Pathophysiology of Diet-Induced Acid Stress
Abstract
:1. Introduction
2. Dietary Contribution to Acid–Base Status
3. Overview of Acid–Base Balance
4. What Is a “Normal” Serum [HCO3−]?
5. Pathophysiology of Late-Phase H+ Stress (Metabolic Acidosis)
5.1. Proximal Renal Tubule Acidosis
5.2. Distal Renal Tubule Acidosis
5.3. Chronic Metabolic Acidosis in Chronic Kidney Disease
6. Pathophysiology of Mid-Phase H+ Stress (Covert Acidosis)
7. Steady-State H+ Accumulation without Decreased Serum [HCO3−], i.e., Covert Acidosis
7.1. How Might Steady-State H+ Accumulation Occur?
7.2. Sequestration of Retained H+ into Non-Serum Fluid Compartments
7.3. Buffering of Retained H+ by HCO3−
7.4. Buffering of H+ by Non-HCO3 Buffers
7.5. H+ Neutralization by Endogenous Organic Acids
7.6. Potential Mechanisms for Organ Injury Associated with Covert Acidosis
8. Potential Strategies for Detecting Covert Acidosis
9. Increased Dietary H+ with Little-to-No Steady-State H+ Retention (Early-Phase Acid Stress)
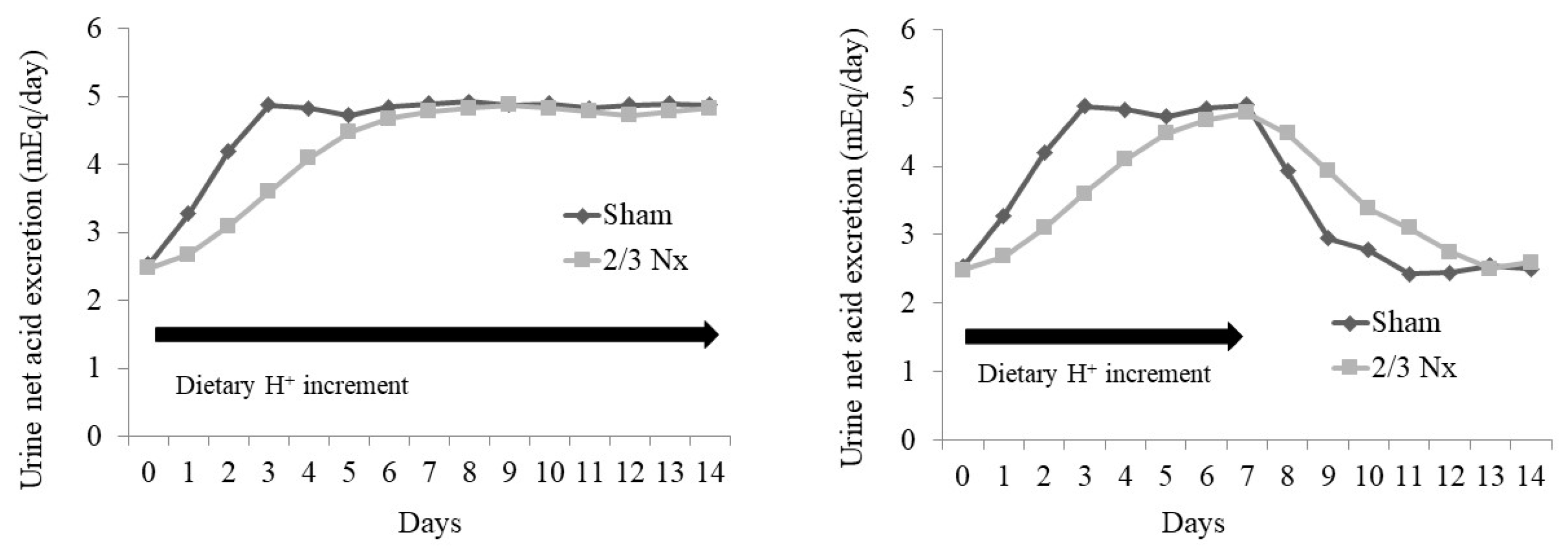
9.1. Effects of an Increment in Dietary H+ on Overall Body H+: Normal vs. Reduced GFR
9.2. Kidney Mechanisms Mediating the Excretion of Dietary H+ That Might Have Adverse Consequences
10. How Clinicians Might Be Alerted to Patients at Risk for Early-Phase H+ Stress
11. Potential Treatment for Early-Phase H+ Stress: Dietary H+ Reduction
11.1. Removing/Limiting H+-Producing Food Components
11.2. Adding Base-Producing Food Components
11.3. Na+-Based Alkali Therapies
11.4. Remove Accumulated Acid: Acid-Binding Polymers
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gropper, S.S. The role of nutrition in chronic disease. Nutrition 2023, 15, 664. [Google Scholar] [CrossRef]
- Mente, A.; Dehghan, M.; Rangarajan, S.; O’Donnel, M.; Hu, W.; Dagenais, G.; Wielgosz, A.; Lear, S.A.; Wei, L.; Diaz, R.; et al. Diet, cardiovascular disease, and mortality in 80 countries. Eur. Heart J. 2023, 44, 2560–2579. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H. Diet and chronic kidney disease. Adv. Nutr. 2019, 10 (Suppl. S4), S367–S379. [Google Scholar] [CrossRef]
- Remer, T.; Manz, F. Potential renal acid load of foods and its influence on urine pH. J. Am. Diet. Assn. 1995, 95, 791–797. [Google Scholar] [CrossRef]
- United State Renal Data System. 2019 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2019. [Google Scholar]
- Adeva, M.M.; Souto, G. Diet-induced metabolic acidosis. Clin. Nutr. 2011, 30, 416–421. [Google Scholar] [CrossRef]
- Navaneethan, S.D.; Schold, J.D.; Arrigain, S.; Jolly, S.E.; Wehbe, E.; Raina, R.; Simon, J.F.; Srinivas, T.R.; Jain, A.; Schreiber, M.J., Jr.; et al. Serum bicarbonate and mortality in stage 3 and stage 4 chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 2395–2402. [Google Scholar] [CrossRef]
- Raphael, K.; Wei, G.; Baird, B.; Greene, T.; Beddhu, S. Higher plasma bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int. 2011, 79, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Dobre, M.; Pajewski, N.M.; Beddhu, D.; Chonchol, M.; Hostetter, T.H.; Li, P.; Rahman, M.; Servilla, K.; Weiner, D.E.; Wright, J.T.; et al. Serum bicarbonate and cardiovascular events in hypertensive adults: Results from the Systolic Blood Pressure Intervention Trial. Nephrol. Dial. Transplant. 2019, 35, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- de Brito-Ashurst, I.; Varagunam, M.; Raftery, M.J.; Yaqoob, M.M. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J. Am. Soc. Nephrol. 2009, 20, 2075–2084. [Google Scholar] [CrossRef]
- Phisitkul, S.; Khanna, A.; Simoni, J.; Broglio, K.; Sheather, S.; Rajab, M.H.; Wesson, D.E. Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int. 2010, 77, 617–623. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.H.; Wesson, D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014, 86, 1031–1038. [Google Scholar] [CrossRef]
- Di Iorio, B.R.; Bellasi, A.; Raphael, K.L.; Santoro, D.; Aucella, F.; Garofano, L.; Ceccarelli, M.; Di Lullo, L.; Capolongo, G.; Di Iorio, M.; et al. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: The UBI Study. J. Nephrol. 2019, 32, 989–1001. [Google Scholar] [CrossRef]
- Wesson, D.E. The continuum of acid stress. Clin. J. Am. Soc. Nephrol. 2021, 16, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Alpern, R.J. Trade-offs in the adaptation to acidosis. Kidney Int. 1995, 47, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Alpern, R.J.; Sakhaee, K. The clinical spectrum of chronic metabolic acidosis: Homeostatic mechanisms produce significant morbidity. Am. J. Kid. Dis. 1997, 29, 291–302. [Google Scholar] [CrossRef] [PubMed]
- DuBose, T.D., Jr. Urine ammonium and pre-clinical acidosis in CKD. J. Am. Soc. Nephrol. 2017, 28, 2258–2260. [Google Scholar] [CrossRef] [PubMed]
- Raphael, K.L. Metabolic acidosis and subclinical metabolic acidosis in CKD. J. Am. Soc. Nephrol. 2018, 29, 376–382. [Google Scholar] [CrossRef]
- JaJoo, R.; Song, L.; Rasmussen, H.; Harris, S.S.; Dawson-Hughes, B. Dietary acid-base balance, bone resorption, and calcium excretion. J. Am. Coll. Nutr. 2006, 25, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; Leung, J.; Woo, J. Association between estimated net endogenous acid production and subsequent decline in muscle mass over four years in ambulatory older Chinese people in Hong Kong: A prospective cohort study. J. Gerentol. A. Biol. Sci. Med. Sci. 2015, 70, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Simoni, J.; Sheather, S.; Broglio, K.; Rajab, M.H.; Wesson, D.E. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int. 2010, 78, 303–309. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Sager, L.N.; Mamun, A.; Madias, N.E.; Wesson, D.E. Urine citrate excretion identifies changes in acid retention as eGFR declines in patients with chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2019, 317, F502–F511. [Google Scholar] [CrossRef]
- Park, M.; Jung, S.J.; Yoon, S.; Yun, J.M.; Yoon, H.J. Association between the markers of metabolic acid load and higher all-cause and cardiovascular mortality in a general population with preserved renal function. Hypertens. Res. 2015, 38, 433–438. [Google Scholar] [CrossRef]
- Han, E.; Kim, G.; Hong, N.; Lee, Y.-H.; Kim, D.W.; Shin, H.J.; Lee, B.-W.; Kang, E.S.; Lee, I.-K.; Cha, B.-S. Association between dietary acid load and the risk of cardiovascular disease: Nationwide surveys (KNHANES 2008–2011). Cardiovasc. Diabetol. 2016, 15, 122. [Google Scholar] [CrossRef]
- Banerjee, T.; Crews, D.; Wesson, D.E.; Tilea, A.; Saran, R.; Rios Burrows, N.; Williams, D.E.; Powe, N.R.; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team. Dietary acid load and chronic kidney disease among adults in the United States. BMC Nephrol. 2014, 15, 137–148. [Google Scholar] [CrossRef]
- Rebholz, C.M.; Coresh, J.; Grams, M.E.; Steffen, L.M.; Anderson, C.A.; Appel, L.J.; Crews, D.C. Dietary acid load and incident chronic kidney disease: Results from the ARIC study. Am. J. Nephrol. 2015, 42, 427–435. [Google Scholar] [CrossRef]
- Banerjee, T.; Crews, D.; Wesson, D.E.; Tilea, A.M.; Saran, R.; Ríos-Burrows, N.; Williams, D.E.; Powe, N.R. High dietary acid load predicts ESRD among US adults with CKD. J. Am. Soc. Nephrol. 2015, 26, 1693–1700. [Google Scholar] [CrossRef]
- Banerjee, T.; Crews, D.C.; Wesson, D.E.; McCulloch, C.E.; Johansen, K.L.; Saydah, S.; Burrows, N.R.; Saran, R.; Gillespie, B.; Bragg-Gresham, J.; et al. Serum Anion Gap a risk factor for ESRD in Adults with Moderate Chronic Kidney Disease. Am. J. Physiology (Ren. Physiol.). 2019, 316, F1244–F1253. [Google Scholar] [CrossRef]
- Kraut, J.A.; Madias, N.E. Metabolic acidosis of CKD: An update. Am. J. Kidney Dis. 2016, 67, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Scialla, J.; Anderson, C.A. Dietary acid load: A novel nutritional target in chronic kidney disease? Adv. Chronic Kid. Dis. 2013, 20, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Martinez-Steel, E.; Du, M.; Pomeranz, J.L.; O’Connor, L.E.; Herrick, K.A.; Luo, H.; Zhang, X.; Mozaffarian, D.; Zhang, F.F. Trends in consumption of ultraprocessed foods among US youths aged 2–19 years, 1999–2018. JAMA 2021, 326, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, L.A.; Morris, R.C., Jr.; Sebastian, A. Dietary sodium chloride intake independently predicts the degree of hyperchloremic metabolic acidosis in healthy humans consuming a net acid-producing diet. Am. J. Physiol. Ren. Physiol. 2007, 293, F521–F525. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R.; Priest, N.; Anderson, N.B. Understanding associations among race, socioeconomic status, and health: Patterns and prospects. Health Psychol. 2016, 35, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Tangri, N.; Mathur, V.; Reaven, N.L.; Funk, S.F.; Wesson, D.E. Relationship Between Metabolic Acidosis and Chronic Kidney Disease Progression Across Racial and Ethnic Groups: An Observational, Retrospective Cohort Study. Am. J. Nephrol. 2022, 53, 603–613. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Sager, L.N.; Madias, N.E.; Wesson, D.E. Urine citrate excretion as a marker of acid retention in patients without overt metabolic acidosis. Kidney Int. 2019, 95, 1190–1196. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.-H.; Wesson, D.E. Dietary acid reduction with fruits and vegetables or sodium bicarbonate reduces kidney injury in subjects with moderately reduced GFR due to hypertensive nephropathy. Kidney Int. 2012, 81, 86–93. [Google Scholar] [CrossRef]
- Nath, K.A.; Hostetter, M.K.; Hostetter, T.H. Pathophysiology of chronic tubulo-interstitial disease in rats-Interactions of dietary acid load, ammonia, and complement component C3. J. Clin. Investig. 1985, 76, 667–675. [Google Scholar] [CrossRef]
- Wesson, D.E. Dietary acid increases blood and renal cortical acid content in rats. Am. J. Physiol. (Ren. Physiol.) 1998, 274, F97–F103. [Google Scholar] [CrossRef]
- Dong, L.; Li, Z.; Leffler, N.R.; Asch, A.S.; Chi, J.T.; Yang, L.V. Acidosis activation of the proton-sensing GPR4 receptor stimulates vascular endothelial cell inflammatory responses revealed by transcriptome analysis. PLoS ONE 2013, 8, e61991. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.E.; Simoni, J.; Broglio, K.; Sheather, S. Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. Am. J. Physiol. Ren. Physiol. 2011, 300, F830–F837. [Google Scholar] [CrossRef]
- Goraya, N.; Madias, N.E.; Mamun, A.; Simoni, J.; Wesson, D.E. Biomarkers of covert acid stress in patients with CKD: A cross-sectional study. Am. J. Nephrol. 2022, 53, 794–805. [Google Scholar] [CrossRef]
- Wesson, D.E. Assessing acid retention in humans. Am. J. Physiol. Ren. Physiol. 2011, 301, F1140–F1142. [Google Scholar] [CrossRef]
- Kraut, J.A.; Lew, V.; Madias, N.E. Re-evaluation of total CO2 concentration in apparently healthy younger adults. Am. J. Nephrol. 2018, 48, 15–20. [Google Scholar] [CrossRef]
- KDIGO Guidelines. Chapter 3: Management of progression and complications of CKD. Kidney Int. 2013, 83 (Suppl. S3), 73–90. [Google Scholar]
- Eustace, J.A.; Astor, B.; Munter, P.M.; Ikizler, T.A.; Coresh, J. Prevalence of acidosis and inflammation and their association with low serum albumin in chronic kidney disease. Kidney Int. 2004, 65, 1031–1040. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Metabolic acidosis and kidney disease: Does bicarbonate therapy slow the progression of CKD? Nephrol. Dial. Transplant. 2012, 27, 3056–3062. [Google Scholar] [CrossRef] [PubMed]
- Raphael, K.L.; Zhang, Y.; Ying, J.; Greene, T. Prevalence of and risk factors for reduced serum bicarbonate in chronic kidney disease. Nephrology 2014, 19, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Moranne, O.; Froissart, M.; Rossert, J.; Gauci, C.; Boffa, J.J.; Haymann, J.P.; M’rad, M.B.; Jacquot, C.; Houillier, P.; Stengel, B.; et al. Timing of onset of CKD-related metabolic complications. J. Am. Soc. Nephrol. 2009, 20, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Goraya, N.; Simoni, J.; Sager, L.N.; Pruszynski, J.; Wesson, D.E. Acid retention in chronic kidney disease is inversely related to GFR. Am. J. Physiol. Ren. Physiol. 2018, 314, F985–F991. [Google Scholar] [CrossRef] [PubMed]
- Bushinsky, D.A.; Krieger, N.S. Effects of acid on bone. Kidney Int. 2022, 101, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.E.; Medina, R.; Grieber, S.; May, R.C.; England, B.K.; Price, S.R.; Bailey, J.L.; Goldberg, A.L. Metabolic acidosis stimulates muscle protein degradation by activating the adenosine triphosphate-dependent pathway involving ubiquitin and proteasomes. J. Clin. Investig. 1994, 93, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- McSherry, E. Renal tubular acidosis in children. Kidney Int. 1981, 20, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Brenner, R.J.; Spring, D.B.; Sebastian, A.; McSherry, E.M.; Palubinskas, A.J.; Morris, R.C., Jr. Incidence of radiographically evident bone disease, nephrocalcinosis, and nephrolithiasis in various types of renal tubular acidosis. N. Engl. J. Med. 1982, 307, 217–221. [Google Scholar] [CrossRef]
- Caruana, R.J.; Buckalew, V.M., Jr. The syndrome of distal (type 1) renal tubular acidosis. Clinical and Laboratory findings in 58 cases. Medicine 1988, 67, 84–89. [Google Scholar] [CrossRef]
- Kurtzman, N.A. Disorders of distal acidification. Kidney Int. 1990, 38, 720–727. [Google Scholar] [CrossRef]
- Coe, F.L.; Parks, J.H. Stone disease in hereditary distal renal tubular acidosis. Ann. Intern. Med. 1980, 93, 60–61. [Google Scholar] [CrossRef] [PubMed]
- McSherry, E.; Morris, R.C., Jr. Attainment and maintenance of normal stature with alkali therapy in infants and children with classic renal tubular acidosis. J. Clin. Investig. 1978, 61, 509–527. [Google Scholar] [CrossRef] [PubMed]
- Domrongkitchaiporn, S.; Pongskul, C.; Sirikulchayanonta, V.; Stitchantrakul, W.; Leeprasert, V.; Ongphiphadhanakul, B.; Radinahamed, P.; Rajatanavin, R. Bone histology and bone mineral density after correction of acidosis in distal renal tubule acidosis. Kidney Int. 2002, 62, 2160–2166. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76 (Suppl. S1), S1–S107. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.E.; Simoni, J. Increased tissue acid mediates progressive GFR decline in animals with reduced nephron mass. Kidney Int. 2009, 75, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.E.; Pruszynki, J.; Cai, W.; Simoni, J. Acid retention with reduced glomerular filtration rate increases urine biomarkers of kidney and bone injury. Kidney Int. 2017, 91, 914–927. [Google Scholar] [CrossRef] [PubMed]
- Lemann, J., Jr.; Bushinsky, D.A.; Hamm, L.L. Bone buffering of acid and base in humans. Am. J. Physiol. 2003, 285, F811–F832. [Google Scholar] [CrossRef]
- Schwartz, W.B.; Jenson, R.L.; Relman, A.S. The disposition of acid administered to sodium-depleted subjects: The renal response and the role of whole-body buffers. J. Clin. Investig. 1954, 33, 587–597. [Google Scholar] [CrossRef]
- Swan, R.C.; Pitts, R.F. Neutralization of infused acid by nephrectomized dogs. J. Clin Investig. 1955, 34, 205–212. [Google Scholar] [CrossRef]
- Bandyopadhyay, U.; Das, D.; Banerjee, R.K. Reactive oxygen species: Oxidative damage and pathogenesis. Curr. Sci. 1999, 77, 658–666. [Google Scholar]
- Levin, A.; Bakris, G.L.; Molitch, M.; Smulders, M.; Tian, J.; Williams, L.A.; Andress, D.L. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorous in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 2007, 71, 31–38. [Google Scholar] [CrossRef]
- Brennan, T.S.; Klahr, S.; Hamm, L.L. Citrate transport in rabbit nephron. Am. J. Physiol. 1986, 251, F683–F689. [Google Scholar] [CrossRef]
- Brennan, S.; Hering-Smith, K.; Hamm, L.L. Effect of pH on citrate reabsorption in the proximal convoluted tubule. Am. J. Physiol. 1988, 255, F301–F306. [Google Scholar] [CrossRef]
- Simpson, D.P. Citrate excretion. A window on renal metabolism. Am. J. Physiol. 1983, 244, F223–F234. [Google Scholar] [CrossRef] [PubMed]
- Hamm, L.L.; Simon, E.E. Roles and mechanisms of urinary buffer excretion. Am. J. Physiol. 1987, 253, F595–F605. [Google Scholar] [CrossRef] [PubMed]
- Bresslau, N.A.; Brinkley, L.; Hill, K.D.; Pack, C.Y. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J. Clin. Endocrinol. Metab. 1988, 66, 140–146. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Sadeghian, R.; Clark, C.C.T.; Abbasi, B. Higher dietary acid load is associated with an increased risk of calcium oxalate kidney stones. J. Ren. Nutr. 2020, 31, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, L.; Sebastian, A. Age and systemic acid-base equilibrium: Analysis of published data. J. Gerontol. 1996, 51, B91–B99. [Google Scholar] [CrossRef] [PubMed]
- Abramowitz, M.K.; Melamed, M.L.; Bauer, C.; Raff, A.C.; Hostetter, T.H. Effects of oral sodium bicarbonate in patients with CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 714–720. [Google Scholar] [CrossRef]
- Wesson, D.E.; Jo, C.-H.; Simoni, J. Angiotensin II receptors mediate increased distal nephron acidification caused by acid retention. Kidney Int. 2012, 82, 1184–1194. [Google Scholar] [CrossRef]
- Wesson, D.E.; Jo, C.-H.; Simoni, J. Angiotensin II-mediated GFR decline in subtotal nephrectomy is due to acid retention associated with reduced GFR. Nephrol. Dial. Transplant. 2015, 30, 762–770. [Google Scholar] [CrossRef]
- Raphael, K.; Gilligan, S.; Hotstetter, T.; Greene, T.; Beddhu, S. Association between urine ammonium and urine TGF-b1 in CKD. Clin. J. Am. Soc. Nephrol. 2018, 13, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Vallet, M.; Metzger, M.; Haymann, J.-P.; Flamant, M.; Gauci, C.; Thervet, E.; Boffa, J.J.; Vrtovsnik, F.; Froissart, M.; Stengel, B.; et al. Urinary ammonia and long-term outcomes in chronic kidney disease. Kidney Int. 2015, 88, 137–145. [Google Scholar] [CrossRef]
- Wesson, D.E. Endogenous endothelins mediate increased distal tubule acidification induced by dietary acid in rats. J. Clin. Investig. 1997, 99, 2203–2211. [Google Scholar] [CrossRef]
- Khanna, A.; Simoni, J.; Wesson, D.E. Endothelin-induced increased aldosterone activity mediates augmented distal nephron acidification as a result of dietary protein. J. Am. Soc. Nephrol. 2005, 16, 1929–1935. [Google Scholar] [CrossRef]
- Wesson, D.E.; Simoni, J. Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney Int. 2010, 78, 1128–1135. [Google Scholar] [CrossRef]
- Izu, Y.; Mizoguchi, F.; Kawmata, A.; Hayata, T.; Kakamoto, T.; Nakashima, K.; Inagami, T.; Ezura, Y.; Noda, M. Angiotensin II type 2 receptor blockade increases bone mass. J. Biol. Chem. 2009, 284, 4857–4864. [Google Scholar] [CrossRef] [PubMed]
- Salcuni, A.S.; Palmieri, S.; Carnevale, V.; Morelli, V.; Battista, C.; Cuarnieri, V.; Guglielmi, G.; Desina, G.; Eller-Vainicher, C.; Beck-Peccoz, P.; et al. Bone involvement in aldosteronism. J. Bone Miner. Res. 2012, 27, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- DuBois, P.; Totola, C.P.; Lodka, D.; Kny, M.; Schmidt, F.; Song, K.; Schmidt, S.; Bassel-Duby, R.; Olson, E.N.; Fielitz, J. Angiotensin II induces skeletal muscle atrophy by activating TFEB-Mediated MuRF1 expression. Circ. Res. 2015, 117, 424–436. [Google Scholar] [CrossRef]
- Burniston, J.G.; Saini, A.S.; Tan, L.-B.; Godspink, D.F. Aldosterone induces myocyte apoptosis in the heart and skeletal muscle of rats in vivo. J. Mol. Cell. Cardiol. 2005, 39, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ortega, M.; Gomez-Garre, D.; Alcazar, R.; Palacios, I.; Bustos, C.; González, S.; Plaza, J.J.; González, E.; Egido, J. Involvement of angiotensin II and endothelin in matrix protein production and renal sclerosis. J. Hypertens. Suppl. 1994, 12, S51–S58. [Google Scholar]
- Greene, E.L.; Kren, S.; Hostetter, T.H. Role of aldosterone in the remnant kidney model in the rat. J. Clin. Investig. 1996, 98, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Remuzzi, G.; Bertani, T. Pathophysiology of progressive nephropathies. N. Eng. J. Med. 1998, 339, 1448–1456. [Google Scholar] [CrossRef]
- Nagami, G.T.; Hamm, L.L. Regulation of Acid-Base Balance in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2017, 24, 274–279. [Google Scholar] [CrossRef]
- Weiner, D.I.; Mitch, M.E.; Sands, J.M. Urea and ammonium metabolism and the control of renal nitrogen excretion. Clin. J. Am. Soc. Nephrol. 2015, 10, 1444–1458. [Google Scholar] [CrossRef]
- Kleger, G.R.; Turgay, M.; Imoberdorf, R.; McNurlan, M.A.; Garlik, P.J.; Ballmer, P.E. Acute metabolic acidosis decreases muscle protein breakdown but not albumin synthesis in humans. Am. J. Kidney Dis. 2001, 38, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Gholami, F.; Bahrampour, N.; Samadi, M.; Rasaei, N.; Yarizadeh, H.; Naghshi, S.; Mirzaei, K. The association of dietary acid load (DAL) with estimated skeletal muscle mass and bone mineral content: A cross-sectional study. BMC Nutr. 2023, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, L.; Banerjee, T.; Powe, N.; Sebastian, A. Acid balance, dietary acid load, and bone effects—A controversial subject. Nutrients 2018, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Bushinsky, D.A.; Hostetter, T.; Klaener, G.; Stasiv, Y.; Lockey, C.; McNulty, S.; Lee, A.; Parsell, D.; Mathur, V.; Li, E.; et al. Randomized, controlled trial of TRC101 to increase serum bicarbonate in patients with CKD. Clin. J. Am. Soc. Nephrol. 2018, 13, 26–35. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 4. Lifestyle Management. Diabetes Care. 2017, 40 (Suppl. S1), S33–S43. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115. [Google Scholar]
- Loniewski, I.; Wesson, D.E. Bicarbonate therapy for kidney disease. Kidney Int. 2014, 85, 529–535. [Google Scholar] [CrossRef]
- Nolan, C.; Califano, J.R.; Butzin, C.A. Influence of calcium acetate or calcium citrate on intestinal aluminum absorption. Kidney Int. 1990, 38, 937–941. [Google Scholar] [CrossRef]
- Wesson, D.E.; Mathur, V.; Tangri, N.; Stasiv, Y.; Parsell, D.; Li, E.; Klaerner, G.; Bushinsky, D.A. Efficacy and safety of veverimer, an oral non-absorbed hydrochloric acid binder, in the treatment of metabolic acidosis associated with chronic kidney disease: A phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Lancet 2019, 393, 1417–1427. [Google Scholar] [CrossRef]
- Wesson, D.E.; Mathur, V.; Tangri, N.; Stasiv, Y.; Parsell, D.; Li, E. A multicentre, randomized, blinded, placebo-controlled 40-week-extension study to assess the long-term safety and efficacy of veverimer for the treatment of metabolic acidosis in chronic kidney disease. Lancet 2019, 394, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Tangri, N.; Mather, V.; Bushinsky, D.A.; Klaener, G.; Li, E.; Parsell, D.; Stasiv, Y.; Walker, M.; Wesson, D.E.; Wheeler, D.C.; et al. Valor CKD: A multicenter, randomized, double-blind placebo-controlled trial evaluating veverimer in slowing progression of chronic kidney disease. J. Am. Soc. Nephrol. 2024. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goraya, N.; Wesson, D.E. Pathophysiology of Diet-Induced Acid Stress. Int. J. Mol. Sci. 2024, 25, 2336. https://doi.org/10.3390/ijms25042336
Goraya N, Wesson DE. Pathophysiology of Diet-Induced Acid Stress. International Journal of Molecular Sciences. 2024; 25(4):2336. https://doi.org/10.3390/ijms25042336
Chicago/Turabian StyleGoraya, Nimrit, and Donald E. Wesson. 2024. "Pathophysiology of Diet-Induced Acid Stress" International Journal of Molecular Sciences 25, no. 4: 2336. https://doi.org/10.3390/ijms25042336



