A549 cells caused by hyperoxia. Exp. Cell Res. 2004, 297, 472–483. [Google Scholar] [CrossRef]
- Kolb, J.F.; Mohamed, A.A.H.; Price, R.O.; Swanson, R.J.; Bowman, A.; Chiavarini, R.L.; Stacey, M.; Schoenbach, K.H. Cold atmospheric pressure air plasma jet for medical applications. Appl. Phys. Lett. 2008, 92, 241501. [Google Scholar] [CrossRef]
- Stoffels, E.; Kieft, I.E.; Sladek, R.E.J.; van den Bedem, L.J.M.; van der Laan, E.P.; Steinbuch, M. Plasma needle for in vivo medical treatment: Recent developments and perspectives. Plasma Sources Sci. Technol. 2006, 15, S169. [Google Scholar] [CrossRef]
- Shashurin, A.; Keidar, M.; Bronnikov, S.; Jurjus, R.A.; Stepp, M.A. Living tissue under treatment of cold plasma atmospheric jet. Appl. Phys. Lett. 2008, 93, 181501. [Google Scholar] [CrossRef]
- Kieft, I.E.; Kurdi, M.; Stoffels, E. Reattachment and Apoptosis After Plasma-Needle Treatment of Cultured Cells. IEEE Trans. Plasma Sci. 2006, 34, 1331–1336. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Gueydan, C.; Han, J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018, 28, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Montie, T.C.; Kelly-Wintenberg, K.; Roth, J.R. An overview of research using the one atmosphere uniform glow discharge plasma (OAUGDP) for sterilization of surfaces and materials. IEEE Trans. Plasma Sci. 2000, 28, 41–50. [Google Scholar] [CrossRef]
- Nuccitelli, R.; Chen, X.; Pakhomov, A.G.; Baldwin, W.H.; Sheikh, S.; Pomicter, J.L.; Ren, W.; Osgood, C.; Swanson, R.J.; Kolb, J.F.; et al. A new pulsed electric field therapy for melanoma disrupts the tumor’s blood supply and causes complete remission without recurrence. Int. J. Cancer 2009, 125, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Dezest, M.; Chavatte, L.; Bourdens, M.; Quinton, D.; Camus, M.; Garrigues, L.; Descargues, P.; Arbault, S.; Burlet-Schiltz, O.; Casteilla, L.; et al. Mechanistic insights into the impact of Cold Atmospheric Pressure Plasma on human epithelial cell lines. Sci. Rep. 2017, 7, 41163. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Chung, T.H.; Bae, S.H.; Leem, S.H. Induction of apoptosis in human breast cancer cells by a pulsed atmospheric pressure plasma jet. Appl. Phys. Lett. 2010, 97, 023702. [Google Scholar] [CrossRef]
- Keidar, M.; Walk, R.; Shashurin, A.; Srinivasan, P.; Sandler, A.; Dasgupta, S.; Ravi, R.; Guerrero-Preston, R.; Trink, B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer 2011, 105, 1295–1301. [Google Scholar] [CrossRef]
- Mumtaz, S.; Rana, J.N.; Lim, J.S.; Javed, R.; Choi, E.H.; Han, I. Effect of Plasma On-Time with a Fixed Duty Ratio on Reactive Species in Plasma-Treated Medium and Its Significance in Biological Applications. Int. J. Mol. Sci. 2023, 24, 5289. [Google Scholar] [CrossRef]
- Canady, J.; Murthy, S.R.K.; Zhuang, T.; Gitelis, S.; Nissan, A.; Ly, L.; Jones, O.Z.; Cheng, X.; Adileh, M.; Blank, A.T.; et al. The First Cold Atmospheric Plasma Phase I Clinical Trial for the Treatment of Advanced Solid Tumors: A Novel Treatment Arm for Cancer. Cancers 2023, 15, 3688. [Google Scholar] [CrossRef]
- Schlosshauer, T.; Kiehlmann, M.; Rothenberger, J.; Sader, R.; Rieger, U.M. Bilateral reduction mammaplasty with pulsed electron avalanche knife PlasmaBlade™ and conventional electrosurgical surgery: A retrospective, randomised controlled clinical trial. Int. Wound J. 2020, 17, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Peprah, K.; Spry, C. Pulsed Electron Avalanche Knife (PEAK) PlasmaBlade versus Traditional Electrocautery for Surgery: A Review of Clinical Effectiveness and Cost-Effectiveness; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, USA, 2019. [Google Scholar]
- Zhou, Z.; Li, X.; Chen, J. Application Experience of Monopolar Low-Temperature Plasma Knife in Unilateral Breast-Conserving Surgery. Front. Med. Sci. Res. 2023, 5, 61–65. [Google Scholar]
- Chen, C.-Y.; Cheng, Y.-C.; Cheng, Y.-J. Synergistic effects of plasma-activated medium and chemotherapeutic drugs in cancer treatment. J. Phys. D Appl. Phys. 2018, 51, 13LT01. [Google Scholar] [CrossRef]
- Boeckmann, L.; Berner, J.; Kordt, M.; Lenz, E.; Schäfer, M.; Semmler, M.L.; Frey, A.; Sagwal, S.K.; Rebl, H.; Miebach, L.; et al. Synergistic effect of cold gas plasma and experimental drug exposure exhibits skin cancer toxicity in vitro and in vivo. J. Adv. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, J.; Kim, H.-J.; Jo, C. Plasma-Induced Degradation of Quercetin Associated with the Enhancement of Biological Activities. J. Agric. Food Chem. 2017, 65, 6929–6935. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Yong, H.I.; Park, S.; Kim, K.; Kim, T.H.; Choe, W.; Jo, C. Effect of atmospheric pressure dielectric barrier discharge plasma on the biological activity of naringin. Food Chem. 2014, 160, 241–245. [Google Scholar] [CrossRef]
- Jeong, G.H.; Park, E.K.; Kim, T.H. Anti-diabetic effects of trans-resveratrol byproducts induced by plasma treatment. Food Res. Int. 2019, 119, 119–125. [Google Scholar] [CrossRef]
- Lilli, M.; Zvonek, M.; Cech, V.; Scheffler, C.; Tirillò, J.; Sarasini, F. Low temperature plasma polymerization: An effective process to enhance the basalt fibre/matrix interfacial adhesion. Compos. Commun. 2021, 27, 100769. [Google Scholar] [CrossRef]
- Cao, Y.; Tang, M.; Yang, P.; Chen, M.; Wang, S.; Hua, H.; Chen, W.; Zhou, X. Atmospheric Low-Temperature Plasma-Induced Changes in the Structure of the Lignin Macromolecule: An Experimental and Theoretical Investigation. J. Agric. Food Chem. 2020, 68, 451–460. [Google Scholar] [CrossRef]
- Choi, S.H.; Jeong, G.H.; Lee, K.-B.; Jo, C.; Kim, T.H. A green chemical oligomerization of phloroglucinol induced by plasma as novel α-glucosidase inhibitors. Biosci. Biotechnol. Biochem. 2018, 82, 2059–2063. [Google Scholar] [CrossRef] [PubMed]
- Dolezalova, E.; Malik, M.A.; Heller, L.; Heller, R. Delivery and expression of plasmid DNA into cells by a novel non-thermal plasma source. Bioelectrochemistry 2021, 140, 107816. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Khajoee, V.; Ogawa, Y.; Kusuhara, K.; Katayama, Y.; Hara, T. A novel transfection method for mammalian cells using gas plasma. J. Biotechnol. 2006, 121, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Edelblute, C.M.; Heller, L.C.; Malik, M.A.; Heller, R. Activated air produced by shielded sliding discharge plasma mediates plasmid DNA delivery to mammalian cells. Biotechnol. Bioeng. 2015, 112, 2583–2590. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Morikawa, N.; Ohkubo-Suzuki, A.; Miyoshi, S.; Arakawa, H.; Kita, Y.; Nishimura, S. An epoch-making application of discharge plasma phenomenon to gene-transfer. Biotechnol. Bioeng. 2005, 92, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Connolly, R.J.; Rey, J.I.; Lambert, V.M.; Wegerif, G.; Jaroszeski, M.J.; Ugen, K.E. Enhancement of antigen specific humoral immune responses after delivery of a DNA plasmid based vaccine through a contact-independent helium plasma. Vaccine 2011, 29, 6781–6784. [Google Scholar] [CrossRef] [PubMed]
- Connolly, R.J.; Lopez, G.A.; Hoff, A.M.; Jaroszeski, M.J. Plasma facilitated delivery of DNA to skin. Biotechnol. Bioeng. 2009, 104, 1034–1040. [Google Scholar] [CrossRef]
- Connolly, R.J.; Chapman, T.; Hoff, A.M.; Kutzler, M.A.; Jaroszeski, M.J.; Ugen, K.E. Non-contact helium-based plasma for delivery of DNA vaccines. Enhancement of humoral and cellular immune responses. Hum. Vaccines Immunother. 2012, 8, 1729–1733. [Google Scholar] [CrossRef]
- Jinno, M.; Ikeda, Y.; Motomura, H.; Kido, Y.; Satoh, S. Investigation of plasma induced electrical and chemical factors and their contribution processes to plasma gene transfection. Arch. Biochem. Biophys. 2016, 605, 59–66. [Google Scholar] [CrossRef]
- Novosel, E.C.; Kleinhans, C.; Kluger, P.J. Vascularization is the key challenge in tissue engineering. Adv. Drug Deliv. Rev. 2011, 63, 300–311. [Google Scholar] [CrossRef]
- Chandra, P.K.; Soker, S.; Atala, A. Chapter 1—Tissue engineering: Current status and future perspectives. In Principles of Tissue Engineering, 5th ed.; Lanza, R., Langer, R., Vacanti, J.P., Atala, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–35. [Google Scholar]
- Morent, R.; De Geyter, N.; Desmet, T.; Dubruel, P.; Leys, C. Plasma Surface Modification of Biodegradable Polymers: A Review. Plasma Process. Polym. 2011, 8, 171–190. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of Polymeric Nanofibers for Tissue Engineering Applications: A Review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Girard-Lauriault, P.-L.; Mwale, F.; Iordanova, M.; Demers, C.; Desjardins, P.; Wertheimer, M.R. Atmospheric Pressure Deposition of Micropatterned Nitrogen-Rich Plasma-Polymer Films for Tissue Engineering. Plasma Process. Polym. 2005, 2, 263–270. [Google Scholar] [CrossRef]
- Heyse, P.; Dams, R.; Paulussen, S.; Houthoofd, K.; Janssen, K.; Jacobs, P.A.; Sels, B.F. Dielectric Barrier Discharge at Atmospheric Pressure as a Tool to Deposit Versatile Organic Coatings at Moderate Power Input. Plasma Process. Polym. 2007, 4, 145–157. [Google Scholar] [CrossRef]
- Lewis, G.T.; Nowling, G.R.; Hicks, R.F.; Cohen, Y. Inorganic Surface Nanostructuring by Atmospheric Pressure Plasma-Induced Graft Polymerization. Langmuir 2007, 23, 10756–10764. [Google Scholar] [CrossRef] [PubMed]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Plasma Methods for the Generation of Chemically Reactive Surfaces for Biomolecule Immobilization and Cell Colonization—A Review. Plasma Process. Polym. 2006, 3, 392–418. [Google Scholar] [CrossRef]
- Drews, J.; Launay, H.; Hansen, C.M.; West, K.; Hvilsted, S.; Kingshott, P.; Almdal, K. Hydrolysis and stability of thin pulsed plasma polymerised maleic anhydride coatings. Appl. Surf. Sci. 2008, 254, 4720–4725. [Google Scholar] [CrossRef]
- Taylor, M.J.; Aitchison, H.; Hawker, M.J.; Mann, M.N.; Fisher, E.R.; Graham, D.J.; Gamble, L.J. Time of flight secondary ion mass spectrometry-A method to evaluate plasma-modified three-dimensional scaffold chemistry. Biointerphases 2018, 13, 03b415. [Google Scholar] [CrossRef]
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal Plasma Technology as a Versatile Strategy for Polymeric Biomaterials Surface Modification: A Review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar] [CrossRef]
- Barry, J.J.A.; Silva, M.M.C.G.; Shakesheff, K.M.; Howdle, S.M.; Alexander, M.R. Using Plasma Deposits to Promote Cell Population of the Porous Interior of Three-Dimensional Poly(D,L-Lactic Acid) Tissue-Engineering Scaffolds. Adv. Funct. Mater. 2005, 15, 1134–1140. [Google Scholar] [CrossRef]
- Choi, Y.-R.; Kwon, J.-S.; Song, D.-H.; Choi, E.H.; Lee, Y.-K.; Kim, K.-N.; Kim, K.-M. Surface modification of biphasic calcium phosphate scaffolds by non-thermal atmospheric pressure nitrogen and air plasma treatment for improving osteoblast attachment and proliferation. Thin Solid Film. 2013, 547, 235–240. [Google Scholar] [CrossRef]
- Safinia, L.; Datan, N.; Höhse, M.; Mantalaris, A.; Bismarck, A. Towards a methodology for the effective surface modification of porous polymer scaffolds. Biomaterials 2005, 26, 7537–7547. [Google Scholar] [CrossRef]
- Liu, F.; Mishbak, H.; Bartolo, P. Hybrid polycaprolactone/hydrogel scaffold fabrication and in-process plasma treatment using PABS. Int. J. Bioprinting 2019, 5, 174. [Google Scholar]
- Zahedi, L.; Ghourchi Beigi, P.; Shafiee, M.; Zare, F.; Mahdikia, H.; Abdouss, M.; Abdollahifar, M.-A.; Shokri, B. Development of plasma functionalized polypropylene wound dressing for betaine hydrochloride controlled drug delivery on diabetic wounds. Sci. Rep. 2021, 11, 9641. [Google Scholar] [CrossRef]
- Brånemark, R.; Emanuelsson, L.; Palmquist, A.; Thomsen, P. Bone response to laser-induced micro- and nano-size titanium surface features. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 220–227. [Google Scholar] [CrossRef]
- Danna, N.R.; Beutel, B.G.; Tovar, N.; Witek, L.; Marin, C.; Bonfante, E.A.; Granato, R.; Suzuki, M.; Coelho, P.G. Assessment of Atmospheric Pressure Plasma Treatment for Implant Osseointegration. BioMed Res. Int. 2015, 2015, 761718. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Coelho, P.G.; Kang, B.-S.; Sul, Y.-T.; Albrektsson, T. Classification of osseointegrated implant surfaces: Materials, chemistry and topography. Trends Biotechnol. 2010, 28, 198–206. [Google Scholar] [CrossRef]
- Shalabi, M.M.; Gortemaker, A.; Hof, M.A.V.T.; Jansen, J.A.; Creugers, N.H.J. Implant Surface Roughness and Bone Healing: A Systematic Review. J. Dent. Res. 2006, 85, 496–500. [Google Scholar] [CrossRef]
- Olivieri, M.P.; Baier, R.E.; Loomis, R.E. Surface properties of mussel adhesive protein component films. Biomaterials 1992, 13, 1000–1008. [Google Scholar] [CrossRef]
- Yan, M.; Hartjen, P.; Gosau, M.; Vollkommer, T.; Grust, A.L.; Fuest, S.; Kluwe, L.; Burg, S.; Smeets, R.; Henningsen, A. Effects of a Novel Cold Atmospheric Plasma Treatment of Titanium on the Proliferation and Adhesion Behavior of Fibroblasts. Int. J. Mol. Sci. 2022, 23, 420. [Google Scholar] [CrossRef]
- Rosa, M.; Albrektsson, T.; Carlos Eduardo, F.; Schwartz-Filho, H.; Wennerberg, A. The influence of surface treatment on the implant roughness pattern. J. Appl. Oral Sci. Rev. FOB 2012, 20, 550–555. [Google Scholar] [CrossRef]
- Zimmerli, W. Clinical presentation and treatment of orthopaedic implant-associated infection. J. Intern. Med. 2014, 276, 111–119. [Google Scholar] [CrossRef]
- Zalieckas, J.; Mondragon, I.R.; Pobedinskas, P.; Kristoffersen, A.S.; Mohamed-Ahmed, S.; Gjerde, C.; Høl, P.J.; Hallan, G.; Furnes, O.N.; Cimpan, M.R.; et al. Polycrystalline Diamond Coating on Orthopedic Implants: Realization and Role of Surface Topology and Chemistry in Adsorption of Proteins and Cell Proliferation. ACS Appl. Mater. Interfaces 2022, 14, 44933–44946. [Google Scholar] [CrossRef]
- Crawford, R.J.; Webb, H.K.; Truong, V.K.; Hasan, J.; Ivanova, E.P. Surface topographical factors influencing bacterial attachment. Adv. Colloid Interface Sci. 2012, 179–182, 142–149. [Google Scholar] [CrossRef]
- Goodman, S.B.; Yao, Z.; Keeney, M.; Yang, F. The future of biologic coatings for orthopaedic implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef]
- Ivanova, E.; Truong, V.K.; Wang, J.; Berndt, C.; Jones, R.; Yusuf, I.; Peake, I.; Schmidt, H.; Fluke, C.; Barnes, D.; et al. Impact of Nanoscale Roughness of Titanium Thin Film Surfaces on Bacterial Retention. Langmuir ACS J. Surf. Colloids 2009, 26, 1973–1982. [Google Scholar] [CrossRef]
- Braem, A.; Van Mellaert, L.; Mattheys, T.; Hofmans, D.; De Waelheyns, E.; Geris, L.; Anné, J.; Schrooten, J.; Vleugels, J. Staphylococcal biofilm growth on smooth and porous titanium coatings for biomedical applications. J. Biomed. Mater. Res. A 2014, 102, 215–224. [Google Scholar] [CrossRef]
- Hahn, H.; Palich, W. Preliminary evaluation of porous metal surfaced titanium for orthopedic implants. J. Biomed. Mater. Res. 1970, 4, 571–577. [Google Scholar] [CrossRef]
- Taddei, E.B.; Henriques, V.A.R.; Silva, C.R.M.; Cairo, C.A.A. Production of new titanium alloy for orthopedic implants. Mater. Sci. Eng. C Biomim. Mater. Sens. Syst. 2004, 24, 683–687. [Google Scholar] [CrossRef]
- Lorenzetti, M.; Dogša, I.; Stošicki, T.; Stopar, D.; Kalin, M.; Kobe, S.; Novak, S. The Influence of Surface Modification on Bacterial Adhesion to Titanium-Based Substrates. ACS Appl. Mater. Interfaces 2015, 7, 1644–1651. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Zhao, H.; Liu, Y.; Liu, J.; Bai, N. Bioactive Effects of Low-Temperature Argon–Oxygen Plasma on a Titanium Implant Surface. ACS Omega 2020, 5, 3996–4003. [Google Scholar] [CrossRef]
- van Hengel, I.A.J.; Tierolf, M.W.A.M.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Antibacterial Titanium Implants Biofunctionalized by Plasma Electrolytic Oxidation with Silver, Zinc, and Copper: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 3800. [Google Scholar] [CrossRef]
- Staruch, R.M.T.; Griffin, M.; Butler, P. Nanoscale Surface Modifications of Orthopaedic Implants: State of the Art and Perspectives. Open Orthop. J. 2016, 10, 920–938. [Google Scholar] [CrossRef]
- Poon, R.W.Y.; Ho, J.P.Y.; Liu, X.; Chung, C.Y.; Chu, P.K.; Yeung, K.W.K.; Lu, W.W.; Cheung, K.M.C. Improvements of anti-corrosion and mechanical properties of NiTi orthopedic materials by acetylene, nitrogen and oxygen plasma immersion ion implantation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 237, 411–416. [Google Scholar] [CrossRef]
- Lu, T.; Qiao, Y.; Liu, X. Surface modification of biomaterials using plasma immersion ion implantation and deposition. Interface Focus 2012, 2, 325–336. [Google Scholar] [CrossRef]
- Han, C.-M.; Lee, E.-J.; Kim, H.-E.; Koh, Y.-H.; Kim, K.N.; Ha, Y.; Kuh, S.-U. The electron beam deposition of titanium on polyetheretherketone (PEEK) and the resulting enhanced biological properties. Biomaterials 2010, 31, 3465–3470. [Google Scholar] [CrossRef]
- Juan, C.; Weimin, S.; Norman, A.J.; Ilavarasan, P. Electrical impact of high-speed bus crossing plane split. In Proceedings of the 2002 IEEE International Symposium on Electromagnetic Compatibility, Minneapolis, MN, USA, 19–23 August 2002; Volume 862, pp. 861–865. [Google Scholar]
- Almasi, D.; Iqbal, N.; Sadeghi, M.; Sudin, I.; Abdul Kadir, M.R.; Kamarul, T. Preparation Methods for Improving PEEK’s Bioactivity for Orthopedic and Dental Application: A Review. Int. J. Biomater. 2016, 2016, 8202653. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef]
- Wakelin, E.A.; Yeo, G.C.; McKenzie, D.R.; Bilek, M.M.M.; Weiss, A.S. Plasma ion implantation enabled bio-functionalization of PEEK improves osteoblastic activity. APL Bioeng. 2018, 2, 026109. [Google Scholar] [CrossRef]
- Poulsson, A.H.C.; Eglin, D.; Geoff Richards, R. Chapter 11—Surface Modification Techniques of PEEK, Including Plasma Surface Treatment. In PEEK Biomaterials Handbook, 2nd ed.; Kurtz, S.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 179–201. [Google Scholar]
- Neděla, O.; Slepička, P.; Švorčík, V. Surface Modification of Polymer Substrates for Biomedical Applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, H.A.; Pickering, K.L.; Mucalo, M.R. A Review on the Use of Hydroxyapatite-Carbonaceous Structure Composites in Bone Replacement Materials for Strengthening Purposes. Materials 2018, 11, 1813. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Akhavan, B.; Wise, S.G.; Bilek, M.M.M. A review of biomimetic surface functionalization for bone-integrating orthopedic implants: Mechanisms, current approaches, and future directions. Prog. Mater. Sci. 2019, 106, 100588. [Google Scholar] [CrossRef]
- Heimann, R.B. Plasma-Sprayed Hydroxylapatite-Based Coatings: Chemical, Mechanical, Microstructural, and Biomedical Properties. J. Therm. Spray Technol. 2016, 25, 827–850. [Google Scholar] [CrossRef]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, W.; Shi, H.; Yang, K.; Wang, G.; Wang, P.; Ji, J.; Chu, P.K. In situ plasma fabrication of ceramic-like structure on polymeric implant with enhanced surface hardness, cytocompatibility and antibacterial capability. J. Biomed. Mater. Res. Part A 2016, 104, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, X. Plasma-Sprayed Ceramic Coatings for Osseointegration. Int. J. Appl. Ceram. Technol. 2013, 10, 1–10. [Google Scholar] [CrossRef]
- Liu, X.; Morra, M.; Carpi, A.; Li, B. Bioactive calcium silicate ceramics and coatings. Biomed. Pharmacother. 2008, 62, 526–529. [Google Scholar] [CrossRef]
- Liu, X.; Ding, C.; Wang, Z. Apatite formed on the surface of plasma-sprayed wollastonite coating immersed in simulated body fluid. Biomaterials 2001, 22, 2007–2012. [Google Scholar] [CrossRef]
- Cerqueira, A.; García-Arnáez, I.; Romero Gavilán, F.J.; Azkargorta Mujika, M.; Elortza, F.; Martín-de-Llano, J.; Carda, C.; Gurruchaga, M.; Goñi, I.; Suay, J. Complex effects of Mg-biomaterials on the osteoblast cell machinery: A proteomic study. Biomater. Adv. 2022, 137, 212826. [Google Scholar] [CrossRef]
- Hoffmann, C.; Berganza, C.; Zhang, J. Cold Atmospheric Plasma: Methods of production and application in dentistry and oncology. Med. Gas Res. 2013, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Goree, J.; Liu, B.; Drake, D.; Stoffels, E. Killing of S. mutans Bacteria Using a Plasma Needle at Atmospheric Pressure. IEEE Trans. Plasma Sci. 2006, 34, 1317–1324. [Google Scholar] [CrossRef]
- Morris, A.D.; McCombs, G.B.; Akan, T.; Hynes, W.; Laroussi, M.; Tolle, S.L. Cold plasma technology: Bactericidal effects on Geobacillus stearothermophilus and Bacillus cereus microorganisms. J. Dent. Hyg. 2009, 83, 55–61. [Google Scholar] [PubMed]
- Yang, B.; Chen, J.; Yu, Q.; Li, H.; Lin, M.; Mustapha, A.; Hong, L.; Wang, Y. Oral bacterial deactivation using a low-temperature atmospheric argon plasma brush. J. Dent. 2011, 39, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Mahasneh, A.; Darby, M.; Tolle, S.L.; Hynes, W.; Laroussi, M.; Karakas, E. Inactivation of Porphyromonas gingivalis by Low-Temperature Atmospheric Pressure Plasma. Plasma Med. 2011, 1, 191–204. [Google Scholar] [CrossRef]
- Rupf, S.; Lehmann, A.; Hannig, M.; Schäfer, B.; Schubert, A.; Feldmann, U.; Schindler, A. Killing of adherent oral microbes by a non-thermal atmospheric plasma jet. J. Med. Microbiol. 2010, 59, 206–212. [Google Scholar] [CrossRef]
- Koban, I.; Holtfreter, B.; Hübner, N.O.; Matthes, R.; Sietmann, R.; Kindel, E.; Weltmann, K.D.; Welk, A.; Kramer, A.; Kocher, T. Antimicrobial efficacy of non-thermal plasma in comparison to chlorhexidine against dental biofilms on titanium discs in vitro—Proof of principle experiment. J. Clin. Periodontol. 2011, 38, 956–965. [Google Scholar] [CrossRef]
- Pan, J.; Sun, K.; Liang, Y.; Sun, P.; Yang, X.; Wang, J.; Zhang, J.; Zhu, W.; Fang, J.; Becker, K.H. Cold Plasma Therapy of a Tooth Root Canal Infected with Enterococcus faecalis Biofilms In Vitro. J. Endod. 2013, 39, 105–110. [Google Scholar] [CrossRef]
- Lata, S.; Chakravorty, S.; Mitra, T.; Pradhan, P.K.; Mohanty, S.; Patel, P.; Jha, E.; Panda, P.K.; Verma, S.K.; Suar, M. Aurora Borealis in dentistry: The applications of cold plasma in biomedicine. Mater. Today Bio 2022, 13, 100200. [Google Scholar] [CrossRef]
- Duske, K.; Koban, I.; Kindel, E.; Schröder, K.; Nebe, B.; Holtfreter, B.; Jablonowski, L.; Weltmann, K.D.; Kocher, T. Atmospheric plasma enhances wettability and cell spreading on dental implant metals. J. Clin. Periodontol. 2012, 39, 400–407. [Google Scholar] [CrossRef]
- Giro, G.; Tovar, N.; Witek, L.; Marin, C.; Silva, N.R.F.; Bonfante, E.A.; Coelho, P.G. Osseointegration assessment of chairside argon-based nonthermal plasma-treated Ca-P coated dental implants. J. Biomed. Mater. Res. Part A 2013, 101, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Carvalho, R.M. Dentine permeability and dentine adhesion. J. Dent. 1997, 25, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Swift, E.J., Jr.; Perdigão, J.; Heymann, H.O. Bonding to enamel and dentin: A brief history and state of the art, 1995. Quintessence Int. 1995, 26, 95–110. [Google Scholar] [PubMed]
- Dong, X.; Chen, M.; Wang, Y.; Yu, Q. A Mechanistic study of Plasma Treatment Effects on Demineralized Dentin Surfaces for Improved Adhesive/Dentin Interface Bonding. Clin. Plasma Med. 2014, 2, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Ritts, A.C.; Li, H.; Yu, Q.; Xu, C.; Yao, X.; Hong, L.; Wang, Y. Dentin surface treatment using a non-thermal argon plasma brush for interfacial bonding improvement in composite restoration. Eur. J. Oral Sci. 2010, 118, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Yavirach, P.; Chaijareenont, P.; Boonyawan, D.; Pattamapun, K.; Tunma, S.; Takahashi, H.; Arksornnukit, M. Effects of plasma treatment on the shear bond strength between fiber-reinforced composite posts and resin composite for core build-up. Dent. Mater. J. 2009, 28, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Nam, S.H.; Kwon, H.C.; Mohamed, A.A.; Lee, J.K.; Kim, G.C. Feasibility of nonthermal atmospheric pressure plasma for intracoronal bleaching. Int. Endod. J. 2011, 44, 170–175. [Google Scholar] [CrossRef]
- Nam, S.H.; Lee, H.W.; Cho, S.H.; Lee, J.K.; Jeon, Y.C.; Kim, G.C. High-efficiency tooth bleaching using non-thermal atmospheric pressure plasma with low concentration of hydrogen peroxide. J. Appl. Oral Sci. 2013, 21, 265–270. [Google Scholar] [CrossRef]
- Tucker, B.S.; Baker, P.A.; Xu, K.G.; Vohra, Y.K.; Thomas, V. Atmospheric pressure plasma jet: A facile method to modify the intimal surface of polymeric tubular conduits. J. Vac. Sci. Technol. A 2018, 36, 04F404. [Google Scholar] [CrossRef]
- Tucker, B.S.; Vijayan, V.M.; Vohra, Y.K.; Thomas, V. Novel magneto-plasma processing for enhanced modification of electrospun biomaterials. Mater. Lett. 2019, 250, 96–98. [Google Scholar] [CrossRef]
- Vijayan, V.M.; Tucker, B.S.; Baker, P.A.; Vohra, Y.K.; Thomas, V. Non-equilibrium hybrid organic plasma processing for superhydrophobic PTFE surface towards potential bio-interface applications. Colloids Surf. B Biointerfaces 2019, 183, 110463. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.M.; Tucker, B.S.; Hwang, P.T.J.; Bobba, P.S.; Jun, H.-W.; Catledge, S.A.; Vohra, Y.K.; Thomas, V. Non-equilibrium organosilane plasma polymerization for modulating the surface of PTFE towards potential blood contact applications. J. Mater. Chem. B 2020, 8, 2814–2825. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.M.; Tucker, B.S.; Dimble, P.S.; Vohra, Y.K.; Thomas, V. Dusty-Plasma-Assisted Synthesis of Silica Nanoparticles for in Situ Surface Modification of 3D-Printed Polymer Scaffolds. ACS Appl. Nano Mater. 2020, 3, 7392–7396. [Google Scholar] [CrossRef]
- Vijayan, V.M.; Walker, M.; Pillai, R.R.; Moreno, G.H.; Vohra, Y.K.; Morris, J.J.; Thomas, V. Plasma Electroless Reduction: A Green Process for Designing Metallic Nanostructure Interfaces onto Polymeric Surfaces and 3D Scaffolds. ACS Appl. Mater. Interfaces 2022, 14, 25065–25079. [Google Scholar] [CrossRef]
- Tucker, B.S.; Surolia, R.; Baker, P.A.; Vohra, Y.; Antony, V.; Thomas, V. Low-Temperature Air Plasma Modification of Electrospun Soft Materials and Bio-interfaces. In Proceedings of the TMS 2019 148th Annual Meeting & Exhibition Supplemental Proceedings, Cham, Switzerland, 10–14 March 2019; pp. 819–826. [Google Scholar]
- Vijayan, V.M.; Walker, M.; Morris, J.J.; Thomas, V. Recent mitigation strategies in engineered healthcare materials towards antimicrobial applications. Curr. Opin. Biomed. Eng. 2022, 22, 100377. [Google Scholar] [CrossRef]
- Tucker, B.S.; Aliakbarshirazi, S.; Vijayan, V.M.; Thukkaram, M.; De Geyter, N.; Thomas, V. Nonthermal plasma processing for nanostructured biomaterials and tissue engineering scaffolds: A mini review. Curr. Opin. Biomed. Eng. 2021, 17, 100259. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karthik, C.; Sarngadharan, S.C.; Thomas, V. Low-Temperature Plasma Techniques in Biomedical Applications and Therapeutics: An Overview. Int. J. Mol. Sci. 2024, 25, 524. https://doi.org/10.3390/ijms25010524
Karthik C, Sarngadharan SC, Thomas V. Low-Temperature Plasma Techniques in Biomedical Applications and Therapeutics: An Overview. International Journal of Molecular Sciences. 2024; 25(1):524. https://doi.org/10.3390/ijms25010524
Chicago/Turabian StyleKarthik, Chandrima, Sarath Chand Sarngadharan, and Vinoy Thomas. 2024. "Low-Temperature Plasma Techniques in Biomedical Applications and Therapeutics: An Overview" International Journal of Molecular Sciences 25, no. 1: 524. https://doi.org/10.3390/ijms25010524







