Use of 3-Deoxy-D-arabino-heptulosonic acid 7-phosphate Synthase (DAHP Synthase) to Enhance the Heterologous Biosynthesis of Diosmetin and Chrysoeriol in an Engineered Strain of Streptomyces albidoflavus
Abstract
:1. Introduction
2. Results
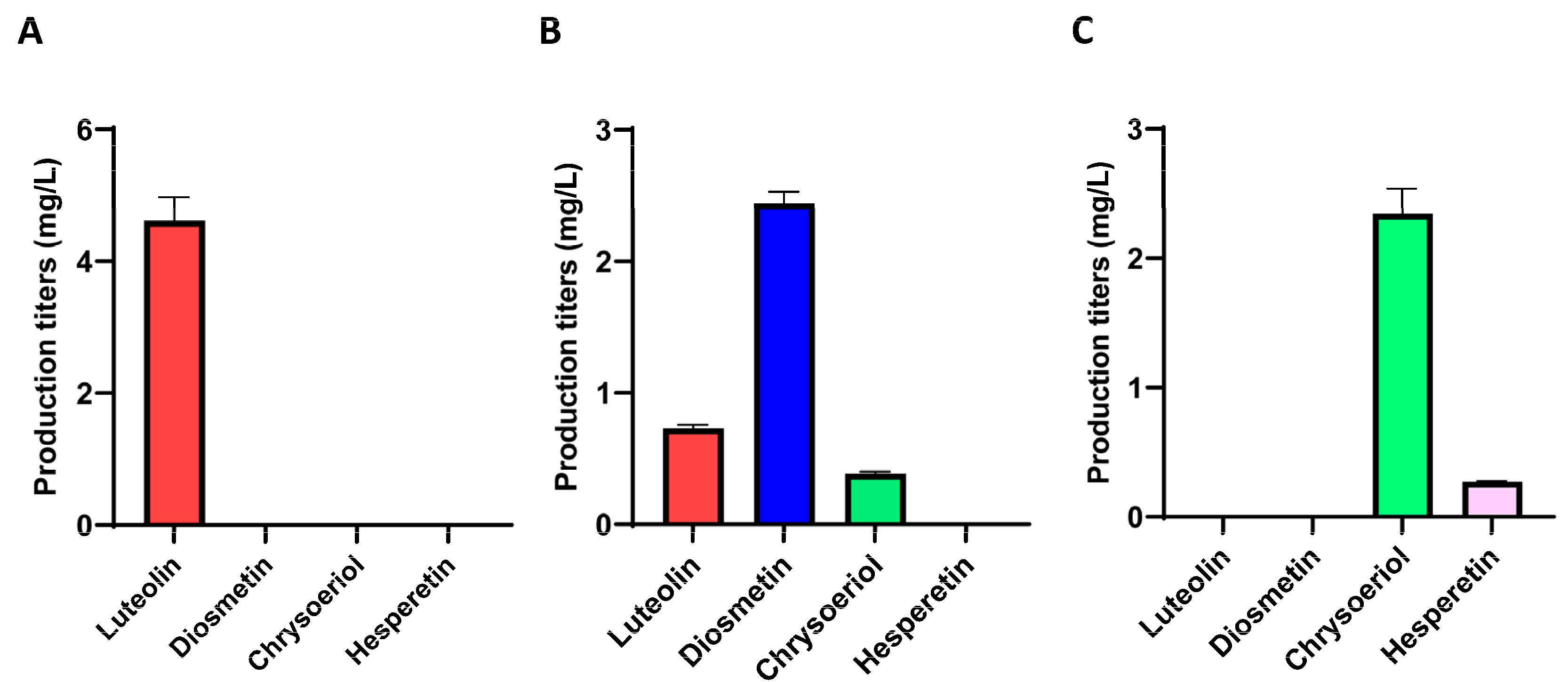
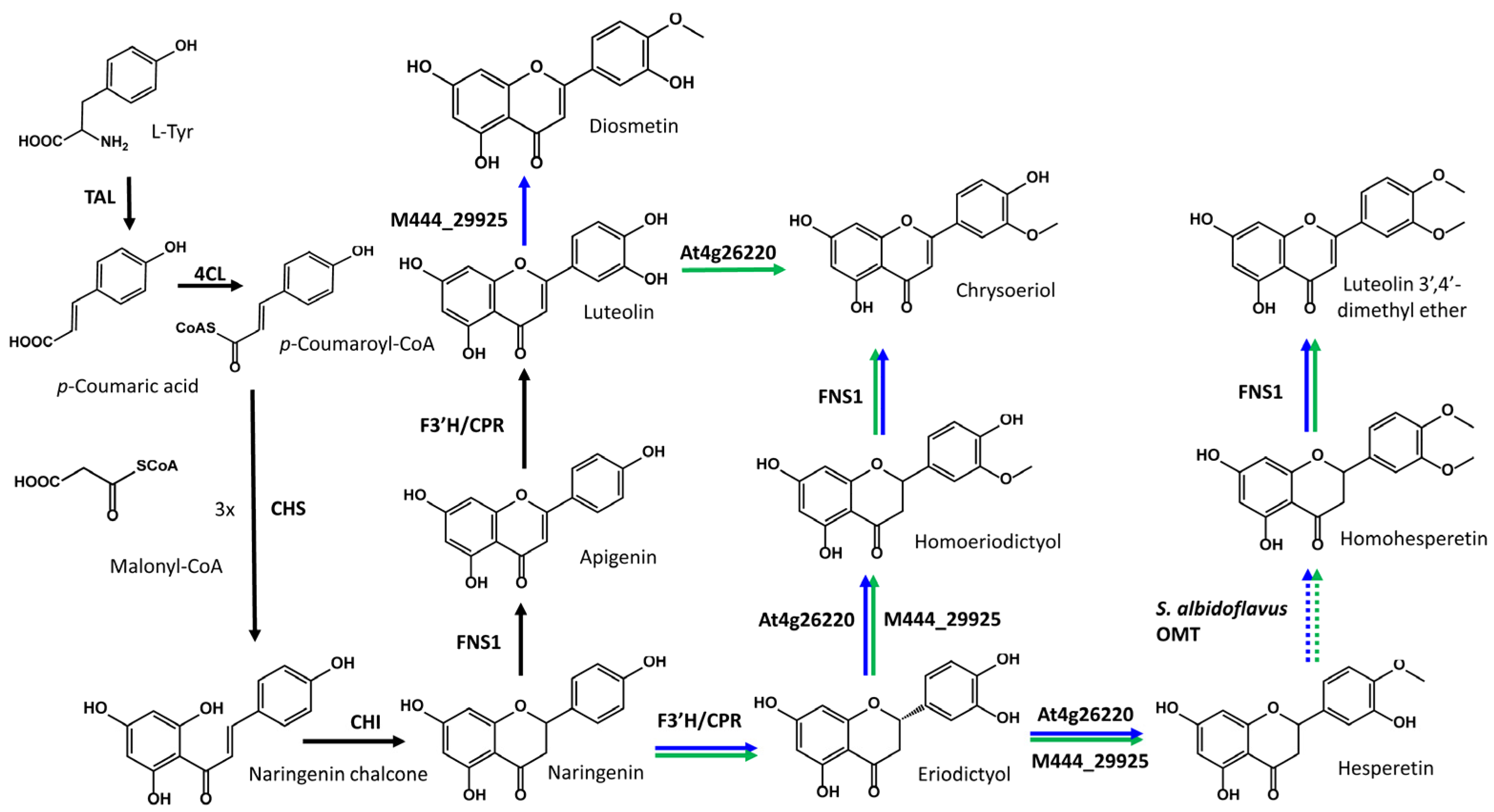
2.1. Heterologous Biosynthesis of Diosmetin
2.2. Heterologous Biosynthesis of Chrysoeriol
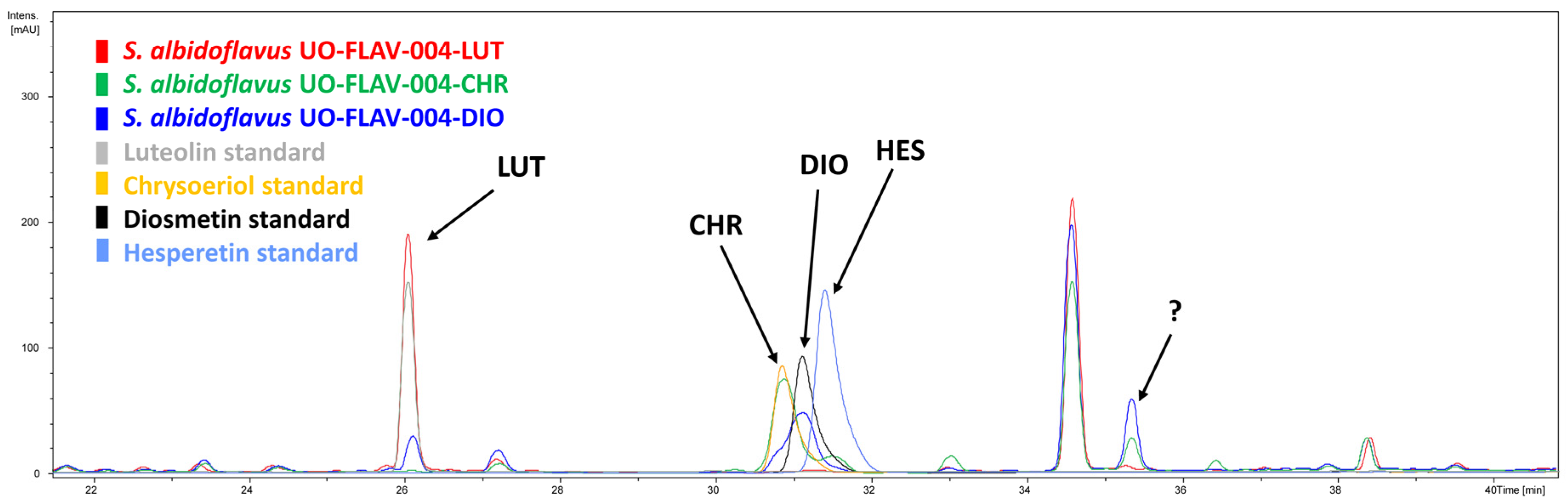
2.3. Identification of Putative Luteolin 3′,4′-Dimethyl Ether in Both Diosmetin- and Chrysoeriol-Producing Strains
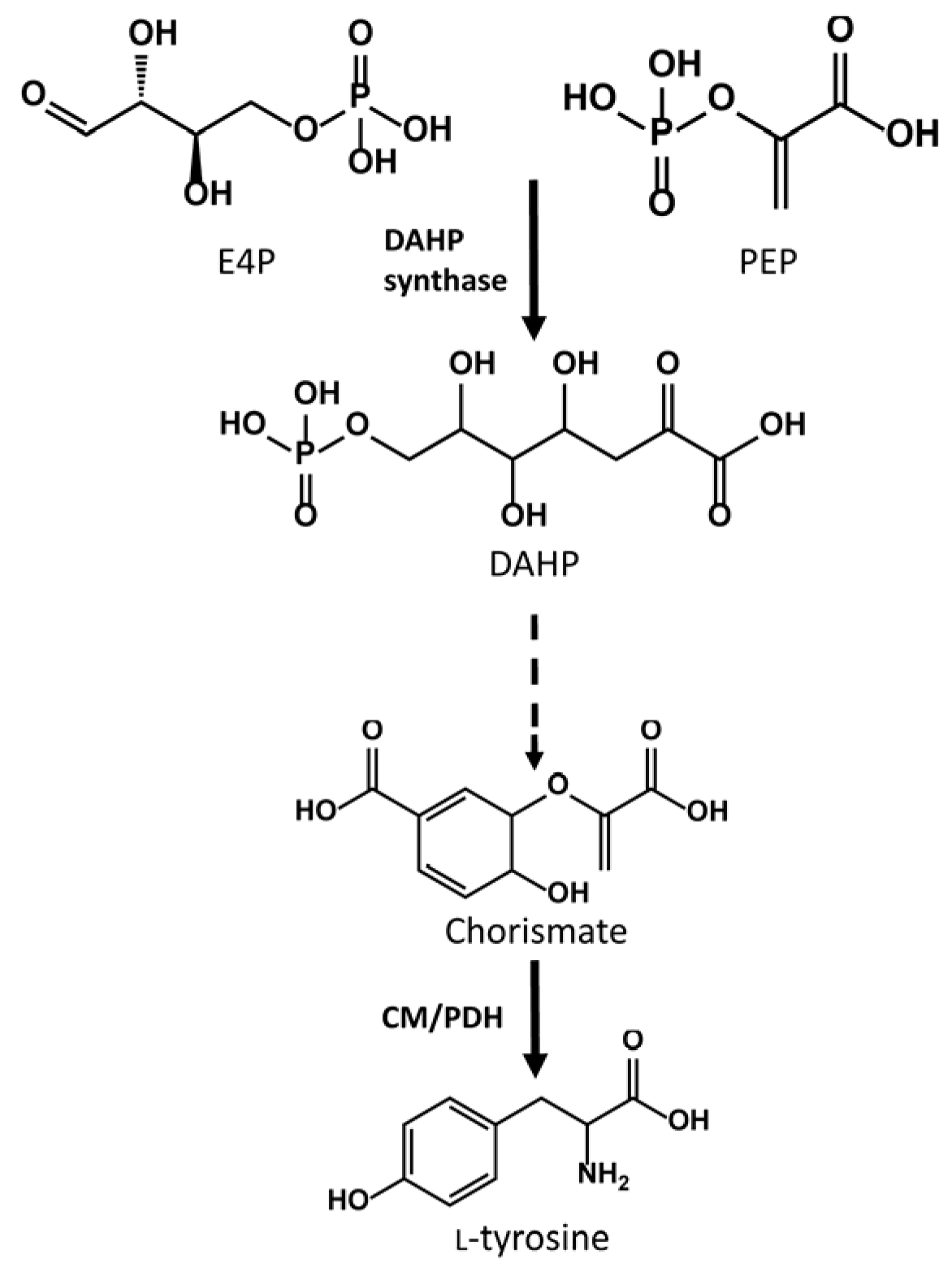
2.4. Use of a DAHP Synthase to Increase the Production Titers of Diosmetin and Chrysoeriol through Precursor Titer Enhancement
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
| Description | Reference | |
|---|---|---|
| Plasmids | ||
| pSEVA181-At4g26220 | Source of At4g26220 (Level 0 MoClo) | This study |
| pSEVA181SP25 | Source of SP25 (Level 0 MoClo) | [21] |
| pSEVA181SP43 | Source of SP43 (Level 0 MoClo) | [21] |
| pSEVA181-M444_29925 | Source of M444_29925 (Level 0 MoClo) | This study |
| pSEVA181RiboJ-RBS | Source of RiboJ-RBS (Level 0 MoClo) | [21] |
| pIDTSMARTttsbib | Source of ttsbib (Level 0 MoClo) | [21] |
| pSEVAUO-M21102 | Level 2 MoClo receptor | [21] |
| pSEVAUO-M31205 | Level 2 MoClo receptor | [21] |
| pSEVAUO-M21206F3H-CPR | Level 1 MoClo harboring F3′H-CPR | [21] |
| PCR-Blunt II-TOPO-FNS1 | Source of FNS1 (Level 0 MoClo) | [24] |
| pSEVAUO-M21102-FNS1 | Level 1 MoClo harboring FNS1 | This study |
| pSEVAUO-M21503-FNS1/F3′H-CPR | Level 2 MoClo harboring FNS1 and F3′H-CPR | This study |
| pSEVAUO-M31105-At4g26220 | Level 1 MoClo plasmid harboring At4g26220 | This study |
| pSEVAUO-M31105-M444_29925 | Level 1 MoClo plasmid harboring M444_29925 | This study |
| pSEVAUO-M31105 | Level 1 MoClo receptor | [21] |
| pSEVAUO-M31205-dahp | Level 1 MoClo plasmid harboring dahp | This study |
| pSEVAUO-M31505 | Level 2 MoClo receptor | [21] |
| pSEVAUO-M31505-At4g26220-dahp | Level 2 MoClo harboring At4g26220 and dahp | This study |
| pSEVAUO-M31505-M444_29925-dahp | Level 2 MoClo harboring M444_29925 and dahp | This study |
| Strains | ||
| E. coli TOP10 | Strain used for routine subcloning | Invitrogen (Waltham, MA, USA) |
| E. coli ET12567/pUZ8002 | Strain used for conjugation | [48] |
| UO-FLAV-004 | S. albidoflavus strain used in this work | [24] |
| UO-FLAV-004-NAR | UO-FLAV-004 harboring TAL, 4CL, CHS and CHI | [24] |
| UO-FLAV-004-LUT | UO-FLAV-004 harboring TAL, 4CL, CHS, CHI, FNS1 and F3′H-CPR | This study |
| UO-FLAV-004-DIO | UO-FLAV-004 harboring TAL, 4CL, CHS, CHI, FNS1, F3′H-CPR and M444_29925 | This study |
| UO-FLAV-004-CHR | UO-FLAV-004 harboring TAL, 4CL, CHS, CHI, FNS1, F3′H-CPR and At4g26220 | This study |
| UO-FLAV-004-DIO-dahp | UO-FLAV-004 harboring TAL, 4CL, CHS, CHI, FNS1, F3′H-CPR, M444_29925 and dahp | This study |
| UO-FLAV-004-CHR-dahp | UO-FLAV-004 harboring TAL, 4CL, CHS, CHI, FNS1, F3′H-CPR, At4g26220 and dahp | This study |
| UO-FLAV-004-FNS1 | UO-FLAV-004 harboring FNS1 | [24] |
4.2. Reagents and Biochemicals
4.3. Genes and Enzymes
4.4. Plasmids Construction
4.4.1. Construction of pSEVAUO-M21503-FNS1/F3′H-CPR
4.4.2. Construction of pSEVAUO-M31105-At4g26220, pSEVAUO-M31105-M444_29925, and pSEVAUO-M31205-dahp
4.4.3. Construction of pSEVAUO-M31505-At4g26220-dahp and pSEVAUO-M31505-M444_29925-dahp
4.5. Flavonoid Extraction and LC-DAD Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.L.A.; Ramzi, A.B.; Baharum, S.N.; Noor, N.M.; Goh, H.H.; Leow, T.C.; Oslan, S.N.; Sabri, S. Recent Advancement of Engineering Microbial Hosts for the Biotechnological Production of Flavonoids. Mol. Biol. Rep. 2019, 46, 6647–6659. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Kaushal, N.; Singh, M.; Singh Sangwan, R. Flavonoids: Food Associations, Therapeutic Mechanisms, Metabolism and Nanoformulations. Food Res. Int. 2022, 157, 111442. [Google Scholar] [CrossRef]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. BioMed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef]
- Zhao, L.; Yuan, X.; Wang, J.; Feng, Y.; Ji, F.; Li, Z.; Bian, J. A Review on Flavones Targeting Serine/Threonine Protein Kinases for Potential Anticancer Drugs. Bioorg. Med. Chem. 2019, 27, 677–685. [Google Scholar] [CrossRef]
- Zhao, K.; Yuan, Y.; Lin, B.; Miao, Z.; Li, Z.; Guo, Q.; Lu, N. LW-215, a Newly Synthesized Flavonoid, Exhibits Potent Anti-Angiogenic Activity In Vitro and In Vivo. Gene 2018, 642, 533–541. [Google Scholar] [CrossRef]
- Camero, C.M.; Germanò, M.P.; Rapisarda, A.; D’Angelo, V.; Amira, S.; Benchikh, F.; Braca, A.; De Leo, M. Anti-Angiogenic Activity of Iridoids from Galium tunetanum. Rev. Bras. Farmacogn. 2018, 28, 374–377. [Google Scholar] [CrossRef]
- Patel, K.; Kumar, V.; Rahman, M.; Verma, A.; Patel, D.K. New Insights into the Medicinal Importance, Physiological Functions and Bioanalytical Aspects of an Important Bioactive Compound of Foods ‘Hyperin’: Health Benefits of the Past, the Present, the Future. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 31–42. [Google Scholar] [CrossRef]
- Wen, X.; Walle, T. Methylated Flavonoids Have Greatly Improved Intestinal Absorption and Metabolic Stability. Drug Metab. Dispos. 2006, 34, 1786–1792. [Google Scholar] [CrossRef]
- Patel, K.; Gadewar, M.; Tahilyani, V.; Patel, D.K. A Review on Pharmacological and Analytical Aspects of Diosmetin: A Concise Report. Chin. J. Integr. Med. 2013, 19, 792–800. [Google Scholar] [CrossRef]
- Chan, B.C.L.; Ip, M.; Gong, H.; Lui, S.L.; See, R.H.; Jolivalt, C.; Fung, K.P.; Leung, P.C.; Reiner, N.E.; Lau, C.B.S. Synergistic Effects of Diosmetin with Erythromycin against ABC Transporter Over-Expressed Methicillin-Resistant Staphylococcus aureus (MRSA) RN4220/PUL5054 and Inhibition of MRSA Pyruvate Kinase. Phytomedicine 2013, 20, 611–614. [Google Scholar] [CrossRef]
- Aboulaghras, S.; Sahib, N.; Bakrim, S.; Benali, T.; Charfi, S.; Guaouguaou, F.E.; El Omari, N.; Gallo, M.; Montesano, D.; Zengin, G.; et al. Health Benefits and Pharmacological Aspects of Chrysoeriol. Pharmaceuticals 2022, 15, 973. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, Biological Functions, and Biotechnological Applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Sharma, K.; Zha, J.; Guleria, S.; Koffas, M.A.G. Recent Advances in the Recombinant Biosynthesis of Polyphenols. Front. Microbiol. 2017, 8, 2259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.; Yu, O. Metabolic Engineering of Flavonoids in Plants and Microorganisms. Appl. Microbiol. Biotechnol. 2011, 91, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Falcone Ferreyra, M.L.; Emiliani, J.; Rodriguez, E.J.; Campos-Bermudez, V.A.; Grotewold, E.; Casati, P. The Identification of Maize and Arabidopsis Type I FLAVONE SYNTHASEs Links Flavones with Hormones and Biotic Interactions. Plant Physiol. 2015, 169, 1090–1107. [Google Scholar] [CrossRef] [PubMed]
- Magadán-Corpas, P.; Ye, S.; Pérez-Valero, Á.; McAlpine, P.L.; Valdés-Chiara, P.; Torres-Bacete, J.; Nogales, J.; Villar, C.J.; Lombó, F. Optimized De Novo Eriodictyol Biosynthesis in Streptomyces albidoflavus Using an Expansion of the Golden Standard Toolkit for Its Use in Actinomycetes. Int. J. Mol. Sci. 2023, 24, 8879. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, B.G.; Ahn, J.H. Biosynthesis of Bioactive O-Methylated Flavonoids in Escherichia coli. Appl. Microbiol. Biotechnol. 2013, 97, 7195–7204. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, J.; Zhu, X.; Zhang, G.; Yang, S.; Guo, X.; Jiang, H.; Ma, Y. De Novo Biosynthesis of Multiple Pinocembrin Derivatives in Saccharomyces cerevisiae. ACS Synth. Biol. 2020, 9, 3042–3051. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Valero, Á.; Ye, S.; Magadán-Corpas, P.; Villar, C.J.; Lombó, F. Metabolic Engineering in Streptomyces albidoflavus for the Biosynthesis of the Methylated Flavonoids Sakuranetin, Acacetin, and Genkwanin. Microb. Cell Factories 2023, 22, 234. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Kaur, R.; Salwan, R. Streptomyces: Host for Refactoring of Diverse Bioactive Secondary Metabolites; Springer International Publishing: Cham, Switzerland, 2021; Volume 11, ISBN 0123456789. [Google Scholar]
- Kuhstoss, S.; Rao, R.N. Analysis of the Integration Function of the Streptomycete Bacteriophage ΦC31. J. Mol. Biol. 1991, 222, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.A.; Till, R.; Smith, M.C.M. Integration Site for Streptomyces Phage ΦBT1 and Development of Site-Specific Integrating Vectors. J. Bacteriol. 2003, 185, 5320–5323. [Google Scholar] [CrossRef] [PubMed]
- Raynal, A.; Friedmann, A.; Tuphile, K.; Guerineau, M.; Pernodet, J.L. Characterization of the AttP Site of the Integrative Element PSAM2 from Streptomyces ambofaciens. Microbiology 2002, 148, 61–67. [Google Scholar] [CrossRef]
- Thykaer, J.; Nielsen, J.; Wohlleben, W.; Weber, T.; Gutknecht, M.; Lantz, A.E.; Stegmann, E. Increased Glycopeptide Production after Overexpression of Shikimate Pathway Genes Being Part of the Balhimycin Biosynthetic Gene Cluster. Metab. Eng. 2010, 12, 455–461. [Google Scholar] [CrossRef]
- Ikeda, M. Towards Bacterial Strains Overproducing L-Tryptophan and Other Aromatics by Metabolic Engineering. Appl. Microbiol. Biotechnol. 2006, 69, 615–626. [Google Scholar] [CrossRef]
- Pandurangan, N. A New Synthesis for Acacetin, Chrysoeriol, Diosmetin, Tricin and Other Hydroxylated Flavones by Modified Baker-Venkataraman Transformation. Lett. Org. Chem. 2014, 11, 225–229. [Google Scholar] [CrossRef]
- Victor, M.M.; David, J.M.; Cortez, M.V.M.; Leite, J.L.; da Silva, G.S.B. A High-Yield Process for Extraction of Hesperidin from Orange (Citrus sinensis L. Osbeck) Peels Waste, and Its Transformation to Diosmetin, A Valuable and Bioactive Flavonoid. Waste Biomass Valorization 2021, 12, 313–320. [Google Scholar] [CrossRef]
- Myronovskyi, M.; Luzhetskyy, A. Native and Engineered Promoters in Natural Product Discovery. Nat. Prod. Rep. 2016, 33, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Darsandhari, S.; Dhakal, D.; Shrestha, B.; Parajuli, P.; Seo, J.-H.; Kim, T.-S.; Sohng, J.K. Characterization of Regioselective Flavonoid O-Methyltransferase from the Streptomyces Sp. KCTC 0041BP. Enzym. Microb. Technol. 2018, 113, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Wils, C.R.; Brandt, W.; Manke, K.; Vogt, T. A Single Amino Acid Determines Position Specificity of an Arabidopsis thaliana CCoAOMT-like O-Methyltransferase. FEBS Lett. 2013, 587, 683–689. [Google Scholar] [CrossRef]
- Schröder, G.; Wehinger, E.; Lukačin, R.; Wellmann, F.; Seefelder, W.; Schwab, W.; Schröder, J. Flavonoid Methylation: A Novel 4′-O-Methyltransferase from Catharanthus Roseus, and Evidence That Partially Methylated Flavanones Are Substrates of Four Different Flavonoid Dioxygenases. Phytochemistry 2004, 65, 1085–1094. [Google Scholar] [CrossRef]
- Aisa, H.A.; Izotova, L.; Karimov, A.; Botirov, E.; Mamadrahimov, A.; Ibragimov, B. Crystal, Molecular Structure and Hirshheld Surface Analysis of 5-Hydroxy-3,6,7,8-Tetramethoxyflavone. Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 1748–1751. [Google Scholar] [CrossRef]
- Shin, W.; Lah, M.S. Structure of (R,S)-Naringenin. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1986, 42, 626–628. [Google Scholar] [CrossRef]
- Lütke-Eversloh, T.; Santos, C.N.S.; Stephanopoulos, G. Perspectives of Biotechnological Production of L-Tyrosine and Its Applications. Appl. Microbiol. Biotechnol. 2007, 77, 751–762. [Google Scholar] [CrossRef]
- Koopman, F.; Beekwilder, J.; Crimi, B.; Van Houwelingen, A.; Hall, R.D.; Bosch, D.; Van Maris, A.J.A.; Pronk, J.T.; Daran, J. De Novo Production of the Flavonoid Naringenin in Engineered Saccharomyces cerevisiae. Microb. Cell Factories 2012, 11, 155. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, T.; Du, G.; Zhou, J.; Chen, J. Modular Optimization of Heterologous Pathways for de Novo Synthesis of (2S)-Naringenin in Escherichia coli. PLoS ONE 2014, 9, e101492. [Google Scholar] [CrossRef]
- Zhou, S.; Hao, T.; Zhou, J. Fermentation and Metabolic Pathway Optimization to De Novo Synthesize (2S)-Naringenin in Escherichia coli. J. Microbiol. Biotechnol. 2020, 30, 1574–1582. [Google Scholar] [CrossRef]
- Lee, H.; Kim, B.G.; Kim, M.; Ahn, J.H. Biosynthesis of Two Flavones, Apigenin and Genkwanin, in Escherichia coli. J. Microbiol. Biotechnol. 2015, 25, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- August, P.R.; Tang, L.; Yoon, Y.J.; Ning, S.; Müller, R.; Yu, T.-W.; Taylor, M.; Hoffmann, D.; Kim, C.-G.; Zhang, X.; et al. Biosynthesis of the Ansamycin Antibiotic Rifamycin: Deductions from the Molecular Analysis of the Rif Biosynthetic Gene Cluster of Amycolatopsis Mediterranei S699. Chem. Biol. 1998, 5, 69–79. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Magarvey, N.; Piraee, M.; Vining, L.C. The Gene Cluster for Chloramphenicol Biosynthesis in Streptomyces venezuelae ISP5230 Includes Novel Shikimate Pathway Homologues and a Monomodular Non-Ribosomal Peptide Synthetase Gene The GenBank Accession Number for the Sequence Reported in This Paper is AF262220. Microbiology 2001, 147, 2817–2829. [Google Scholar] [CrossRef]
- Dyer, W.E.; Weaver, L.M.; Zhao, J.M.; Kuhn, D.N.; Weller, S.C.; Herrmann, K.M. A CDNA Encoding 3-Deoxy-D-Arabino-Heptulosonate 7-Phosphate Synthase from Solanum tuberosum L. J. Biol. Chem. 1990, 265, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.E.B.P.; Suzich, J.A.; Herrmann, K.M. 3-Deoxy-d- Arabino -Heptulosonate 7-Phosphate Synthase from Potato Tuber (Solanum tuberosum L.). Plant Physiol. 1986, 82, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Macneil, D.J.; Gewain, K.M.; Ruby, C.L.; Dezeny, G.; Gibbons, P.H.; Maeneil, T. Analysis of Streptomyces avermitilis Genes Required for Avermectin Biosynthesis Utilizing a Novel Inte-Gration Vector. Gene 1992, 111, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A.; John Innes Foundation. Practical Streptomyces Genetics; John Innes Foundation: Norwich, UK, 2000; Volume 291. [Google Scholar]
- Fernández, E.; Weissbach, U.; Sánchez Reillo, C.; Braña, A.F.; Méndez, C.; Rohr, J.; Salas, J.A. Identification of Two Genes from Streptomyces argillaceus Encoding Glycosyltransferases Involved in Transfer of a Disaccharide during Biosynthesis of the Antitumor Drug Mithramycin. J. Bacteriol. 1998, 180, 4929–4937. [Google Scholar] [CrossRef]
- Myronovskyi, M.; Tokovenko, B.; Brötz, E.; Rückert, C.; Kalinowski, J.; Luzhetskyy, A. Genome Rearrangements of Streptomyces Albus J1074 Lead to the Carotenoid Gene Cluster Activation. Appl. Microbiol. Biotechnol. 2014, 98, 795–806. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Valero, Á.; Serna-Diestro, J.; Villar, C.J.; Lombó, F. Use of 3-Deoxy-D-arabino-heptulosonic acid 7-phosphate Synthase (DAHP Synthase) to Enhance the Heterologous Biosynthesis of Diosmetin and Chrysoeriol in an Engineered Strain of Streptomyces albidoflavus. Int. J. Mol. Sci. 2024, 25, 2776. https://doi.org/10.3390/ijms25052776
Pérez-Valero Á, Serna-Diestro J, Villar CJ, Lombó F. Use of 3-Deoxy-D-arabino-heptulosonic acid 7-phosphate Synthase (DAHP Synthase) to Enhance the Heterologous Biosynthesis of Diosmetin and Chrysoeriol in an Engineered Strain of Streptomyces albidoflavus. International Journal of Molecular Sciences. 2024; 25(5):2776. https://doi.org/10.3390/ijms25052776
Chicago/Turabian StylePérez-Valero, Álvaro, Juan Serna-Diestro, Claudio J. Villar, and Felipe Lombó. 2024. "Use of 3-Deoxy-D-arabino-heptulosonic acid 7-phosphate Synthase (DAHP Synthase) to Enhance the Heterologous Biosynthesis of Diosmetin and Chrysoeriol in an Engineered Strain of Streptomyces albidoflavus" International Journal of Molecular Sciences 25, no. 5: 2776. https://doi.org/10.3390/ijms25052776





