3-(Methylthio)Propionic Acid from Bacillus thuringiensis Berliner Exhibits High Nematicidal Activity against the Root Knot Nematode Meloidogyne incognita (Kofoid and White) Chitwood
Abstract
:1. Introduction
2. Results
2.1. Nematicidal Effects of B. thuringiensis NBIN-863 against M. incognita
2.2. Identification of the VOCs Produced by B. thuringiensis NBIN-863
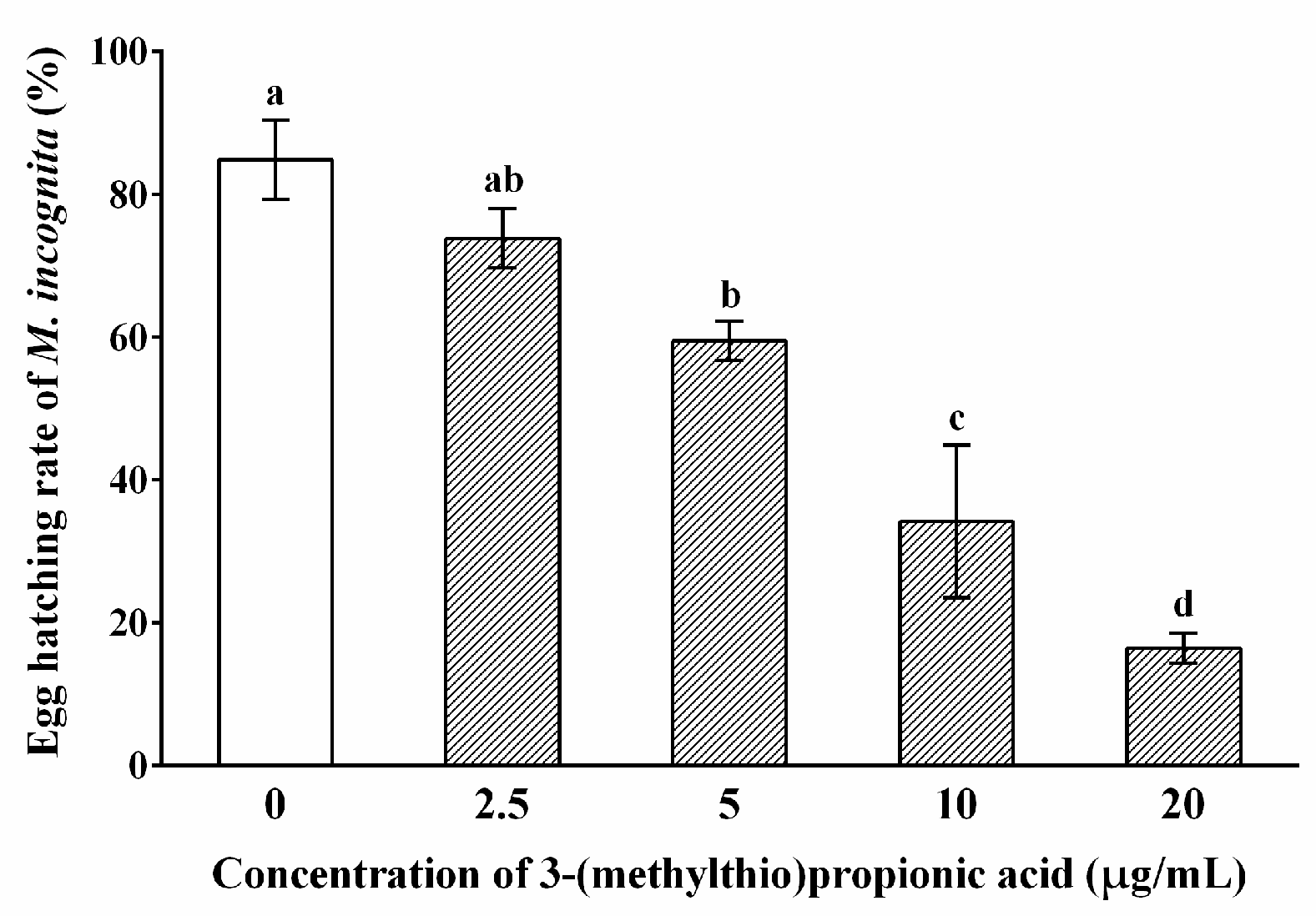
2.3. Nematicidal Activity of 3-(Methylthio)Propionic Acid against M. incognita
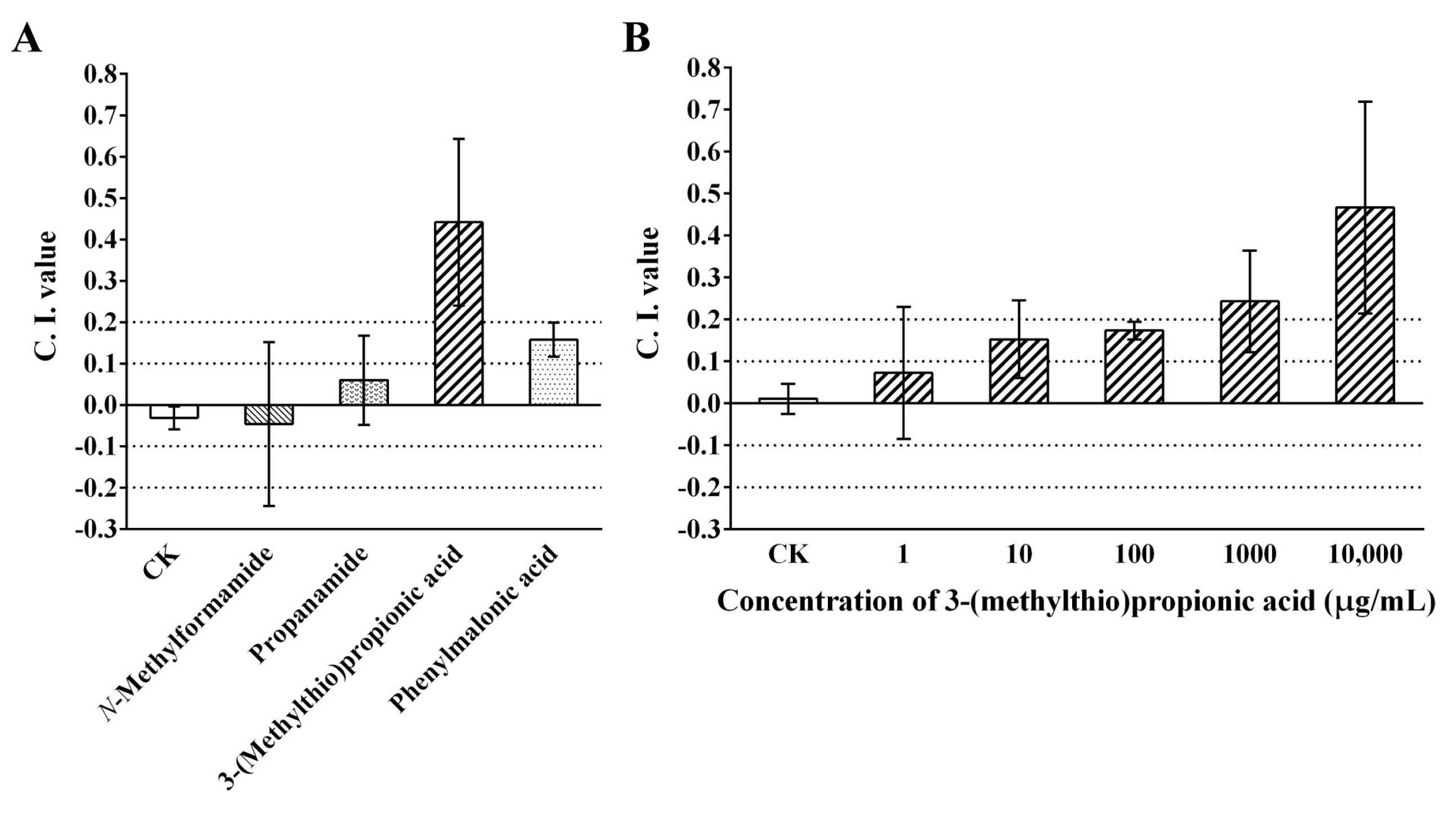
2.4. Chemotaxis of M. incognita toward 3-(Methylthio)Propionic Acid
2.5. Antagonistic Effect of 3-(Methylthio)Propionic Acid against M. incognita in Pot Experiments
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Nematode Material
4.3. Identification of VOCs of the Bacterial Fermentation Supernatant
4.4. Commercial Volatiles Compounds
4.5. Direct Contact Nematicidal Bioassay of the Fermentation Supernatant and VOCs of NBIN-863
4.6. Fumigant Nematicidal Bioassay of the Fermentation Supernatant and VOCs of NBIN-863
4.7. Chemotaxis of M. incognita toward the Fermentation Broth and VOCs of NBIN-863
4.8. Inhibitory Effect of 3-(Methylthio)Propionic Acid on Egg Hatching of M. incognita
4.9. Efficacy of 3-(Methylthio)Propionic Acid against M. incognita in the Pot Experiment
4.10. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abad, P.; Gouzy, J.; Aury, J.M.; Castagnone-Sereno, P.; Danchin, E.G.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C.; et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Elling, A.A. Major emerging problems with minor Meloidogyne species. Phytopathology 2013, 103, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.; Gaur, H.S.; Helder, J.; Jones, M.G.; Kikuchi, T.; Manzanilla-Lopez, R.; Palomares-Rius, J.E.; Wesemael, W.M.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Dai, M.; Gao, S.; Mi, Y.; Zhang, S.; Wei, J.; Zhao, H.; Duan, F.; Liang, C.; Shi, Q. Volatile organic compounds produced by Paenibacillus polymyxa J2-4 exhibit toxic activity against Meloidogyne incognita. Pest Manag. Sci. 2023. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Xie, C.; Zhou, Y.; Bo, D.; Zhang, S.; Mao, S.; Liao, Y.; Cui, S.; Zhu, Z.; Wang, X.; et al. Unzipped chromosome-level genomes reveal allopolyploid nematode origin pattern as unreduced gamete hybridization. Nat. Commun. 2023, 14, 7156. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.L.; Hussey, R.S.; Baum, T.J. Getting to the roots of parasitism by nematodes. Trends Parasitol. 2004, 20, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Philbrick, A.N.; Adhikari, T.B.; Louws, F.J.; Gorny, A.M. Meloidogyne enterolobii, a major threat to tomato production: Current status and future prospects for its management. Front. Plant Sci. 2020, 11, 606395. [Google Scholar] [CrossRef]
- Mejias, J.; Truong, N.M.; Abad, P.; Favery, B.; Quentin, M. Plant proteins and processes targeted by parasitic nematode effectors. Front. Plant Sci. 2019, 10, 970. [Google Scholar] [CrossRef]
- Favery, B.; Quentin, M.; Jaubert-Possamai, S.; Abad, P. Gall-forming root-knot nematodes hijack key plant cellular functions to induce multinucleate and hypertrophied feeding cells. J. Insect Physiol. 2016, 84, 60–69. [Google Scholar] [CrossRef]
- Hewezi, T. Epigenetic mechanisms in nematode-plant interactions. Annu. Rev. Phytopathol. 2020, 58, 119–138. [Google Scholar] [CrossRef]
- Huang, D.; Yu, C.; Shao, Z.; Cai, M.; Li, G.; Zheng, L.; Yu, Z.; Zhang, J. Identification and characterization of nematicidal volatile organic compounds from deep-sea Virgibacillus dokdonensis MCCC 1A00493. Molecules 2020, 25, 744. [Google Scholar] [CrossRef]
- Oka, Y. Attraction of second-stage juveniles of Meloidogyne species to fluopyram. Pest Manag. Sci. 2023, 79, 2696–2703. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K.; Williams, J.; Wang, N.Y.; Cicerone, R.J. Agricultural soil fumigation as a source of atmospheric methyl bromide. Proc. Natl. Acad. Sci. USA 1993, 90, 8420–8423. [Google Scholar] [CrossRef] [PubMed]
- Whorton, M.D.; Foliart, D.E. Mutagenicity, carcinogenicity and reproductive effects of dibromochloropropane (DBCP). Mutat. Res. 1983, 123, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.M.; Rosskopf, E.N.; Leesch, J.G.; Chellemi, D.O.; Bull, C.T.; Mazzola, M. United States department of agriculture-agricultural research service research on alternatives to methyl bromide: Pre-plant and post-harvest. Pest Manag. Sci. 2003, 59, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, W.; Wang, Y.; Liu, H.; Zhang, S.; Dong, B.; Ji, X.; Qiao, K. Management of Meloidogyne incognita on cucumber with a new nonfumigant nematicide fluopimomide. Plant Dis. 2022, 106, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Xu, S.; Zhang, W.; Xu, C.; Li, B.; Zhang, D.; Mu, W.; Liu, F. Nematicidal activity of trans-2-Hexenal against southern root-knot nematode (Meloidogyne incognita) on tomato plants. J. Agric. Food Chem. 2017, 65, 544–550. [Google Scholar] [CrossRef]
- Berini, F.; Katz, C.; Gruzdev, N.; Casartelli, M.; Tettamanti, G.; Marinelli, F. Microbial and viral chitinases: Attractive biopesticides for integrated pest management. Biotechnol. Adv. 2018, 36, 818–838. [Google Scholar] [CrossRef]
- Riga, E. The effects of Brassica green manures on plant parasitic and free living nematodes used in combination with reduced rates of synthetic nematicides. J. Nematol. 2011, 43, 119–121. [Google Scholar]
- Westerdahl, B.B.; Hasey, J.; Grant, J.; Beem, L.W. Bionematicides as an alternative to methyl bromide fumigation. J. Nematol. 2021, 53, 1–10. [Google Scholar] [CrossRef]
- Zhu, M.C.; Zhao, N.; Liu, Y.K.; Li, X.M.; Zhen, Z.Y.; Zheng, Y.Q.; Zhang, K.Q.; Yang, J.K. The cAMP-PKA signalling pathway regulates hyphal growth, conidiation, trap morphogenesis, stress tolerance, and autophagy in Arthrobotrys oligospora. Environ. Microbiol. 2022, 24, 6524–6538. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Tanaka, K. Purpureocillium lilacinum for plant growth promotion and biocontrol against root-knot nematodes infecting eggplant. PLoS ONE 2023, 18, e0283550. [Google Scholar] [CrossRef] [PubMed]
- Nasiou, E.; Thoden, T.; Pardavella, I.V.; Tzortzakakis, E.A. Compatibility of fluazaindolizine and oxamyl with Pasteuria penetrans on spore attachment to juveniles of Meloidogyne javanica and M. incognita. J. Nematol. 2020, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Gao, Y.; Li, X.; Chen, S.; Yan, S.; Tian, X. Identification and nematicidal characterization of proteases secreted by endophytic bacteria Bacillus cereus BCM2. Phytopathology 2020, 110, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Lin, J.; Zheng, J.; Wang, S.; Zhou, H.; Sun, M. Alcaligenes faecalis ZD02, a novel nematicidal bacterium with an extracellular serine protease virulence factor. Appl. Environ. Microbiol. 2016, 82, 2112–2120. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Yan, R.; Li, X.; Lin, Y.; Yang, Z.; Ma, Y.; Ding, Z. Biocontrol potential of Pseudomonas rhodesiae GC-7 against the root-knot nematode Meloidogyne graminicola through both antagonistic effects and induced plant resistance. Front. Microbiol. 2022, 13, 1025727. [Google Scholar] [CrossRef]
- Schalchli, H.; Tortella, G.R.; Rubilar, O.; Parra, L.; Hormazabal, E.; Quiroz, A. Fungal volatiles: An environmentally friendly tool to control pathogenic microorganisms in plants. Crit. Rev. Biotechnol. 2016, 36, 144–152. [Google Scholar] [CrossRef]
- Rani, A.; Rana, A.; Dhaka, R.K.; Singh, A.P.; Chahar, M.; Singh, S.; Nain, L.; Singh, K.P.; Minz, D. Bacterial volatile organic compounds as biopesticides, growth promoters and plant-defense elicitors: Current understanding and future scope. Biotechnol. Adv. 2023, 63, 108078. [Google Scholar] [CrossRef]
- Deng, X.; Wang, X.; Li, G. Nematicidal effects of volatile organic compounds from microorganisms and plants on plant-parasitic nematodes. Microorganisms 2022, 10, 1201. [Google Scholar] [CrossRef]
- Tahir, H.A.S.; Gu, Q.; Wu, H.; Raza, W.; Safdar, A.; Huang, Z.; Rajer, F.U.; Gao, X. Effect of volatile compounds produced by Ralstonia solanacearum on plant growth promoting and systemic resistance inducing potential of Bacillus volatiles. BMC Plant Biol. 2017, 17, 133. [Google Scholar] [CrossRef]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Pare, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Shao, Z.; Cai, M.; Zheng, L.; Li, G.; Huang, D.; Cheng, W.; Thomashow, L.S.; Weller, D.M.; Yu, Z.; et al. Multiple modes of nematode control by volatiles of Pseudomonas putida 1A00316 from antarctic soil against Meloidogyne incognita. Front. Microbiol. 2018, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ran, Y.; Yang, H.; Mo, M.; Li, G. Volatile metabolites from Brevundimonas diminuta and nematicidal esters inhibit Meloidogyne javanica. Microorganisms 2023, 11, 966. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wang, J.Y.; Liu, X.F.; Guan, Q.; Dou, N.X.; Li, J.; Zhang, Q.; Gao, Y.M.; Wang, M.; Li, J.S.; et al. Nematicidal activity of volatile organic compounds produced by Bacillus altitudinis AMCC 1040 against Meloidogyne incognita. Arch. Microbiol. 2022, 204, 521. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.-Q.; Mo, M.-H.; Zhou, J.-P.; Zou, C.-S.; Zhang, K.-Q. Evaluation and identification of potential organic nematicidal volatiles from soil bacteria. Soil Biol. Biochem. 2007, 39, 2567–2575. [Google Scholar] [CrossRef]
- Chen, J.; Song, B. Natural nematicidal active compounds: Recent research progress and outlook. J. Integr. Agric. 2021, 20, 2015–2031. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Lu, H.; Wang, X.; Zhang, K.Q.; Li, G.H. Effect of volatile organic compounds from bacteria on nematodes. Chem. Biodivers. 2015, 12, 1415–1421. [Google Scholar] [CrossRef]
- Wu, W.; Zeng, Y.; Yan, X.; Wang, Z.; Guo, L.; Zhu, Y.; Wang, Y.; He, X. Volatile organic compounds of Bacillus velezensis GJ-7 against Meloidogyne hapla through multiple prevention and control modes. Molecules 2023, 28, 3182. [Google Scholar] [CrossRef]
- Ayaz, M.; Ali, Q.; Farzand, A.; Khan, A.R.; Ling, H.; Gao, X. Nematicidal Volatiles from Bacillus atrophaeus GBSC56 promote growth and stimulate induced systemic resistance in tomato against Meloidogyne incognita. Int. J. Mol. Sci. 2021, 22, 5049. [Google Scholar] [CrossRef]
- Geng, C.; Liu, Y.; Li, M.; Tang, Z.; Muhammad, S.; Zheng, J.; Wan, D.; Peng, D.; Ruan, L.; Sun, M. Dissimilar crystal proteins Cry5Ca1 and Cry5Da1 synergistically act against Meloidogyne incognita and delay Cry5Ba-based nematode resistance. Appl. Environ. Microbiol. 2017, 83, e03505-16. [Google Scholar] [CrossRef]
- Luo, X.; Chen, L.; Huang, Q.; Zheng, J.; Zhou, W.; Peng, D.; Ruan, L.; Sun, M. Bacillus thuringiensis metalloproteinase Bmp1 functions as a nematicidal virulence factor. Appl. Environ. Microbiol. 2013, 79, 460–468. [Google Scholar] [CrossRef]
- Peng, D.; Lin, J.; Huang, Q.; Zheng, W.; Liu, G.; Zheng, J.; Zhu, L.; Sun, M. A novel metalloproteinase virulence factor is involved in Bacillus thuringiensis pathogenesis in nematodes and insects. Environ. Microbiol. 2016, 18, 846–862. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.Z.; Hale, K.; Carta, L.; Platzer, E.; Wong, C.; Fang, S.C.; Aroian, R.V. Bacillus thuringiensis crystal proteins that target nematodes. Proc. Natl. Acad. Sci. USA 2003, 100, 2760–2765. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Cao, S.; Shi, X.; Nie, X.; Zheng, J.; Deng, Y.; Ruan, L.; Peng, D.; Sun, M. Genetic and biochemical characterization of a gene operon for trans-aconitic acid, a novel nematicide from Bacillus thuringiensis. J. Biol. Chem. 2017, 292, 3517–3530. [Google Scholar] [CrossRef] [PubMed]
- Kahn, T.W.; Duck, N.B.; McCarville, M.T.; Schouten, L.C.; Schweri, K.; Zaitseva, J.; Daum, J. A Bacillus thuringiensis Cry protein controls soybean cyst nematode in transgenic soybean plants. Nat. Commun. 2021, 12, 3380. [Google Scholar] [CrossRef]
- Bravo, A.; Likitvivatanavong, S.; Gill, S.S.; Soberon, M. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 2011, 41, 423–431. [Google Scholar] [CrossRef]
- Ahmad, G.; Khan, A.; Khan, A.A.; Ali, A.; Mohhamad, H.I. Biological control: A novel strategy for the control of the plant parasitic nematodes. Antonie Van Leeuwenhoek 2021, 114, 885–912. [Google Scholar] [CrossRef] [PubMed]
- Griffitts, J.S.; Haslam, S.M.; Yang, T.; Garczynski, S.F.; Mulloy, B.; Morris, H.; Cremer, P.S.; Dell, A.; Adang, M.J.; Aroian, R.V. Glycolipids as receptors for Bacillus thuringiensis crystal toxin. Science 2005, 307, 922–925. [Google Scholar] [CrossRef]
- Zhang, F.; Peng, D.; Cheng, C.; Zhou, W.; Ju, S.; Wan, D.; Yu, Z.; Shi, J.; Deng, Y.; Wang, F.; et al. Bacillus thuringiensis crystal protein Cry6Aa triggers Caenorhabditis elegans necrosis pathway mediated by aspartic protease (ASP-1). PLoS Pathog. 2016, 12, e1005389. [Google Scholar] [CrossRef]
- Wan, L.; Lin, J.; Du, H.; Zhang, Y.; Bravo, A.; Soberon, M.; Sun, M.; Peng, D. Bacillus thuringiensis targets the host intestinal epithelial junctions for successful infection of Caenorhabditis elegans. Environ. Microbiol. 2019, 21, 1086–1098. [Google Scholar] [CrossRef]
- Miyashita, N.; Koga, H. Three-dimensional ultrastructure of feeding tubes and interconnected endoplasmic reticulum in root-knot nematode-induced giant cells in rose balsam. Protoplasma 2017, 254, 1941–1951. [Google Scholar] [CrossRef]
- Niu, Q.; Huang, X.; Zhang, L.; Xu, J.; Yang, D.; Wei, K.; Niu, X.; An, Z.; Bennett, J.W.; Zou, C.; et al. A Trojan horse mechanism of bacterial pathogenesis against nematodes. Proc. Natl. Acad. Sci. USA 2010, 107, 16631–16636. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, P.V.M.; Campos, V.P.; Terra, W.C.; Pedroso, M.P.; de Paula, L.L.; da Silva, M.S.G.; Monteiro, T.S.A.; de Freitas, L.G. Attraction and toxicity: Ways volatile organic compounds released by Pochonia chlamydosporia affect Meloidogyne incognita. Microbiol. Res. 2021, 255, 126925. [Google Scholar] [CrossRef] [PubMed]
- Grahovac, J.; Pajcin, I.; Vlajkov, V. Bacillus VOCs in the context of biological control. Antibiotics 2023, 12, 581. [Google Scholar] [CrossRef] [PubMed]
- Tahir, H.A.; Gu, Q.; Wu, H.; Niu, Y.; Huo, R.; Gao, X. Bacillus volatiles adversely affect the physiology and ultra-structure of Ralstonia solanacearum and induce systemic resistance in tobacco against bacterial wilt. Sci. Rep. 2017, 7, 40481. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Yoon, M.Y.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Shin, T.S.; Park, H.W.; Yu, N.H.; Kim, Y.H.; Kim, J.C. Diffusible and volatile antifungal compounds produced by an antagonistic Bacillus velezensis G341 against various phytopathogenic fungi. Plant Pathol. J. 2017, 33, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Chavarria-Quicaño, E.; Contreras-Jácquez, V.; Carrillo-Fasio, A.; De la Torre-González, F.; Asaff-Torres, A. Native Bacillus paralicheniformis isolate as a potential agent for phytopathogenic nematodes control. Front. Microbiol. 2023, 14, 1213306. [Google Scholar] [CrossRef] [PubMed]
- He, C.N.; Ye, W.Q.; Zhu, Y.Y.; Zhou, W.W. Antifungal activity of volatile organic compounds produced by Bacillus methylotrophicus and Bacillus thuringiensis against five common spoilage fungi on loquats. Molecules 2020, 25, 3360. [Google Scholar] [CrossRef]
- Chaouachi, M.; Marzouk, T.; Jallouli, S.; Elkahoui, S.; Gentzbittel, L.; Ben, C.; Djébali, N. Activity assessment of tomato endophytic bacteria bioactive compounds for the postharvest biocontrol of Botrytis cinerea. Postharvest Biol. Technol. 2021, 172, 111389. [Google Scholar] [CrossRef]
- Zheng, M.; Shi, J.; Shi, J.; Wang, Q.; Li, Y. Antimicrobial effects of volatiles produced by two antagonistic Bacillus strains on the anthracnose pathogen in postharvest mangos. Biol. Control 2013, 65, 200–206. [Google Scholar] [CrossRef]
- Liarzi, O.; Bucki, P.; Braun Miyara, S.; Ezra, D. Bioactive Volatiles from an endophytic Daldinia cf. concentrica isolate affect the viability of the plant parasitic nematode Meloidogyne javanica. PLoS ONE 2016, 11, e0168437. [Google Scholar] [CrossRef]
- Seo, H.J.; Park, A.R.; Kim, S.; Yeon, J.; Yu, N.H.; Ha, S.; Chang, J.Y.; Park, H.W.; Kim, J.C. Biological control of root-knot nematodes by organic acid-producing Lactobacillus brevis WiKim0069 isolated from kimchi. Plant Pathol. J. 2019, 35, 662–673. [Google Scholar] [CrossRef]
- Pacule, H.B.; Vanegas, J.A.G.; Terra, W.C.; Campos, V.P.; Oliveira, D.F. (R)-Carvone is a potential soil fumigant against Meloidogyne incognita whose likely enzymatic target in the nematode is acetylcholinesterase. Exp. Parasitol. 2022, 241, 108359. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Ferrari, F.; Giannakou, I.; Menkissoglu-Spiroudi, U. Synergistic and antagonistic interactions of terpenes against Meloidogyne incognita and the nematicidal activity of essential oils from seven plants indigenous to Greece. Pest Manag. Sci. 2011, 67, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Parida, L.; Neogi, S.; Padmanabhan, V. Effect of temperature pre-exposure on the locomotion and chemotaxis of C. elegans. PLoS ONE 2014, 9, e111342. [Google Scholar] [CrossRef] [PubMed]
- Bargmann, C.I.; Hartwieg, E.; Horvitz, H.R. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 1993, 74, 515–527. [Google Scholar] [CrossRef]
- Wang, P.; Sun, Y.; Yang, L.; Hu, Y.; Li, J.; Wang, J.; Zhang, F.; Liu, Y. Chemotactic responses of the root-knot nematode Meloidogyne incognita to Streptomyces plicatus. FEMS Microbiol. Lett. 2019, 366, fnz234. [Google Scholar] [CrossRef] [PubMed]

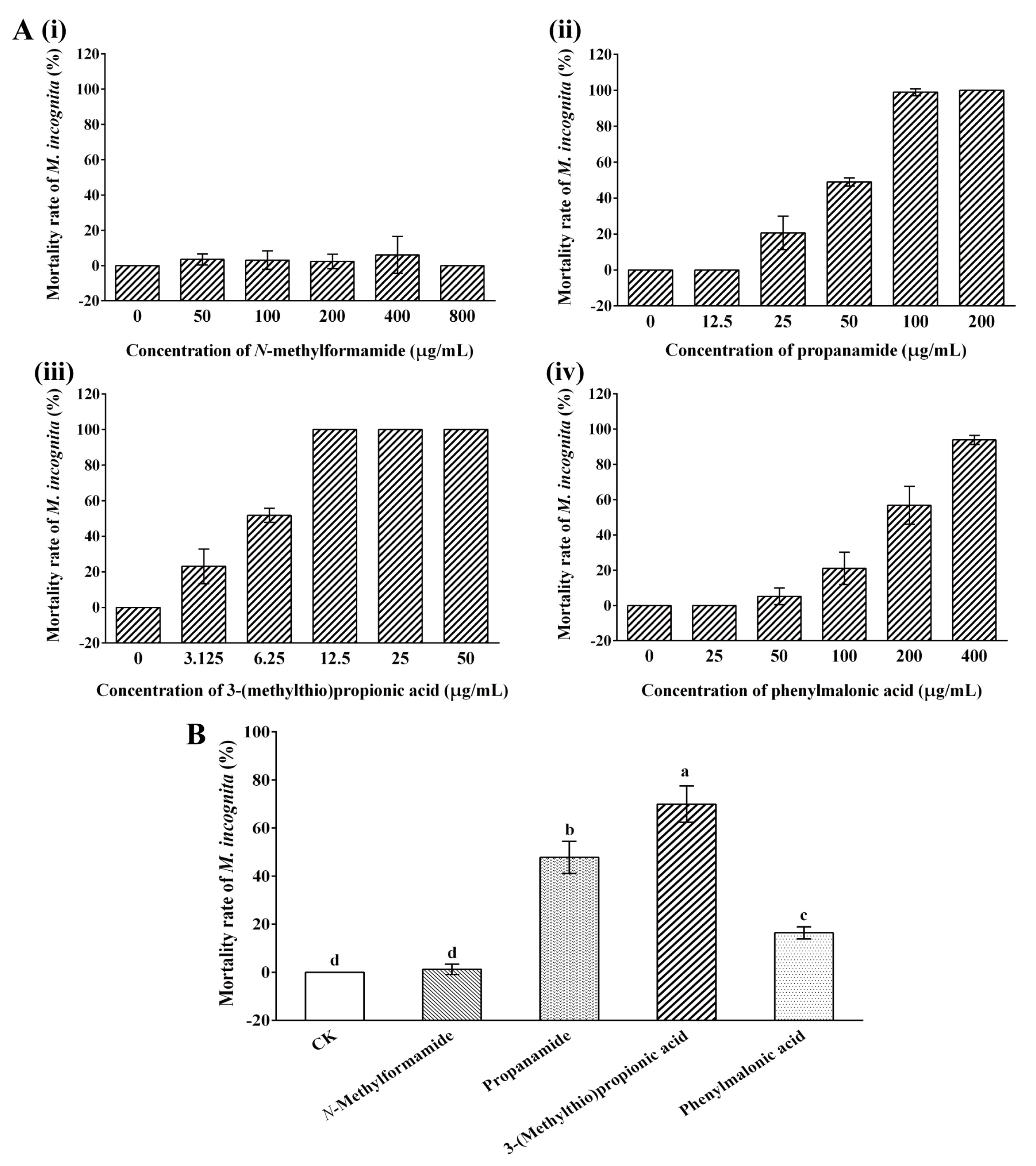

| Peak Number | Retention Time (min) | Compound | CAS Number | Area Percentage(%) |
|---|---|---|---|---|
| 6 | 12.43 | N-Methylformamide | 123-39-7 | 61.78 |
| 7 | 12.64 | Propanamide | 79-05-0 | 4.57 |
| 11 | 15.77 | 3-(Methylthio)propionic acid | 646-01-5 | 7.32 |
| 14 | 17.75 | Phenylmalonic acid | 2613-89-0 | 5.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Wang, Y.; Zhu, L.; Min, Y.; Tian, Y.; Gong, Y.; Liu, X. 3-(Methylthio)Propionic Acid from Bacillus thuringiensis Berliner Exhibits High Nematicidal Activity against the Root Knot Nematode Meloidogyne incognita (Kofoid and White) Chitwood. Int. J. Mol. Sci. 2024, 25, 1708. https://doi.org/10.3390/ijms25031708
Chen L, Wang Y, Zhu L, Min Y, Tian Y, Gong Y, Liu X. 3-(Methylthio)Propionic Acid from Bacillus thuringiensis Berliner Exhibits High Nematicidal Activity against the Root Knot Nematode Meloidogyne incognita (Kofoid and White) Chitwood. International Journal of Molecular Sciences. 2024; 25(3):1708. https://doi.org/10.3390/ijms25031708
Chicago/Turabian StyleChen, Ling, Yueying Wang, Lei Zhu, Yong Min, Yuxi Tian, Yan Gong, and Xiaoyan Liu. 2024. "3-(Methylthio)Propionic Acid from Bacillus thuringiensis Berliner Exhibits High Nematicidal Activity against the Root Knot Nematode Meloidogyne incognita (Kofoid and White) Chitwood" International Journal of Molecular Sciences 25, no. 3: 1708. https://doi.org/10.3390/ijms25031708





