Retinoic Acid Action in Cumulus Cells: Implications for Oocyte Development and In Vitro Fertilization
Abstract
:1. The Search for High Quality Oocytes
2. Retinoic Acid Levels in Cumulus Cells as a Non-Invasive Predictor of Oocyte Quality
3. RA Can Promote Oocyte Competency via Regulation of Cx43 in CGC
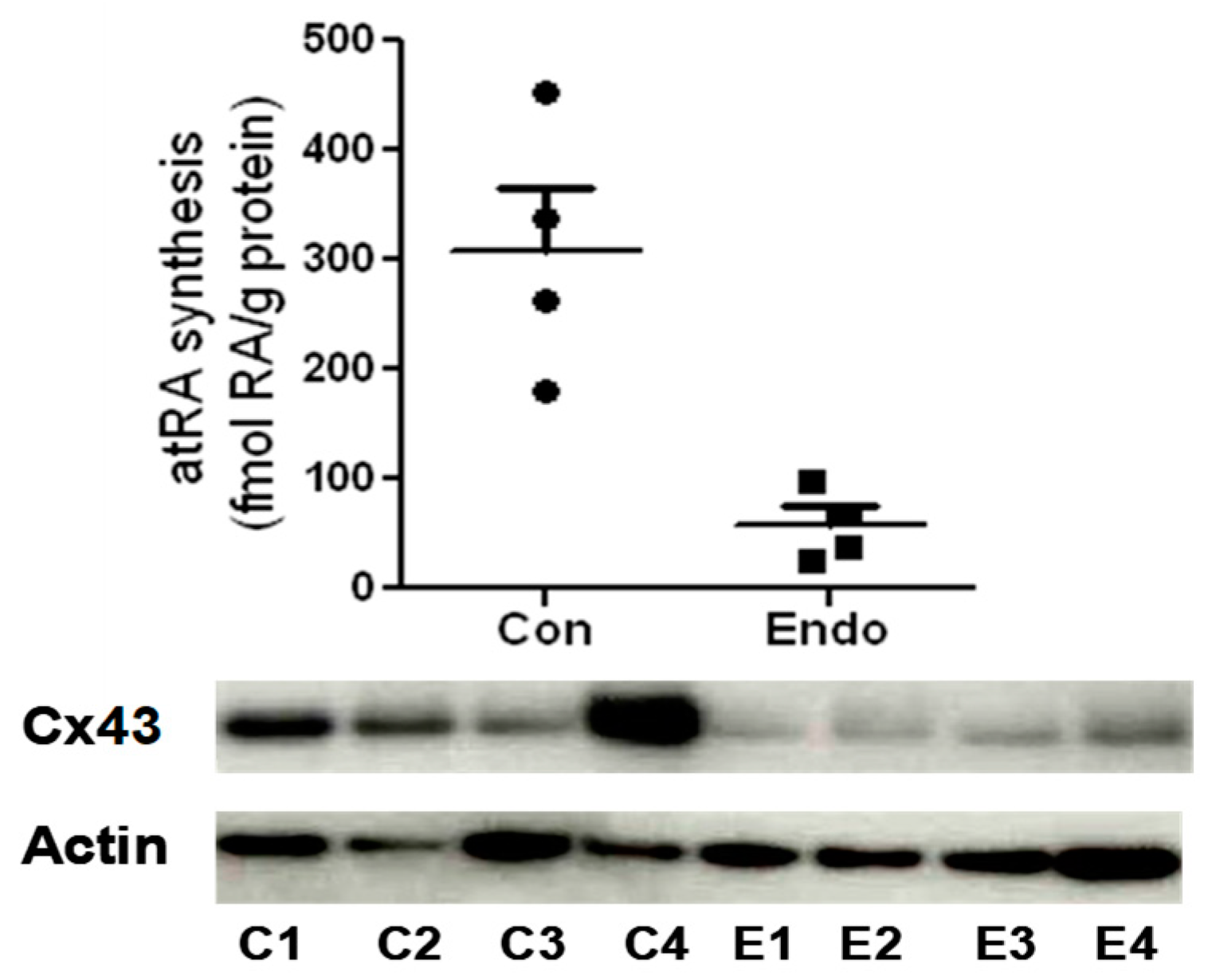
4. A Role for RA in Endometriosis-Associated Infertility via Action on Cx43
5. Concluding Remarks
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Del Collado, M.; Andrade, G.M.; Goncalves, N.J.N.; Fortini, S.; Perecin, F.; Carriero, M.M. The embryo non-invasive pre-implantation diagnosis era: How far are we? Anim. Reprod. 2023, 20, e20230069. [Google Scholar] [CrossRef] [PubMed]
- Neal, S.A.; Morin, S.J.; Franasiak, J.M.; Goodman, L.R.; Juneau, C.R.; Forman, E.J.; Werner, M.D.; Scott, R.T., Jr. Preimplantation genetic testing for aneuploidy is cost-effective, shortens treatment time, and reduces the risk of failed embryo transfer and clinical miscarriage. Fertil. Steril. 2018, 110, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Paulson, R.J. Preimplantation genetic screening: What is the clinical efficiency? Fertil. Steril. 2017, 108, 228–320. [Google Scholar] [CrossRef] [PubMed]
- Assou, S.; Haouzi, D.; De Vos, J.; Hamamah, S. Human cumulus cells as biomarkers for embryo and pregnancy outcomes. Mol. Hum. Reprod. 2010, 16, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.A.; Tan, L.; Suraj, V.; Pera, R.R.; Shen, S. Biomarkers identified with time-lapse imaging: Discovery, validation, and practical application. Fertil. Steril. 2013, 99, 1035–1043. [Google Scholar] [CrossRef]
- Nagy, B.; Poto, L.; Farkas, N.; Koppan, M.; Varnagy, A.; Kovacs, K.; Papp, S.; Bohonyi, N.; Bodis, J. Follicular fluid progesterone concentration is associated with fertilization outcome after ivf: A systematic review and meta-analysis. Reprod. Biomed. Online 2019, 38, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Gagliardi, A.; Landi, C.; Focarelli, R.; De Leo, V.; Luddi, A.; Bini, L.; Piomboni, P. Protein pathways working in human follicular fluid: The future for tailored ivf? Expert. Rev. Mol. Med. 2016, 18, e9. [Google Scholar] [CrossRef] [PubMed]
- Green, K.A.; JFranasiak, M.; Werner, M.D.; Tao, X.; Landis, J.N.; Scott, R.T., Jr.; Treff, N.R. Cumulus cell transcriptome profiling is not predictive of live birth after in vitro fertilization: A paired analysis of euploid sibling blastocysts. Fertil. Steril. 2018, 109, 460–466.e2. [Google Scholar] [CrossRef]
- Armstrong, S.; Bhide, P.; Jordan, V.; Pacey, A.; Farquhar, C. Time-lapse systems for embryo incubation and assessment in assisted reproduction. Cochrane Database Syst. Rev. 2018, 5, CD011320. [Google Scholar] [CrossRef]
- Goldberg, G.S.; Valiunas, V.; Brink, P.R. Selective permeability of gap junction channels. Biochim. Biophys. Acta 2004, 1662, 96–101. [Google Scholar] [CrossRef]
- Wang, H.X.; Tong, D.; El-Gehani, F.; Tekpetey, F.R.; Kidder, G.M. Connexin expression and gap junctional coupling in human cumulus cells: Contribution to embryo quality. J. Cell. Mol. Med. 2009, 13, 972–984. [Google Scholar] [CrossRef]
- Brown, J.A.; Eberhardt, D.M.; Schrick, F.N.; Roberts, M.P.; Godkin, J.D. Expression of retinol-binding protein and cellular retinol-binding protein in the bovine ovary. Mol. Reprod. Dev. 2003, 64, 261–269. [Google Scholar] [CrossRef]
- Ikeda, S.; Kitagawa, M.; Imai, H.; Yamada, M. The roles of vitamin a for cytoplasmic maturation of bovine oocytes. J. Reprod. Dev. 2005, 51, 23–35. [Google Scholar] [CrossRef]
- Livera, G.; Rouiller-Fabre, V.; Valla, J.; Habert, R. Effects of retinoids on the meiosis in the fetal rat ovary in culture. Mol. Cell Endocrinol. 2000, 165, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.L.; Bucco, R.A.; Sierra-Rievera, E.; Osteen, K.G.; Melner, M.H.; Ong, D.E. Synthesis of retinoic acid by rat ovarian cells that express cellular retinoic acid-binding protein-ii. Biol. Reprod. 1999, 60, 110–114. [Google Scholar] [CrossRef]
- Eberhardt, D.M.; Will, W.A.; Godkin, J.D. Retinol administration to superovulated ewes improves in vitro embryonic viability. Biol. Reprod. 1999, 60, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
- Whaley, S.L.; Hedgpeth, V.S.; Farin, C.E.; Martus, N.S.; Jayes, F.C.; Britt, J.H. Influence of vitamin a injection before mating on oocyte development, follicular hormones, and ovulation in gilts fed high-energy diets. J. Anim. Sci. 2000, 78, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Alminana, C.; Gil, M.A.; Cuello, C.; Caballero, I.; Roca, J.; Vazquez, J.M.; Gomez, E.; Martinez, E.A. In vitro maturation of porcine oocytes with retinoids improves embryonic development. Reprod. Fertil. Dev. 2008, 20, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, E.; Mahmoudi, R.; Bahadori, M.H.; Amiri, I. The effect of retinoic acid on in vitro maturation and fertilization rate of mouse germinal vesicle stage oocytes. Cell J. 2011, 13, 19–24. [Google Scholar] [PubMed]
- Tahaei, L.S.; Eimani, H.; Yazdi, P.E.; Ebrahimi, B.; Fathi, R. Effects of retinoic acid on maturation of immature mouse oocytes in the presence and absence of a granulosa cell co-culture system. J. Assist. Reprod. Genet. 2011, 28, 553–558. [Google Scholar] [CrossRef]
- Best, M.W.; Wu, J.; Pauli, S.A.; Kane, M.A.; Pierzchalski, K.; Session, D.R.; Woods, D.C.; Shang, W.; Taylor, R.N.; Sidell, N. A role for retinoids in human oocyte fertilization: Regulation of connexin 43 by retinoic acid in cumulus granulosa cells. Mol. Hum. Reprod. 2015, 21, 527–534. [Google Scholar] [CrossRef]
- Read, C.C.; Dyce, P.W. All-trans retinoic acid exposure increases connexin 43 expression in cumulus cells and improves embryo development in bovine oocytes. Mol. Reprod. Dev. 2019, 86, 1865–1873. [Google Scholar] [CrossRef]
- Tanmahasamut, P.; Sidell, N. Up-regulation of gap junctional intercellular communication and connexin43 expression by retinoic acid in human endometrial stromal cells. J. Clin. Endocrinol. Metab. 2005, 90, 4151–4156. [Google Scholar] [CrossRef]
- Wu, J.; Taylor, R.N.; Sidell, N. Retinoic acid regulates gap junction intercellular communication in human endometrial stromal cells through modulation of the phosphorylation status of connexin 43. J. Cell Physiol. 2013, 228, 903–910. [Google Scholar] [CrossRef]
- Haliloglu, S.; Baspinar, N.; Serpek, B.; Erdem, H.; Bulut, Z. Vitamin a and beta-carotene levels in plasma, corpus luteum and follicular fluid of cyclic and pregnant cattle. Reprod. Domest. Anim. 2002, 37, 96–99. [Google Scholar] [CrossRef]
- Schweigert, F.J.; Steinhagen, B.; Raila, J.; Siemann, A.; Peet, D.; Buscher, U. Concentrations of carotenoids, retinol and alpha-tocopherol in plasma and follicular fluid of women undergoing ivf. Hum. Reprod. 2003, 18, 1259–1264. [Google Scholar] [CrossRef]
- Schweigert, F.J.; Zucker, H. Concentrations of vitamin a, beta-carotene and vitamin e in individual bovine follicles of different quality. J. Reprod. Fertil. 1988, 82, 575–579. [Google Scholar] [CrossRef]
- Pauli, S.A.; Session, D.R.; Shang, W.; Easley, K.; Wieser, F.; Taylor, R.N.; Pierzchalski, K.; Napoli, J.L.; Kane, M.A.; Sidell, N. Analysis of follicular fluid retinoids in women undergoing in vitro fertilization: Retinoic acid influences embryo quality and is reduced in women with endometriosis. Reprod. Sci. 2013, 20, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Pierzchalski, K.; Taylor, R.N.; Nezhat, C.; Jones, J.W.; Napoli, J.L.; Yang, G.; Kane, M.A.; Sidell, N. Retinoic acid biosynthesis is impaired in human and murine endometriosis. Biol. Reprod. 2014, 91, 84. [Google Scholar] [CrossRef] [PubMed]
- Pierzchalski, K.; Yu, J.; Norman, V.; Kane, M.A. Crbpi regulates mammary retinoic acid homeostasis and the mammary microenvironment. FASEB J. 2013, 27, 1904–1916. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.L.; Ong, D.E. Spatial and temporal patterns of expression of cellular retinol-binding protein and cellular retinoic acid-binding proteins in rat uterus during early pregnancy. Biol. Reprod. 1998, 58, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.A.; Shen, K.; Wang, Y.; Brooks, S.C. Retinoic acid signaling is required for proper morphogenesis of mammary gland. Dev. Dyn. 2005, 234, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Gittens, J.E.; Mhawi, A.A.; Lidington, D.; Ouellette, Y.; Kidder, G.M. Functional analysis of gap junctions in ovarian granulosa cells: Distinct role for connexin43 in early stages of folliculogenesis. Am. J. Physiol. Cell Physiol. 2003, 284, C880-7. [Google Scholar] [CrossRef]
- Tong, D.; Gittens, J.E.; Kidder, G.M.; Bai, D. Patch-clamp study reveals that the importance of connexin43-mediated gap junctional communication for ovarian folliculogenesis is strain specific in the mouse. Am. J. Physiol. Cell Physiol. 2006, 290, C290-7. [Google Scholar] [CrossRef]
- De Los Reyes, M.; Palomino, J.; Gallegos, C.; Espinoza, R.; Dettleff, P.; Peralta, O.A.; Parraguez, V.H.; Ramirez, G. Gene and protein expression of connexins 37 and 43 in cumulus-oocytes complexes throughout the canine oestrous cycle. Reprod. Fertil. Dev. 2020, 32, 976–987. [Google Scholar] [CrossRef]
- Veitch, G.I.; Gittens, J.E.; Shao, Q.; Laird, D.W.; Kidder, G.M. Selective assembly of connexin37 into heterocellular gap junctions at the oocyte/granulosa cell interface. J. Cell Sci. 2004, 117, 2699–2707. [Google Scholar] [CrossRef] [PubMed]
- Read, C.C.; Willhelm, G.; Dyce, P.W. Connexin 43 coupling in bovine cumulus cells, during the follicular growth phase, and its relationship to in vitro embryo outcomes. Mol. Reprod. Dev. 2018, 85, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Norris, R.P.; Freudzon, M.; Mehlmann, L.M.; Cowan, A.E.; Simon, A.M.; Paul, D.L.; Lampe, P.D.; Jaffe, L.A. Luteinizing hormone causes map kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: One of two paths to meiotic resumption. Development 2008, 135, 3229–3238. [Google Scholar] [CrossRef] [PubMed]
- Sela-Abramovich, S.; Chorev, E.; Galiani, D.; Dekel, N. Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology 2005, 146, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Dekel, N. Cellular, biochemical and molecular mechanisms regulating oocyte maturation. Mol. Cell. Endocrinol. 2005, 234, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Ackert, C.L.; Gittens, J.E.; O’Brien, M.J.; Eppig, J.J.; Kidder, G.M. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev. Biol. 2001, 233, 258–270. [Google Scholar] [CrossRef]
- Solan, J.L.; Lampe, P.D. Connexin43 phosphorylation: Structural changes and biological effects. Biochem. J. 2009, 419, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Lampe, P.D.; Lau, A.F. Regulation of gap junctions by phosphorylation of connexins. Arch. Biochem. Biophys. 2000, 384, 205–215. [Google Scholar] [CrossRef]
- Lampe, P.D.; Lau, A.F. The effects of connexin phosphorylation on gap junctional communication. Int. J. Biochem. Cell Biol. 2004, 36, 1171–1186. [Google Scholar] [CrossRef] [PubMed]
- Pahujaa, M.; Anikin, M.; Goldberg, G.S. Phosphorylation of connexin43 induced by src: Regulation of gap junctional communication between transformed cells. Exp. Cell Res. 2007, 313, 4083–4090. [Google Scholar] [CrossRef]
- Weng, S.; Lauven, M.; Schaefer, T.; Polontchouk, L.; Grover, R.; Dhein, S. Pharmacological modification of gap junction coupling by an antiarrhythmic peptide via protein kinase c activation. FASEB J. 2002, 16, 1114–1116. [Google Scholar] [CrossRef] [PubMed]
- Imanaga, I.; Hai, L.; Ogawa, K.; Matsumura, K.; Mayama, T. Phosphorylation of connexin in functional regulation of the cardiac gap junction. Exp. Clin. Cardiol. 2004, 9, 161–164. [Google Scholar]
- Meilleur, M.A.; Akpovi, C.D.; Pelletier, R.M.; Vitale, M.L. Tumor necrosis factor-alpha-induced anterior pituitary folliculostellate ttt/gf cell uncoupling is mediated by connexin 43 dephosphorylation. Endocrinology 2007, 148, 5913–5924. [Google Scholar] [CrossRef]
- Chang, H.K.; Hou, W.S. Retinoic acid modulates interferon-gamma production by hepatic natural killer t cells via phosphatase 2a and the extracellular signal-regulated kinase pathway. J. Interferon Cytokine Res. 2015, 35, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Cho, D.H.; Lee, J.Y.; Lee, H.J.; Ha, Y.; Ahn, J.H.; Jo, I. B56delta subunit of protein phosphatase 2a decreases phosphorylation of endothelial nitric oxide synthase at serine 116: Mechanism underlying aphidicolin-stimulated no production. Nitric Oxide 2015, 50, 46–51. [Google Scholar] [CrossRef]
- Sirnes, S.; Kjenseth, A.; Leithe, E.; Rivedal, E. Interplay between pkc and the map kinase pathway in connexin43 phosphorylation and inhibition of gap junction intercellular communication. Biochem. Biophys. Res. Commun. 2009, 382, 41–45. [Google Scholar] [CrossRef]
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef]
- Nezhat, C.; Littman, E.D.; Lathi, R.B.; Berker, B.; Westphal, L.M.; Giudice, L.C.; Milki, A.A. The dilemma of endometriosis: Is consensus possible with an enigma? Fertil. Steril. 2005, 84, 1587–1588. [Google Scholar] [CrossRef] [PubMed]
- Al-Fadhli, R.; Kelly, S.M.; Tulandi, T.; Tan, S.L. Effects of different stages of endometriosis on the outcome of in vitro fertilization. J. Obstet. Gynaecol. Can. 2006, 28, 888–891. [Google Scholar] [CrossRef]
- Garrido, N.; Pellicer, A.; Remohi, J.; Simon, C. Uterine and ovarian function in endometriosis. Semin. Reprod. Med. 2003, 21, 183–192. [Google Scholar] [CrossRef]
- Kumbak, B.; Kahraman, S.; Karlikaya, G.; Lacin, S.; Guney, A. In vitro fertilization in normoresponder patients with endometriomas: Comparison with basal simple ovarian cysts. Gynecol. Obstet. Investig. 2008, 65, 212–216. [Google Scholar] [CrossRef]
- Hull, M.G.; Williams, J.A.; Ray, B.; McLaughlin, E.A.; Akande, V.A.; Ford, W.C. The contribution of subtle oocyte or sperm dysfunction affecting fertilization in endometriosis-associated or unexplained infertility: A controlled comparison with tubal infertility and use of donor spermatozoa. Hum. Reprod. 1998, 13, 1825–1830. [Google Scholar] [CrossRef]
- Navarro, J.; Garrido, N.; Remohi, J.; Pellicer, A. How does endometriosis affect infertility? Obstet. Gynecol. Clin. N. Am. 2003, 30, 181–192. [Google Scholar] [CrossRef]
- Napoli, J.L. Physiological insights into all-trans-retinoic acid biosynthesis. Biochim. Biophys. Acta 2012, 1821, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, A.; Kane, M.A.; Yu, J.; Jones, J.W.; Qu, H.; Badell, M.; Taylor, R.N.; Sidell, N. Alternatively activated macrophages are the primary retinoic acid-producing cells in human decidua. Reprod. Sci. 2020, 27, 334–341. [Google Scholar] [CrossRef]
- Sanders, T.J.; McCarthy, N.E.; Giles, E.M.; Davidson, K.L.; Haltalli, M.L.; Hazell, S.; Lindsay, J.O.; Stagg, A.J. Increased production of retinoic acid by intestinal macrophages contributes to their inflammatory phenotype in patients with crohn’s disease. Gastroenterology 2014, 146, 1278–1288.e2. [Google Scholar] [CrossRef]
- Cai, S.; Chen, M.; Xue, B.; Zhu, Z.; Wang, X.; Li, J.; Wang, H.; Zeng, X.; Qiao, S.; Zeng, X. Retinoic acid enhances ovarian steroidogenesis by regulating granulosa cell proliferation and mesp2/star/cyp11a1 pathway. J. Adv. Res. 2023, in press. [Google Scholar] [CrossRef]
- Lourenco, B.; Sousa, A.P.; Almeida-Santos, T.; Ramalho-Santos, J. Relation of cumulus cell status with single oocyte maturity, fertilization capability and patient age. J. Reprod. Infertil. 2014, 15, 15–21. [Google Scholar] [PubMed]
- Blomhoff, R. Transport and metabolism of vitamin a. Nutr. Rev. 1994, 52, S13–S23. [Google Scholar] [CrossRef]
- Kayser, S.; Schlenk, R.F.; Platzbecker, U. Management of patients with acute promyelocytic leukemia. Leukemia 2018, 32, 1277–1294. [Google Scholar] [CrossRef]
- Ortiz, N.E.; Nijhawan, R.I.; Weinberg, J.M. Acitretin. Dermatol. Ther. 2013, 26, 390–399. [Google Scholar] [CrossRef]
- Vallerand, I.A.; Lewinson, R.T.; Farris, M.S.; Sibley, C.D.; Ramien, M.L.; Bulloch, A.G.M.; Patten, S.B. Efficacy and adverse events of oral isotretinoin for acne: A systematic review. Br. J. Dermatol. 2018, 178, 76–85. [Google Scholar] [CrossRef]
- Adamson, P.C. Clinical and pharmacokinetic studies of all-trans-retinoic acid in pediatric patients with cancer. Leukemia 1994, 8 (Suppl. S3), S22–S25. [Google Scholar] [PubMed]
- Parmar, S.; Tallman, M.S. Acute promyelocytic leukaemia: A review. Expert. Opin. Pharmacother. 2003, 4, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Nellessen, C.M.; Janzen, V.; Mayer, K.; Giovannini, G.; Gembruch, U.; Brossart, P.; Merz, W.M. Successful treatment of acute promyelocytic leukemia in pregnancy with single-agent all-trans retinoic acid. Arch. Gynecol. Obstet. 2018, 297, 281–284. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidell, N.; Rajakumar, A. Retinoic Acid Action in Cumulus Cells: Implications for Oocyte Development and In Vitro Fertilization. Int. J. Mol. Sci. 2024, 25, 1709. https://doi.org/10.3390/ijms25031709
Sidell N, Rajakumar A. Retinoic Acid Action in Cumulus Cells: Implications for Oocyte Development and In Vitro Fertilization. International Journal of Molecular Sciences. 2024; 25(3):1709. https://doi.org/10.3390/ijms25031709
Chicago/Turabian StyleSidell, Neil, and Augustine Rajakumar. 2024. "Retinoic Acid Action in Cumulus Cells: Implications for Oocyte Development and In Vitro Fertilization" International Journal of Molecular Sciences 25, no. 3: 1709. https://doi.org/10.3390/ijms25031709



