Synthesis and Investigations of the Antitumor Effects of First-Row Transition Metal(II) Complexes Supported by Two Fluorinated and Non-Fluorinated β-Diketonates
Abstract
:1. Introduction
2. Results and Discussion
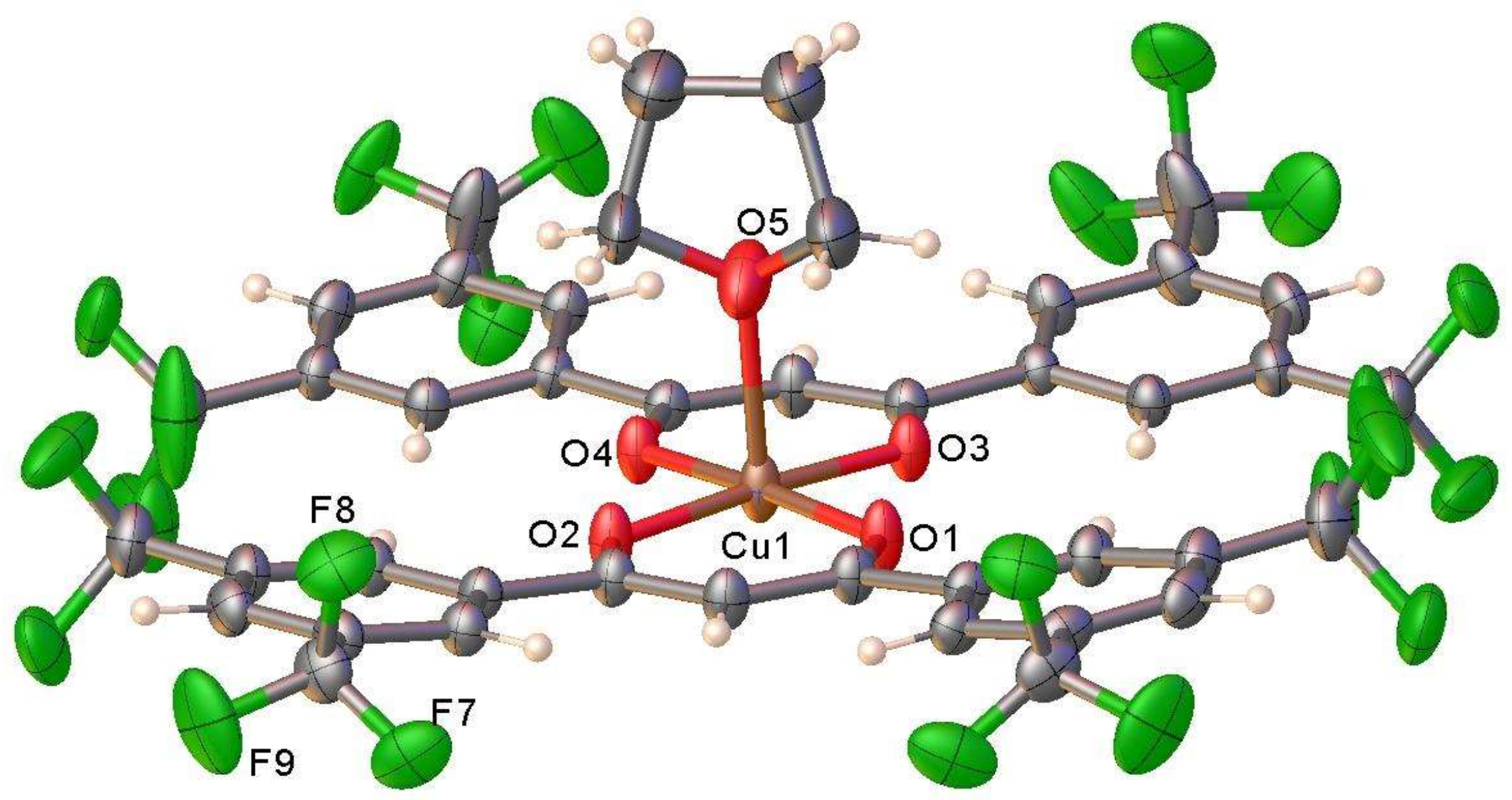
2.1. Synthesis and Characterization
2.2. Biological Studies
3. Materials and Methods
3.1. Chemistry
3.1.1. Materials and General Methods
3.1.2. Synthesis of [Mn(LCF3)2(H2O)2] (1)
3.1.3. Synthesis of [Fe(LCF3)2] (2)
3.1.4. Synthesis of [Co(LCF3)2(H2O)2] (3)
3.1.5. Synthesis of [Ni(LCF3)2(H2O)2] (4)
3.1.6. Synthesis of [Cu(LCF3)2] (5)
3.1.7. Synthesis of [Zn(LCF3)2] (6)
3.1.8. Synthesis of [Mn(LMes)2(H2O)2] (7)
3.1.9. Synthesis of [Fe(LMes)2] (8)
3.1.10. Synthesis of [Co(LMes)2(H2O)2] (9)
3.1.11. Synthesis of [Ni(LMes)2(H2O)2] (10)
3.1.12. Synthesis of [Cu(LMes)2] (11)
3.1.13. Synthesis of [Zn(LMes)2] (12)
3.1.14. Synthesis of [Mn(LPh)2(H2O)2] (13)
3.1.15. Synthesis of [Fe(LPh)2] (14)
3.1.16. Synthesis of [Co(LPh)2(H2O)2] (15)
3.1.17. Synthesis of [Ni(LPh)2(H2O)2] (16)
3.1.18. Synthesis of [Cu(LPh)2] (17)
3.1.19. Synthesis of [Zn(LPh)2].2H2O (18)
3.2. X-ray Data Collection and Structure Determination
3.3. Biology
3.4. Cell Cultures
3.5. MTT Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crossman, A.S.; Marshak, M.P. 1.11—β-Diketones: Coordination and Application. In Comprehensive Coordination Chemistry III; Constable, E.C., Parkin, G., Que, L., Jr., Eds.; Elsevier: Oxford, UK, 2021; pp. 331–365. [Google Scholar]
- Stalpaert, M.; De Vos, D. Stabilizing Effect of Bulky β-Diketones on Homogeneous Mo Catalysts for Deoxydehydration. ACS Sustain. Chem. Eng. 2018, 6, 12197–12204. [Google Scholar] [CrossRef]
- Lo, J.C.; Gui, J.; Yabe, Y.; Pan, C.M.; Baran, P.S. Functionalized olefin cross-coupling to construct carbon-carbon bonds. Nature 2014, 516, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Shafir, A.; Buchwald, S.L. Highly selective room-temperature copper-catalyzed C-N coupling reactions. J. Am. Chem. Soc. 2006, 128, 8742–8743. [Google Scholar] [CrossRef] [PubMed]
- Baik, T.G.; Luis, A.L.; Wang, L.C.; Krische, M.J. Diastereoselective cobalt-catalyzed Aldol and Michael cycloreductions. J. Am. Chem. Soc. 2001, 123, 5112–5113. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Lee, S.H.; Lee, D. Copper-catalyzed N-arylation of amines with hypervalent iodonium salts. Synlett 2000, 7, 1022–1024. [Google Scholar]
- Isayama, S.; Mukaiyama, T. Hydration of Olefins with Molecular Oxygen and Triethylsilane Catalyzed by Bis(trifluoroacetylacetonato)cobalt(II). Chem. Lett. 1989, 18, 569–572. [Google Scholar] [CrossRef]
- Krajewski, S.M.; Crossman, A.S.; Akturk, E.S.; Suhrbier, T.; Scappaticci, S.J.; Staab, M.W.; Marshak, M.P. Sterically encumbered beta-diketonates and base metal catalysis. Dalton Trans. 2019, 48, 10714–10722. [Google Scholar] [CrossRef] [PubMed]
- Akturk, E.S.; Scappaticci, S.J.; Seals, R.N.; Marshak, M.P. Bulky beta-Diketones Enabling New Lewis Acidic Ligand Platforms. Inorg. Chem. 2017, 56, 11466–11469. [Google Scholar] [CrossRef]
- Crossman, A.S.; Larson, A.T.; Shi, J.X.; Krajewski, S.M.; Akturk, E.S.; Marshak, M.P. Synthesis of Sterically Hindered beta-Diketones via Condensation of Acid Chlorides with Enolates. J. Org. Chem. 2019, 84, 7434–7442. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lien, J.C.; Chen, C.Y.; Hung, C.C.; Lin, H.C. Design, synthesis and evaluation of novel derivatives of curcuminoids with cytotoxicity. Int. J. Mol. Sci. 2021, 22, 12171. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as Curecumin: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef]
- Krishnankutty, K.; Venugopalan, P. Metal chelates of curcuminoids. Synth. React. Inorg. Met. Org. Chem. 1998, 28, 1313–1325. [Google Scholar] [CrossRef]
- Meza-Morales, W.; Alejo-Osorio, Y.; Alvarez-Ricardo, Y.; Obregon-Mendoza, M.A.; Machado-Rodriguez, J.C.; Arenaza-Corona, A.; Toscano, R.A.; Ramirez-Apan, M.T.; Enriquez, R.G. Homoleptic Complexes of Heterocyclic Curcuminoids with Mg(II) and Cu(II): First Conformationally Heteroleptic Case, Crystal Structures, and Biological Properties. Molecules 2023, 28, 1434. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; DuBourdieu, D.; Srivastava, A.; Kumar, P.; Lall, R. Metal-Curcumin Complexes in Therapeutics: An Approach to Enhance Pharmacological Effects of Curcumin. Int. J. Mol. Sci. 2021, 22, 7094. [Google Scholar] [CrossRef] [PubMed]
- Meza-Morales, W.; Estevez-Carmona, M.M.; Alvarez-Ricardo, Y.; Obregon-Mendoza, M.A.; Cassani, J.; Ramirez-Apan, M.T.; Escobedo-Martinez, C.; Soriano-Garcia, M.; Reynolds, W.F.; Enriquez, R.G. Full Structural Characterization of Homoleptic Complexes of Diacetylcurcumin with Mg, Zn, Cu, and Mn: Cisplatin-level Cytotoxicity in Vitro with Minimal Acute Toxicity in Vivo. Molecules 2019, 24, 1598. [Google Scholar] [CrossRef]
- Meza-Morales, W.; Machado-Rodriguez, J.C.; Alvarez-Ricardo, Y.; Obregon-Mendoza, M.A.; Nieto-Camacho, A.; Toscano, R.A.; Soriano-Garcia, M.; Cassani, J.; Enriquez, R.G. A New Family of Homoleptic Copper Complexes of Curcuminoids: Synthesis, Characterization and Biological Properties. Molecules 2019, 24, 910. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, C.M.; Shi, H.F.; Yang, M.Y.; Liu, Y.; Ji, P.; Chen, H.J.; Tan, R.X.; Li, E.G. Curcumin is a biologically active copper chelator with antitumor activity. Phytomedicine 2016, 23, 1–8. [Google Scholar] [CrossRef]
- Mendiguchia, B.S.; Aiello, I.; Crispini, A. Zn(ii) and Cu(ii) complexes containing bioactive O,O-chelated ligands: Homoleptic and heteroleptic metal-based biomolecules. Dalton Trans. 2015, 44, 9321–9334. [Google Scholar] [CrossRef]
- Wanninger, S.; Lorenz, V.; Subhan, A.; Edelmann, F.T. Metal complexes of curcumin—Synthetic strategies, structures and medicinal applications. Chem. Soc. Rev. 2015, 44, 4986–5002. [Google Scholar] [CrossRef]
- Leung, M.H.M.; Harada, T.; Kee, T.W. Delivery of curcumin and medicinal effects of the copper(II)-curcumin complexes. Curr. Pharm. Des. 2013, 19, 2070–2083. [Google Scholar]
- Zhou, S.; Xue, X.; Jiang, B.; Tian, Y. Metal complexes of a novel bis-β-diketone-type ligand and its copper(II) complexes of two-photon biological imaging. Sci. China Chem. 2012, 55, 334–340. [Google Scholar] [CrossRef]
- Aliaga-Alcalde, N.; Marques-Gallego, P.; Kraaijkamp, M.; Herranz-Lancho, C.; den Dulk, H.; Gorner, H.; Roubeau, O.; Teat, S.J.; Weyhermuller, T.; Reedijk, J. Copper Curcuminoids Containing Anthracene Groups: Fluorescent Molecules with Cytotoxic Activity. Inorg. Chem. 2010, 49, 9655–9663. [Google Scholar] [CrossRef] [PubMed]
- Barik, A.; Mishra, B.; Kunwar, A.; Kadam, R.M.; Shen, L.; Dutta, S.; Padhye, S.; Satpati, A.K.; Zhang, H.Y.; Priyadarsini, K.I. Comparative study of copper(II)-curcumin complexes as superoxide dismutase mimics and free radical scavengers. Eur. J. Med. Chem. 2007, 42, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.H.; Bohmerle, K.; Polishchuk, E.; Martins, C.; Toleikis, P.; Tse, J.; Yuen, V.; McNeill, J.H.; Orvig, C. Complementary inhibition of synoviocyte, smooth muscle cell or mouse lymphoma cell proliferation by a vanadyl curcumin complex compared to curcumin alone. J. Inorg. Biochem. 2004, 98, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Krishnankutty, K.; John, V.D. Synthesis, characterization, and antitumour studies of metal chelates of some synthetic curcuminoids. Synt. React. Inorg. Met. Org. Chem. 2003, 33, 343–358. [Google Scholar] [CrossRef]
- Schilling, T.; Keppler, K.B.; Heim, M.E.; Niebch, G.; Dietzfelbinger, H.; Rastetter, J.; Hanauske, A.R. Clinical phase I and pharmacokinetic trial of the new titanium complex budotitane. Investig. New Drugs 1995, 13, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, H.; Berger, M.R.; Keppler, B.K.; Schmähl, D. Efficacy of β-diketonato complexes of titanium, zirconium, and hafnium against chemically induced autochthonous colonic tumors in rats. J. Cancer Res. Clin. Oncol. 1987, 113, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Kljun, J.; Turel, I. β-Diketones as Scaffolds for Anticancer Drug Design—From Organic Building Blocks to Natural Products and Metallodrug Components. Eur. J. Inorg. Chem. 2017, 2017, 1655–1666. [Google Scholar] [CrossRef]
- Seršen, S.; Kljun, J.; Kryeziu, K.; Panchuk, R.; Alte, B.; Körner, W.; Heffeter, P.; Berger, W.; Turel, I. Structure-related mode-of-action differences of anticancer organoruthenium complexes with β-diketonates. J. Med. Chem. 2015, 58, 3984–3996. [Google Scholar] [CrossRef]
- Aliende, C.; Pérez-Manrique, M.; Jalón, F.A.; Manzano, B.R.; Rodríguez, A.M.; Cuevas, J.V.; Espino, G.; Martínez, M.Á.; Massaguer, A.; González-Bártulos, M.; et al. Preparation of new half sandwich ruthenium arene complexes with aminophosphines as potential chemotherapeutics. J. Inorg. Biochem. 2012, 117, 171–188. [Google Scholar] [CrossRef]
- Vock, C.A.; Renfrew, A.K.; Scopelliti, R.; Juillerat-Jeanneret, L.; Dyson, P.J. Influence of the diketonato ligand on the cytotoxicities of [Ru(η6-p-cymene)-(R2acac)(PTA)]+ complexes (PTA = 1,3,5-triaza-7-phosphaadamantane). Eur. J. Inorg. Chem. 2008, 2008, 1661–1671. [Google Scholar] [CrossRef]
- Melchart, M.; Habtemariam, A.; Parsons, S.; Sadler, P.J. Chlorido-, aqua-, 9-ethylguanine- and 9-ethyladenine-adducts of cytotoxic ruthenium arene complexes containing O,O-chelating ligands. J. Inorg. Biochem. 2007, 101, 1903–1912. [Google Scholar] [CrossRef]
- Fernández, R.; Melchart, M.; Habtemariam, A.; Parsons, S.; Sadler, P.J. Use of chelating ligands to tune the reactive site of half-sandwich ruthenium(II)-arene anticancer complexes. Chem. Eur. J. 2004, 10, 5173–5179. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Kennedy, D.C.; Patrick, B.O.; James, B.R. Ruthenium(II) acetylacetonato-sulfoxide complexes. Inorg. Chem. Commun. 2003, 6, 996–1000. [Google Scholar] [CrossRef]
- Do Couto Almeida, J.; Marzano, I.M.; De Paula, F.C.S.; Pivatto, M.; Lopes, N.P.; De Souza, P.C.; Pavan, F.R.; Formiga, A.L.B.; Pereira-Maia, E.C.; Guerra, W. Complexes of platinum and palladium with β-diketones and DMSO: Synthesis, characterization, molecular modeling, and biological studies. J. Mol. Struct. 2014, 1075, 370–376. [Google Scholar] [CrossRef]
- Wilson, J.J.; Lippard, S.J. In vitro anticancer activity of cis-diammineplatinum(II) complexes with β-diketonate leaving group ligands. J. Med. Chem. 2012, 55, 5326–5336. [Google Scholar] [CrossRef] [PubMed]
- Muscella, A.; Calabriso, N.; Vetrugno, C.; Fanizzi, F.P.; De Pascali, S.A.; Marsigliante, S. The signalling axis mediating neuronal apoptosis in response to [Pt(O,O′-acac)(γ-acac)(DMS)]. Biochem. Pharmacol. 2011, 81, 1271–1285. [Google Scholar] [CrossRef] [PubMed]
- Muscella, A.; Calabriso, N.; Vetrugno, C.; Fanizzi, F.P.; De Pascali, S.A.; Storelli, C.; Marsigliante, S. The platinum (II) complex [Pt(O,O’-acac)(γ-acac)(DMS)] alters the intracellular calcium homeostasis in MCF-7 breast cancer cells. Biochem. Pharmacol. 2011, 81, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Muscella, A.; Calabriso, N.; Vetrugno, C.; Urso, L.; Fanizzi, F.P.; De Pascali, S.A.; Marsigliante, S. Sublethal concentrations of the platinum(II) complex [Pt(O,O′-acac) (γ-acac)(DMS)] alter the motility and induce anoikis in MCF-7 cells. Br. J. Pharmacol. 2010, 160, 1362–1377. [Google Scholar] [CrossRef]
- Muscella, A.; Calabriso, N.; Fanizzi, F.P.; De Pascali, S.A.; Urso, L.; Ciccarese, A.; Migoni, D.; Marsigliante, S. [Pt(O,O′-acac)(γ-acac)(DMS)], a new Pt compound exerting fast cytotoxicity in MCF-7 breast cancer cells via the mitochondrial apoptotic pathway. Br. J. Pharmacol. 2008, 153, 34–49. [Google Scholar] [CrossRef]
- Schwartz, P.; Meischen, S.J.; Gale, G.R.; Atkins, L.M.; Smith, A.B.; Walker, E.M., Jr. Preparation and antitumor evaluation of water-soluble derivatives of dichloro(1,2-diaminocyclohexane)platinum(II). Cancer Treat. Rep. 1977, 61, 1519–1525. [Google Scholar] [PubMed]
- Cleare, M.J.; Hoeschele, J.D. Anti-tumour platinum compounds. Relationship between structure and activity. Platin. Met. Rev. 1973, 17, 2–13. [Google Scholar]
- Figueroa-DePaz, Y.; Resendiz-Acevedo, K.; Dávila-Manzanilla, S.G.; García-Ramos, J.C.; Ortiz-Frade, L.; Serment-Guerrero, J.; Ruiz-Azuara, L. DNA, a target of mixed chelate copper(II) compounds (Casiopeinas®) studied by electrophoresis, UV–vis and circular dichroism techniques. J. Inorg. Biochem. 2022, 231, 111772. [Google Scholar] [CrossRef] [PubMed]
- Serment-Guerrero, J.; Bravo-Gomez, M.E.; Lara-Rivera, E.; Ruiz-Azuara, L. Genotoxic assessment of the copper chelated compounds Casiopeinas: Clues about their mechanisms of action. J. Inorg. Biochem. 2017, 166, 68–75. [Google Scholar] [CrossRef]
- Correia, I.; Borovic, S.; Cavaco, I.; Matos, C.P.; Roy, S.; Santos, H.M.; Fernandes, L.; Capelo, J.L.; Ruiz-Azuara, L.; Pessoa, J.C. Evaluation of the binding of four anti-tumor Casiopeínas® to human serum albumin. J. Inorg. Biochem. 2017, 175, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Gómez, M.E.; de la Paz, A.L.H.; Gracia-Mora, I. Antineoplastic evaluation of two mixed chelate copper complexes (casiopeínas®) in HCT-15 xenograft model. J. Mex. Chem. Soc. 2013, 57, 205–211. [Google Scholar] [CrossRef]
- García-Ramos, J.C.; Tovar-Tovar, A.; Hernández-Lima, J.; Cortés-Guzmán, F.; Moreno-Esparza, R.; Ruiz-Azuara, L. A new kind of intermolecular stacking interaction between copper (II) mixed chelate complex (Casiopeína III-ia) and adenine. Polyhedron 2011, 30, 2697–2703. [Google Scholar] [CrossRef]
- Ruiz-Azuara, L.; Bravo-Gomez, M.E. Copper Compounds in Cancer Chemotherapy. Curr. Med. Chem. 2010, 17, 3606–3615. [Google Scholar] [CrossRef]
- Aguilar-Jiménez, Z.; Espinoza-Guillén, A.; Resendiz-Acevedo, K.; Fuentes-Noriega, I.; Mejía, C.; Ruiz-Azuara, L. The Importance of Being Casiopeina as Polypharmacologycal Profile (Mixed Chelate– Copper (II) Complexes and Their In Vitro and In Vivo Activities). Inorganics 2023, 11, 394. [Google Scholar]
- Paixao, D.A.; de Oliveira, B.C.A.; Almeida, J.D.; Sousa, L.M.; Lopes, C.D.; Carneiro, Z.A.; Tezuka, D.Y.; Clavijo, J.C.T.; Ellena, J.; Polloni, L.; et al. Crystal structure, anti-Trypanosoma cruzi and cytotoxic activities of Cu(II) complexes bearing beta-diketone and alpha-diimine ligands. Inorg. Chim. Acta 2020, 499, 119164. [Google Scholar] [CrossRef]
- Polloni, L.; Silva, A.C.D.; Teixeira, S.C.; Azevedo, F.V.D.; Zoia, M.A.P.; da Silva, M.S.; Lima, P.; Correia, L.I.V.; Almeida, J.D.; da Silva, C.V.; et al. Action of copper(II) complex with beta-diketone and 1,10-phenanthroline (CBP-01) on sarcoma cells and biological effects under cell. Biomed. Pharmacother. 2019, 112, 108586. [Google Scholar] [CrossRef]
- Malekshah, R.E.; Salehi, M.; Kubicki, M.; Khaleghian, A. Biological studies and computational modeling of two new copper complexes derived from beta-diketones and their nano-complexes. J. Coord. Chem. 2019, 72, 1697–1714. [Google Scholar] [CrossRef]
- Malekshah, R.E.; Salehi, M.; Kubicki, M.; Khaleghian, A. New mononuclear copper(II) complexes from β-diketone and β-keto ester N-donor heterocyclic ligands: Structure, bioactivity, and molecular simulation studies. J. Coord. Chem. 2018, 71, 952–968. [Google Scholar] [CrossRef]
- Maghool, F.; Emami, M.H.; Alipour, R.; Mohammadzadeh, S.; Sereshki, N.; Dehkordi, S.A.E.; Fahim, A.; Tayarani-Najaran, Z.; Sheikh, A.; Kesharwani, P.; et al. Rescue effect of curcumin against copper toxicity. J. Trace Elem. Med. Biol. 2023, 78, 127153. [Google Scholar] [CrossRef]
- Meza-Morales, W.; Alvarez-Ricardo, Y.; Obregón-Mendoza, M.A.; Arenaza-Corona, A.; Ramírez-Apan, M.T.; Toscano, R.A.; Poveda-Jaramillo, J.C.; Enríquez, R.G. Three new coordination geometries of homoleptic Zn complexes of curcuminoids and their high antiproliferative potential. RSC Adv. 2023, 13, 8577–8585. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Li, J.B.; Yu, J.; Wang, Y.Q.; Liu, H.Z.; Lin, G.M.; He, Z.G.; Wang, Y.J. Unique flower-like Cur-metal complexes loaded liposomes for primary and metastatic breast cancer therapy. Mater. Sci. Eng. C 2021, 121, 111835. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.P.; Wei, Z.Z.; Wang, Z.F.; Huang, X.L.; Tan, M.X.; Zou, H.H.; Liang, H. Imaging and therapeutic applications of Zn(ii)-cryptolepine-curcumin molecular probes in cell apoptosis detection and photodynamic therapy. Chem. Commun. 2020, 56, 3999–4002. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.H.; Mei, X.T.; Ye, Y.B.; Xue, T.; Wang, J.S.; Sun, W.J.; Lin, C.X.; Xue, R.X.; Zhang, J.B.; Xu, D.H. Zn(II)-curcumin solid dispersion impairs hepatocellular carcinoma growth and enhances chemotherapy by modulating gut microbiota-mediated zinc homeostasis. Pharmacol. Res. 2019, 150, 104454–104463. [Google Scholar] [CrossRef] [PubMed]
- Greish, K.; Pittala, V.; Taurin, S.; Taha, S.; Bahman, F.; Mathur, A.; Jasim, A.; Mohammed, F.; El-Deeb, I.M.; Fredericks, S.; et al. Curcumin-Copper Complex Nanoparticles for the Management of Triple-Negative Breast Cancer. Nanomaterials 2018, 8, 884. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Chakravarty, A.R. Metal Complexes of Curcumin for Cellular Imaging, Targeting, and Photoinduced Anticancer Activity. Acc. Chem. Res. 2015, 48, 2075–2083. [Google Scholar] [CrossRef]
- Goswami, T.K.; Gadadhar, S.; Gole, B.; Karande, A.A.; Chakravarty, A.R. Photocytotoxicity of copper(II) complexes of curcumin and N-ferrocenylmethyl-L-amino acids. Eur. J. Med. Chem. 2013, 63, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Vajragupta, O.; Boonchoong, P.; Watanabe, H.; Tohda, M.; Kummasud, N.; Sumanont, Y. Manganese complexes of curcumin and its derivatives: Evaluation for the radical scavenging ability and neuroprotective activity. Free Radic. Biol. Med. 2003, 35, 1632–1644. [Google Scholar] [CrossRef]
- Hema, M.K.; Karthik, C.S.; Warad, I.; Lokanath, N.K.; Zarrouk, A.; Kumara, K.; Pampa, K.J.; Mallu, P. Regular square planer bis-(4,4,4-trifluoro-1-(thiophen-2-yl)butane-1,3-dione)/copper(II) complex: Trans/cis-DFT isomerization, crystal structure, thermal, solvatochromism, hirshfeld surface and DNA-binding analysis. J. Mol. Struct. 2018, 1157, 69–77. [Google Scholar] [CrossRef]
- Pramanik, A.; Laha, D.; Pramanik, P.; Karmakar, P. A novel drug copper acetylacetonate loaded in folic acid-tagged chitosan nanoparticle for efficient cancer cell targeting. J. Drug Target. 2014, 22, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.F.; Shen, Z.H.; Shi, Y.; He, Q.; Xia, Q.C. Synthesis, characterization, crystal structure, and biological activity of the copper complex. Russ. J. Coord. Chem. 2010, 36, 458–462. [Google Scholar] [CrossRef]
- Pellei, M.; Bagnarelli, L.; Gabrielli, S.; Lupidi, G.; Cimarelli, C.; Stella, F.; Dolmella, A.; Santini, C. Copper(II) complexes based on isopropyl ester derivatives of bis(pyrazol-1-yl)acetate ligands with catalytic potency in organic macro(molecules) synthesis. Inorg. Chim. Acta 2023, 544, 121234. [Google Scholar] [CrossRef]
- Del Bello, F.; Pellei, M.; Bagnarelli, L.; Santini, C.; Giorgioni, G.; Piergentili, A.; Quaglia, W.; Battocchio, C.; Iucci, G.; Schiesaro, I.; et al. Cu(I) and Cu(II) Complexes Based on Lonidamine-Conjugated Ligands Designed to Promote Synergistic Antitumor Effects. Inorg. Chem. 2022, 61, 4919–4937. [Google Scholar] [CrossRef]
- Pellei, M.; Bagnarelli, L.; Luciani, L.; Del Bello, F.; Giorgioni, G.; Piergentili, A.; Quaglia, W.; De Franco, M.; Gandin, V.; Marzano, C.; et al. Synthesis and Cytotoxic Activity Evaluation of New Cu(I) Complexes of Bis(pyrazol-1-yl) Acetate Ligands Functionalized with an NMDA Receptor Antagonist. Int. J. Mol. Sci. 2020, 21, 2616. [Google Scholar] [CrossRef]
- Gabrielli, S.; Pellei, M.; Venditti, I.; Fratoddi, I.; Battocchio, C.; Iucci, G.; Schiesaro, I.; Meneghini, C.; Palmieri, A.; Marcantoni, E.; et al. Development of new and efficient copper(II) complexes of hexyl bis(pyrazolyl)acetate ligands as catalysts for allylic oxidation. Dalton Trans. 2020, 49, 15622–15632. [Google Scholar] [CrossRef]
- Morelli, M.B.; Amantini, C.; Santoni, G.; Pellei, M.; Santini, C.; Cimarelli, C.; Marcantoni, E.; Petrini, M.; Del Bello, F.; Giorgioni, G.; et al. Novel antitumor copper(ii) complexes designed to act through synergistic mechanisms of action, due to the presence of an NMDA receptor ligand and copper in the same chemical entity. New J. Chem. 2018, 42, 11878–11887. [Google Scholar] [CrossRef]
- Gandin, V.; Ceresa, C.; Esposito, G.; Indraccolo, S.; Porchia, M.; Tisato, F.; Santini, C.; Pellei, M.; Marzano, C. Therapeutic potential of the phosphino Cu(I) complex (HydroCuP) in the treatment of solid tumors. Sci. Rep. 2017, 7, 13936. [Google Scholar] [CrossRef] [PubMed]
- Tisato, F.; Marzano, C.; Peruzzo, V.; Tegoni, M.; Giorgetti, M.; Damjanovic, M.; Trapananti, A.; Bagno, A.; Santini, C.; Pellei, M.; et al. Insights into the cytotoxic activity of the phosphane copper(I) complex Cu(thp)(4) PF6. J. Inorg. Biochem. 2016, 165, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Pellei, M.; Papini, G.; Trasatti, A.; Giorgetti, M.; Tonelli, D.; Minicucci, M.; Marzano, C.; Gandin, V.; Aquilanti, G.; Dolmella, A.; et al. Nitroimidazole and glucosamine conjugated heteroscorpionate ligands and related copper(II) complexes. Syntheses, biological activity and XAS studies. Dalton Trans. 2011, 40, 9877–9888. [Google Scholar] [CrossRef] [PubMed]
- Papini, G.; Bandoli, G.; Dolmella, A.; Gioia Lobbia, G.; Pellei, M.; Santini, C. New homoleptic carbene transfer ligands and related coinage metal complexes. Inorg. Chem. Commun. 2008, 11, 1103–1106. [Google Scholar] [CrossRef]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef]
- Swallow, S. Chapter Two—Fluorine in Medicinal Chemistry. In Progress in Medicinal Chemistry; Lawton, G., Witty, D.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 54, pp. 65–133. [Google Scholar]
- Wang, J.; Sanchez-Rosello, M.; Acena, J.L.; del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef]
- Shah, P.; Westwell, A.D. The role of fluorine in medicinal chemistry. J. Enzym. Inhib. Med. Chem. 2007, 22, 527–540. [Google Scholar] [CrossRef]
- Isanbor, C.; O’Hagan, D. Fluorine in medicinal chemistry: A review of anti-cancer agents. J. Fluor. Chem. 2006, 127, 303–319. [Google Scholar] [CrossRef]
- Doerrer, L.H.; Dias, H.V.R. Fluorinated ligands and their effects on physical properties and chemical reactivity. Dalton Trans. 2023, 52, 7770–7771. [Google Scholar] [CrossRef] [PubMed]
- Abdou, I.M.; Saleh, A.M.; Zohdi, H.F. Synthesis and antitumor activity of 5-trifluoromethyl-2,4-dihydropyrazol-3- one nucleosides. Molecules 2004, 9, 109–116. [Google Scholar] [CrossRef]
- Edwards, P.N. Uses of Fluorine in Chemotherapy. In Organofluorine Chemistry: Principles and Commercial Applications; Banks, R.E., Smart, B.E., Tatlow, J.C., Eds.; Springer: Boston, MA, USA, 1994; pp. 501–541. [Google Scholar]
- Lakhi, J.S.; Patterson, M.R.; Dias, H.V.R. Coinage metal metallacycles involving a fluorinated 3,5-diarylpyrazolate. New J. Chem. 2020, 44, 14814–14822. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, P.; Yang, Y.; Huang, X.; Yang, X.-J.; Wu, B. High-Yield Synthesis of 1,3-Dimesityl-propane-1,3-dione: Isolation of Its Aluminum Complex as a Stable Intermediate. Synth. Commun. 2008, 38, 2349–2356. [Google Scholar] [CrossRef]
- Pellei, M.; Del Gobbo, J.; Caviglia, M.; Karade, D.V.; Gandin, V.; Marzano, C.; Noonikara Poyil, A.; Dias, H.V.R.; Santini, C. Synthesis and cytotoxicity studies of Cu(I) and Ag(I) complexes based on sterically hindered β-diketonates with different degrees of fluorination. Dalton Trans. 2023, 52, 12098–12111. [Google Scholar] [CrossRef]
- Crowder, J.M.; Han, H.X.; Wei, Z.; Dikarev, E.V.; Petrukhina, M.A. Unsolvated homo- and heterometallic highly fluorinated β-diketonate complexes of copper(II). Polyhedron 2019, 157, 33–38. [Google Scholar] [CrossRef]
- Larson, A.T.; Crossman, A.S.; Krajewski, S.M.; Marshak, M.P. Copper(II) as a Platform for Probing the Steric Demand of Bulky beta-Diketonates. Inorg. Chem. 2020, 59, 423–432. [Google Scholar] [CrossRef]
- He, C.; Zhang, G.; Ke, J.; Zhang, H.; Miller, J.T.; Kropf, A.J.; Lei, A. Labile Cu(I) Catalyst/Spectator Cu(II) Species in Copper-Catalyzed C–C Coupling Reaction: Operando IR, in Situ XANES/EXAFS Evidence and Kinetic Investigations. J. Am. Chem. Soc. 2013, 135, 488–493. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Layek, S.; Kumari, S.; Anuradha; Agrahari, B.; Ganguly, R.; Pathak, D.D. Synthesis, characterization and crystal structure of a diketone based Cu(II) complex and its catalytic activity for the synthesis of 1,2,3-triazoles. Inorg. Chim. Acta 2016, 453, 735–741. [Google Scholar] [CrossRef]
- Soldatov, D.V.; Henegouwen, A.T.; Enright, G.D.; Ratcliffe, C.I.; Ripmeester, J.A. Nickel(II) and zinc(II) dibenzoylmethanates: Molecular and crystal structure, polymorphism, and guest- or temperature-induced oligomerization. Inorg. Chem. 2001, 40, 1626–1636. [Google Scholar] [CrossRef] [PubMed]
- Abbati, G.L.; Cornia, A.; Fabretti, A.C.; Caneschi, A.; Gatteschi, D. Structure and Magnetic Properties of a Mixed-Valence Heptanuclear Manganese Cluster. Inorg. Chem. 1998, 37, 3759–3766. [Google Scholar] [CrossRef] [PubMed]
- Takashima, Y.; Hanamura, T.; Maeda, Y. The Mössbauer spectra of iron-dibenzoylmethane complexes. J. Inorg. Nucl. Chem. 1970, 32, 2101–2104. [Google Scholar] [CrossRef]
- Lu, H.J.; Gao, J.; Du, C.X.; Fan, Y.T.; Hou, H.W.; Ding, D.G.; Zhai, J.L. Cobalt (II) complexes of dibenzoylmethane (Hdbm): Crystal structures and axial metathetical reaction of the complexes with pyridine or its derivatives. Chin. J. Inorg. Chem. 2003, 19, 174–178. [Google Scholar]
- Ma, B.Q.; Gao, S.; Wang, Z.M.; Liao, C.S.; Yan, C.H.; Xu, G.X. Synthesis and structure of bis(dibenzoylmethane) copper(II). J. Chem. Crystallogr. 1999, 29, 793–796. [Google Scholar] [CrossRef]
- Knyazeva, A.N.; Shugam, E.A.; Shkol’nikova, L.M. Crystal chemical data on inner complexes of β-diketones—IV. Crystal and molecular structure of copper dibenzoylmethanate. J. Mol. Struct. 1969, 10, 76–79. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Pellei, M.; Santini, C.; Bagnarelli, L.; Caviglia, M.; Sgarbossa, P.; De Franco, M.; Zancato, M.; Marzano, C.; Gandin, V. Novel Silver Complexes Based on Phosphanes and Ester Derivatives of Bis(pyrazol-1-yl)acetate Ligands Targeting TrxR: New Promising Chemotherapeutic Tools Relevant to SCLC Management. Int. J. Mol. Sci. 2023, 24, 4091. [Google Scholar] [CrossRef] [PubMed]



| IC50 (µM) ± S.D. | |||||
|---|---|---|---|---|---|
| HCT-15 | U-1285 | NTERA-2 | BxPC-3 | MCF-7 | |
| [Mn(LCF3)2(H2O)2] (1) | 2.9 ± 0.4 | 17.2 ± 3.0 | 2.8 ± 1.2 | 3.7 ± 0.7 | 2.7 ± 0.6 |
| [Fe(LCF3)2] (2) | >50 | >50 | >50 | >50 | >50 |
| [Co(LCF3)2(H2O)2] (3) | 26.4 ± 3.8 | 39.5 ± 4.6 | 18.5 ± 3.8 | 10.5 ± 2.3 | 14.2 ± 4.2 |
| [Ni(LCF3)2(H2O)2] (4) | 26.1 ± 5.2 | 37.2 ± 3.3 | 16.4 ± 3.2 | 19.5 ± 3.0 | 21.3 ± 3.2 |
| [Cu(LCF3)2] (5) | 15.8 ± 3.2 | 12.1 ± 2.3 | 12.2 ± 1.5 | 15.5 ± 2.4 | 25.3 ± 4.1 |
| [Zn(LCF3)2] (6) | 11.2 ± 3.2 | 16.5 ± 2.7 | 10.0 ± 1.2 | 11.2 ± 0.9 | 8.2 ± 2.5 |
| [Mn(LMes)2(H2O)2] (7) | 1.2 ± 0.7 | 5.3 ± 1.2 | 1.3 ± 0.6 | 2.9 ± 0.6 | 2.3 ± 0.4 |
| [Fe(LMes)2] (8) | >50 | >50 | >50 | >50 | >50 |
| [Co(LMes)2(H2O)2] (9) | 6.8 ± 2.1 | 9.2 ± 2.2 | 3.1 ± 0.7 | 8.4 ± 2.5 | 11.8 ± 2.6 |
| [Ni(LMes)2(H2O)2] (10) | 31.7 ± 4.3 | 33.3 ± 6.4 | 15.5 ± 3.7 | 11.2 ± 1.1 | 23.2 ± 2.8 |
| [Cu(LMes)2] (11) | 2.5 ± 1.0 | 4.1 ± 1.2 | 3.0 ± 0.5 | 1.2 ± 0.4 | 3.2 ± 0.6 |
| [Zn(LMes)2] (12) | 8.9 ± 2.1 | 16.1 ± 3.5 | 2.5 ± 0.5 | 2.0 ± 0.6 | 3.8 ± 1.0 |
| [Mn(LPh)2(H2O)2] (13) | 13.3 ± 2.0 | 8.7 ± 1.6 | 4.8 ± 1.4 | 6.0 ± 1.3 | 5.6 ± 1.2 |
| [Fe(LPh)2] (14) | >50 | >50 | 41.3 ± 6.5 | >50 | 49.9 ± 5.2 |
| [Co(LPh)2(H2O)2] (15) | 20.4 ± 6.1 | 44.8 ± 5.2 | 25.5 ± 3.9 | 10.8 ± 3.1 | 16.5 ± 3.3 |
| [Ni(LPh)2(H2O)2] (16) | 18.7 ± 3.9 | 15.4 ± 2.1 | 6.9 ± 1.3 | 12.4 ± 3.2 | 7.2 ± 2.7 |
| [Cu(LPh)2] (17) | 4.9 ± 1.0 | 9.6 ± 1.3 | 4.8 ± 0.9 | 6.7 ± 0.6 | 5.4 ± 0.5 |
| [Zn(LPh)2].2H2O (18) | 7.2 ± 2.1 | 10.6 ± 1.8 | 11.2 ± 2.2 | 10.9 ± 2.2 | 13.2 ± 2.5 |
| Cisplatin | 18.5 ± 2.2 | 8.3 ± 1.4 | 14.6 ± 3.0 | 11.9 ± 1.3 | 11.0 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellei, M.; Del Gobbo, J.; Caviglia, M.; Gandin, V.; Marzano, C.; Karade, D.V.; Noonikara Poyil, A.; Dias, H.V.R.; Santini, C. Synthesis and Investigations of the Antitumor Effects of First-Row Transition Metal(II) Complexes Supported by Two Fluorinated and Non-Fluorinated β-Diketonates. Int. J. Mol. Sci. 2024, 25, 2038. https://doi.org/10.3390/ijms25042038
Pellei M, Del Gobbo J, Caviglia M, Gandin V, Marzano C, Karade DV, Noonikara Poyil A, Dias HVR, Santini C. Synthesis and Investigations of the Antitumor Effects of First-Row Transition Metal(II) Complexes Supported by Two Fluorinated and Non-Fluorinated β-Diketonates. International Journal of Molecular Sciences. 2024; 25(4):2038. https://doi.org/10.3390/ijms25042038
Chicago/Turabian StylePellei, Maura, Jo’ Del Gobbo, Miriam Caviglia, Valentina Gandin, Cristina Marzano, Deepika V. Karade, Anurag Noonikara Poyil, H. V. Rasika Dias, and Carlo Santini. 2024. "Synthesis and Investigations of the Antitumor Effects of First-Row Transition Metal(II) Complexes Supported by Two Fluorinated and Non-Fluorinated β-Diketonates" International Journal of Molecular Sciences 25, no. 4: 2038. https://doi.org/10.3390/ijms25042038







