Gut Microbiota and Inflammation Modulation in a Rat Model for Ulcerative Colitis after the Intraperitoneal Administration of Apigenin, Luteolin, and Xanthohumol
Abstract
:1. Introduction
2. Results
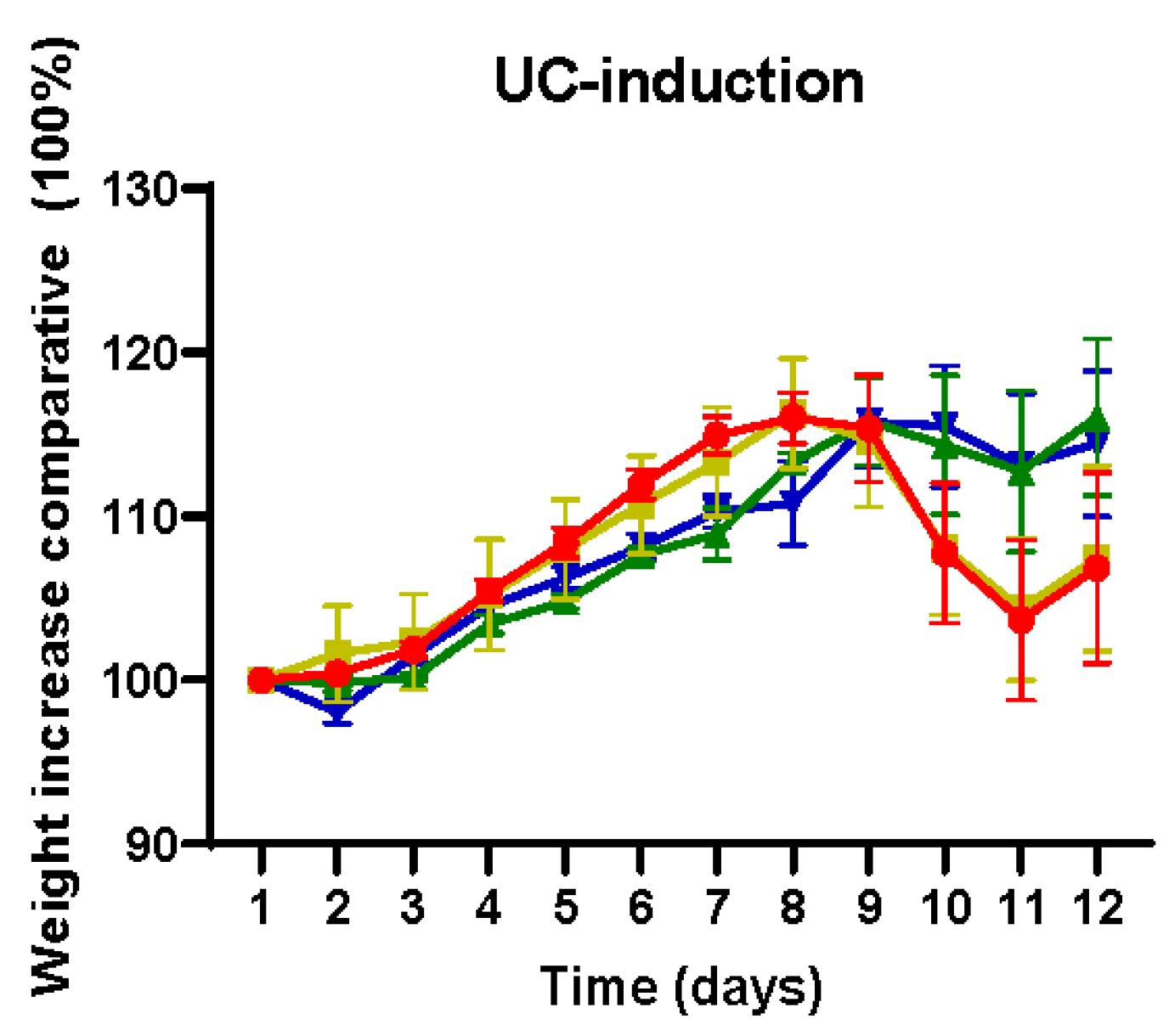
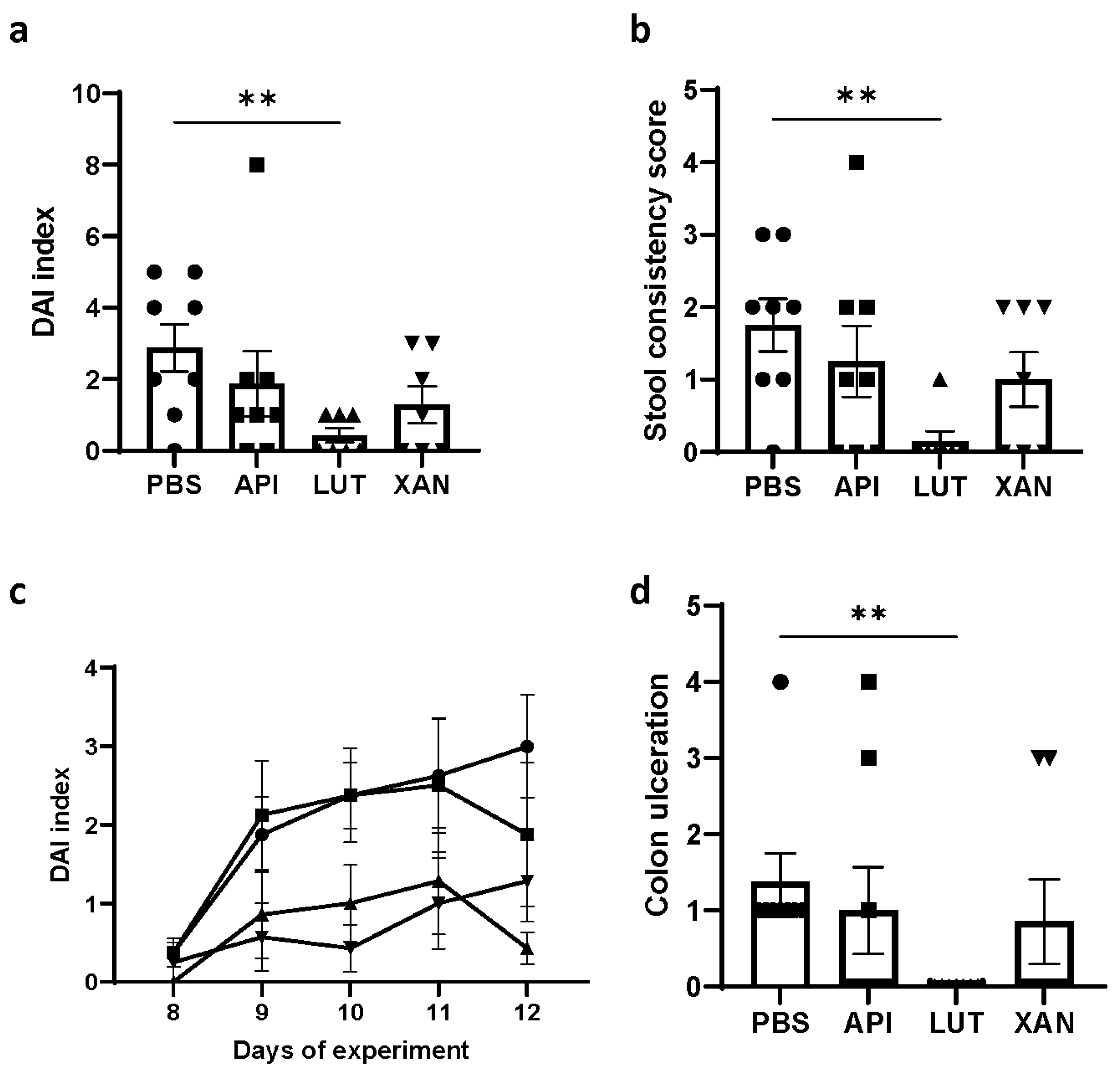
2.1. Effect of Flavonoid Treatments on Body Weight and DAI
2.2. Effect of Flavonoid Treatments on Hyperplastic Peyer’s Patches, Colon Length, and Colon Ulceration
2.3. Effect of Flavonoid Treatments on Pro-Inflammatory Cytokines and Myeloperoxidase (MPO)
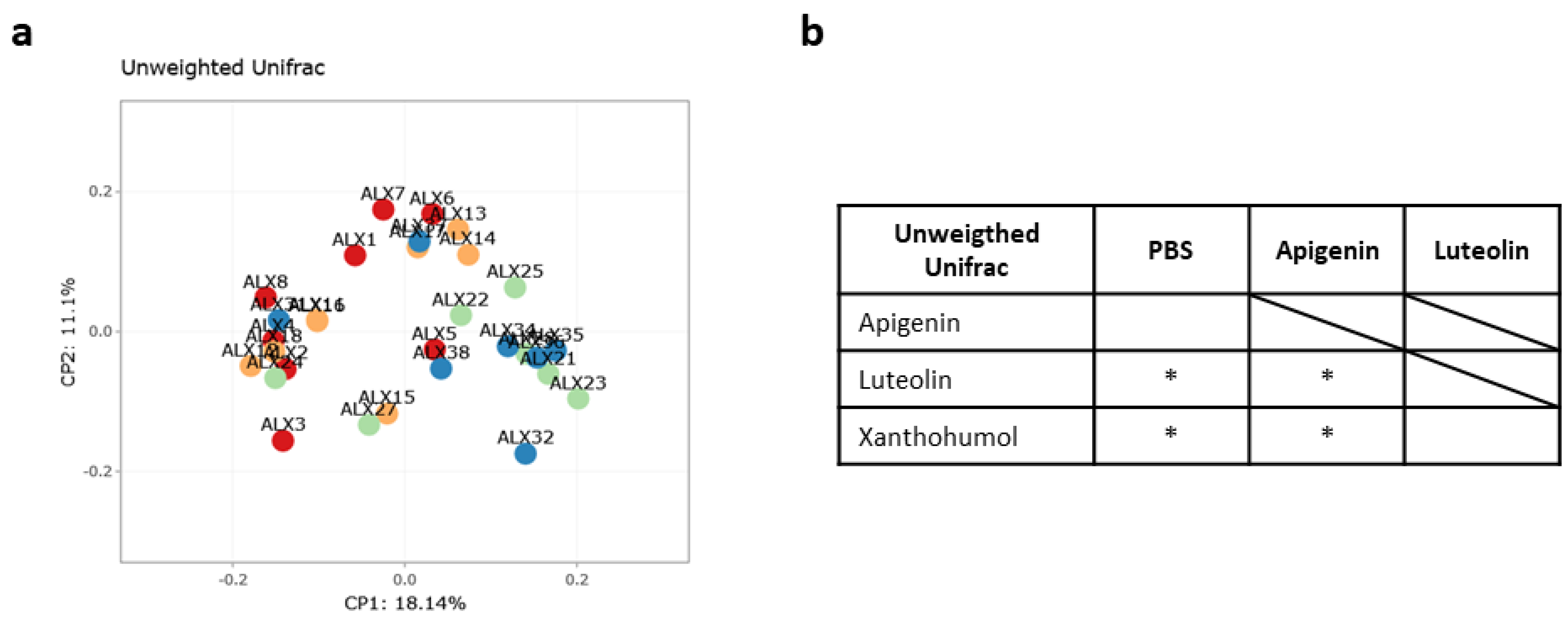
2.4. Caecum Weight and Metataxonomic Analyses of the Gut Microbiota
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Animals
4.3. Experimental Design
4.4. Tissue Sample Collection
4.5. Histological Studies
4.6. Assessment of the Disease Activity Index (DAI)
4.7. Pro-Inflammatory Cytokine Analysis in Plasma
4.8. Myeloperoxidase (MPO) Assays
4.9. 16S rRNA Sequencing and Gut Microbiota Analysis
4.10. Microbiota Analysis
4.11. Statistical Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, B.; Du, P.; Du, Y.; Zhao, D.; Cai, Y.; Yang, Q.; Guo, Z. Luteolin Alleviates Inflammation and Modulates Gut Microbiota in Ulcerative Colitis Rats. Life Sci. 2021, 269, 119008. [Google Scholar] [CrossRef] [PubMed]
- De Souza, H.S.P.; Fiocchi, C.; Iliopoulos, D. The IBD Interactome: An Integrated View of Aetiology, Pathogenesis and Therapy. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 739–749. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the Gut Microbiota in Disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef] [PubMed]
- Mosca, A.; Leclerc, M.; Hugot, J.P. Gut Microbiota Diversity and Human Diseases: Should We Reintroduce Key Predators in Our Ecosystem? Front. Microbiol. 2016, 7, 455. [Google Scholar] [CrossRef] [PubMed]
- Oligschlaeger, Y.; Yadati, T.; Houben, T.; Condello Oliván, C.M.; Shiri-Sverdlov, R. Inflammatory Bowel Disease: A Stressed “Gut/Feeling”. Cells 2019, 8, 659. [Google Scholar] [CrossRef] [PubMed]
- Hirano, A.; Umeno, J.; Okamoto, Y.; Shibata, H.; Ogura, Y.; Moriyama, T.; Torisu, T.; Fujioka, S.; Fuyuno, Y.; Kawarabayasi, Y.; et al. Comparison of the Microbial Community Structure between Inflamed and Non-inflamed Sites in Patients with Ulcerative Colitis. J. Gastroenterol. Hepatol. 2018, 33, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Nishino, K.; Nishida, A.; Inoue, R.; Kawada, Y.; Ohno, M.; Sakai, S.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; et al. Analysis of Endoscopic Brush Samples Identified Mucosa-Associated Dysbiosis in Inflammatory Bowel Disease. J. Gastroenterol. 2018, 53, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Atreya, R.; Neurath, M.F. Involvement of IL-6 in the Pathogenesis of Inflammatory Bowel Disease and Colon Cancer. Clin. Rev. Allergy Immunol. 2005, 28, 187–196. [Google Scholar] [CrossRef]
- Eastaff-Leung, N.; Mabarrack, N.; Barbour, A.; Cummins, A.; Barry, S. Foxp3+ Regulatory T Cells, Th17 Effector Cells, and Cytokine Environment in Inflammatory Bowel Disease. J. Clin. Immunol. 2010, 30, 80–89. [Google Scholar] [CrossRef]
- Liso, M.; Verna, G.; Cavalcanti, E.; De Santis, S.; Armentano, R.; Tafaro, A.; Lippolis, A.; Campiglia, P.; Gasbarrini, A.; Mastronardi, M.; et al. Interleukin 1β Blockade Reduces Intestinal Inflammation in a Murine Model of Tumor Necrosis Factor–Independent Ulcerative Colitis. Cell Mol. Gastroenterol. Hepatol. 2022, 14, 151–171. [Google Scholar] [CrossRef]
- Shahini, A.; Shahini, A. Role of Interleukin-6-Mediated Inflammation in the Pathogenesis of Inflammatory Bowel Disease: Focus on the Available Therapeutic Approaches and Gut Microbiome. J. Cell Commun. Signal 2023, 17, 55–74. [Google Scholar] [CrossRef]
- Lee, M.; Chang, E.B. Inflammatory Bowel Diseases (IBD) and the Microbiome—Searching the Crime Scene for Clues. Gastroenterology 2021, 160, 524–537. [Google Scholar] [CrossRef]
- Himmel, M.E.; Yao, Y.; Orban, P.C.; Steiner, T.S.; Levings, M.K. Regulatory T-cell Therapy for Inflammatory Bowel Disease: More Questions than Answers. Immunology 2012, 136, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Jochum, L.; Stecher, B. Label. or Concept—What Is a Pathobiont? Trends Microbiol. 2020, 28, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.S.; Krishnan, S.; Ramakrishna, B.S.; Mathan, M.; Pulimood, A.B.; Murthy, S.N. Butyrate and Glucose Metabolism by Colonocytes in Experimental Colitis in Mice. Gut 2000, 46, 493–499. [Google Scholar] [CrossRef]
- Belzer, C.; Chia, L.W.; Aalvink, S.; Chamlagain, B.; Piironen, V.; Knol, J.; de Vos, W.M. Microbial Metabolic Networks at the Mucus Layer Lead to Diet-Independent Butyrate and Vitamin B 12 Production by Intestinal Symbionts. mBio 2017, 8, e00770-17. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.; Macfarlane, G.T. Regulation of Short-Chain Fatty Acid Production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Cheng, N.; Zhou, W.; Chen, S.; Wang, Q.; Gao, H.; Xue, X.; Wu, L.; Cao, W. Honey Polyphenols Ameliorate DSS-Induced Ulcerative Colitis via Modulating Gut Microbiota in Rats. Mol. Nutr. Food Res. 2019, 63, e1900638. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Silván, B.; Entrialgo-Cadierno, R.; Villar, C.J.; Capasso, R.; Uranga, J.A.; Lombó, F.; Abalo, R. Antiproliferative and Palliative Activity of Flavonoids in Colorectal Cancer. Biomed. Pharmacother. 2021, 143, 112241. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Wang, L.; Meng, Y.; Xue, W.; Liang, J.; Peng, Z.; Meng, J.; Zhang, M. Apigenin Remodels the Gut Microbiota to Ameliorate Ulcerative Colitis. Front. Nutr. 2022, 9, 1062961. [Google Scholar] [CrossRef]
- Ai, X.-Y.; Qin, Y.; Liu, H.-J.; Cui, Z.-H.; Li, M.; Yang, J.-H.; Zhong, W.-L.; Liu, Y.-R.; Chen, S.; Sun, T.; et al. Apigenin Inhibits Colonic Inflammation and Tumorigenesis by Suppressing STAT3-NF-ΚB Signaling. Oncotarget 2017, 8, 100216–100226. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Jin, X.; Ji, T.; Li, R.; Zhuge, X.; Xu, F.; Quan, Z.; Tong, H.; Yu, W. Luteolin Ameliorates DSS-Induced Colitis in Mice via Suppressing Macrophage Activation and Chemotaxis. Int. Immunopharmacol. 2023, 124, 110996. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-M.; Yun, S.-M.; Choi, Y.-H.; Heo, J.; Kim, N.-J.; Kim, S.-H.; Kim, E.-H. Xanthohumol Prevents Dextran Sulfate Sodium-Induced Colitis via Inhibition of IKKβ/NF-ΚB Signaling in Mice. Oncotarget 2018, 9, 866–880. [Google Scholar] [CrossRef]
- Braune, A.; Gutschow, M.; Engst, W.; Blaut, M. Degradation of Quercetin and Luteolin by Eubacterium Ramulus. Appl. Environ. Microbiol. 2001, 67, 5558–5567. [Google Scholar] [CrossRef] [PubMed]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal Route of Drug Administration: Should It Be Used in Experimental Animal Studies? Pharm. Res. 2020, 37, 12. [Google Scholar] [CrossRef] [PubMed]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; García, L.; Monte, J.; Villar, C.; Lombó, F. Functional Anthocyanin-Rich Sausages Diminish Colorectal Cancer in an Animal Model and Reduce Pro-Inflammatory Bacteria in the Intestinal Microbiota. Genes 2018, 9, 133. [Google Scholar] [CrossRef]
- Chan, S.-T.; Lin, Y.-C.; Chuang, C.-H.; Shiau, R.-J.; Liao, J.-W.; Yeh, S.-L. Oral and Intraperitoneal Administration of Quercetin Decreased Lymphocyte DNA Damage and Plasma Lipid Peroxidation Induced by TSA In Vivo. Biomed. Res. Int. 2014, 2014, 580626. [Google Scholar] [CrossRef]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. Biomed. Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef]
- Zieniuk, B. Dihydrocaffeic Acid—Is It the Less Known but Equally Valuable Phenolic Acid? Biomolecules 2023, 13, 859. [Google Scholar] [CrossRef]
- Li, H.; Christman, L.M.; Li, R.; Gu, L. Synergic Interactions between Polyphenols and Gut Microbiota in Mitigating Inflammatory Bowel Diseases. Food Funct. 2020, 11, 4878–4891. [Google Scholar] [CrossRef]
- Nishihara, Y.; Ogino, H.; Tanaka, M.; Ihara, E.; Fukaura, K.; Nishioka, K.; Chinen, T.; Tanaka, Y.; Nakayama, J.; Kang, D.; et al. Mucosa-Associated Gut Microbiota Reflects Clinical Course of Ulcerative Colitis. Sci. Rep. 2021, 11, 13743. [Google Scholar] [CrossRef]
- Qiu, P.; Ishimoto, T.; Fu, L.; Zhang, J.; Zhang, Z.; Liu, Y. The Gut Microbiota in Inflammatory Bowel Disease. Front. Cell Infect. Microbiol. 2022, 12, 733992. [Google Scholar] [CrossRef]
- Cui, L.; Guan, X.; Ding, W.; Luo, Y.; Wang, W.; Bu, W.; Song, J.; Tan, X.; Sun, E.; Ning, Q.; et al. Scutellaria baicalensis Georgi Polysaccharide Ameliorates DSS-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Modulating Gut Microbiota. Int. J. Biol. Macromol. 2021, 166, 1035–1045. [Google Scholar] [CrossRef]
- Gophna, U.; Sommerfeld, K.; Gophna, S.; Doolittle, W.F.; Veldhuyzen van Zanten, S.J.O. Differences between Tissue-Associated Intestinal Microfloras of Patients with Crohn’s Disease and Ulcerative Colitis. J. Clin. Microbiol. 2006, 44, 4136–4141. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Liu, H.; Yu, H.; Chen, M.; Yang, T.; Zeng, X.; Qiao, S. Core Altered Microorganisms in Colitis Mouse Model: A Comprehensive Time-Point and Fecal Microbiota Transplantation Analysis. Antibiotics 2021, 10, 643. [Google Scholar] [CrossRef]
- Bajer, L.; Kverka, M.; Kostovcik, M.; Macinga, P.; Dvorak, J.; Stehlikova, Z.; Brezina, J.; Wohl, P.; Spicak, J.; Drastich, P. Distinct Gut Microbiota Profiles in Patients with Primary Sclerosing Cholangitis and Ulcerative Colitis. World J. Gastroenterol. 2017, 23, 4548. [Google Scholar] [CrossRef]
- Zou, J.; Shen, Y.; Chen, M.; Zhang, Z.; Xiao, S.; Liu, C.; Wan, Y.; Yang, L.; Jiang, S.; Shang, E.; et al. Lizhong Decoction Ameliorates Ulcerative Colitis in Mice via Modulating Gut Microbiota and Its Metabolites. Appl. Microbiol. Biotechnol. 2020, 104, 5999–6012. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, M.; Wen, X.; He, L.; Zhang, M.; Zhang, J.; Yang, X. Cepharanthine Ameliorates Dextran Sulphate Sodium-induced Colitis through Modulating Gut Microbiota. Microb. Biotechnol. 2022, 15, 2208–2222. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, H.; Wu, S.; Lu, N.; Wang, Y.; Liu, H.; Dong, L.; Liu, T.; Shen, X. Parasutterella, in Association with Irritable Bowel Syndrome and Intestinal Chronic Inflammation. J. Gastroenterol. Hepatol. 2018, 33, 1844–1852. [Google Scholar] [CrossRef]
- Li, W.; Cheng, F.; Zhang, J.; Li, C.; Yu, D.; Simayijiang, H.; Liu, H.; Li, S.; Yan, J. Changes in Gut Microbiota and Metabolites in Papillary Thyroid Carcinoma Patients Following Radioactive Iodine Therapy. Int. J. Gen. Med. 2023, 16, 4453–4464. [Google Scholar] [CrossRef]
- Yu, Z.; Xiaojia, L.; Wei, Z.; Jian, Z.; Aiting, W.; Jing, W.; Lin, Y.; Bangwei, C.; Dan, Y. Baicalin Circumvents Anti-PD-1 Resistance by Regulating the Gut Microbiota Metabolite Short-Chain Fatty Acids. Pharmacol. Res. 2024, 199, 107033. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.-C.; Roquim, M.; Dudonné, S.; Pilon, G.; Levy, E.; Marette, A.; Roy, D.; Desjardins, Y. Berry Polyphenols and Fibers Modulate Distinct Microbial Metabolic Functions and Gut Microbiota Enterotype-Like Clustering in Obese Mice. Front. Microbiol. 2020, 11, 2032. [Google Scholar] [CrossRef]
- Taladrid, D.; Zorraquín-Peña, I.; Molinero, N.; Silva, M.; Manceñido, N.; Pajares, R.; Bartolomé, B.; Moreno-Arribas, M.V. Polyphenols and Ulcerative Colitis: An Exploratory Study of the Effects of Red Wine Consumption on Gut and Oral Microbiome in Active-Phase Patients. Mol. Nutr. Food Res. 2022, 66, e2101073. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Gu, Q. Chimonanthus salicifolius Extract Alleviates DSS-induced Colitis and Regulates Gut Microbiota in Mice. Food Sci. Nutr. 2023, 11, 3019–3030. [Google Scholar] [CrossRef]
- Rowan, F.E.; Docherty, N.G.; Coffey, J.C.; O’Connell, P.R. Sulphate-Reducing Bacteria and Hydrogen Sulphide in the Aetiology of Ulcerative Colitis. Br. J. Surg. 2009, 96, 151–158. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Feng, Z.; Long, W.; Hao, B.; Ding, D.; Ma, X.; Zhao, L.; Pang, X. A Human Stool-Derived Bilophila wadsworthia Strain Caused Systemic Inflammation in Specific-Pathogen-Free Mice. Gut Pathog. 2017, 9, 59. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, B.; Cong, W.; Zhang, M.; Li, Z.; Li, Y.; Liang, S.; Chen, K.; Yang, D.; Wu, Z. Amelioration of AOM/DSS-Induced Murine Colitis-Associated Cancer by Evodiamine Intervention Is Primarily Associated with Gut Microbiota-Metabolism-Inflammatory Signaling Axis. Front. Pharmacol. 2021, 12, 797605. [Google Scholar] [CrossRef]
- Delday, M.; Mulder, I.; Logan, E.T.; Grant, G. Bacteroides Thetaiotaomicron Ameliorates Colon Inflammation in Preclinical Models of Crohn’s Disease. Inflamm. Bowel Dis. 2019, 25, 85–96. [Google Scholar] [CrossRef]
- Yoshida, N.; Emoto, T.; Yamashita, T.; Watanabe, H.; Hayashi, T.; Tabata, T.; Hoshi, N.; Hatano, N.; Ozawa, G.; Sasaki, N.; et al. Bacteroides vulgatus and Bacteroides dorei Reduce Gut Microbial Lipopolysaccharide Production and Inhibit Atherosclerosis. Circulation 2018, 138, 2486–2498. [Google Scholar] [CrossRef]
- He, X.; Bai, Y.; Zhou, H.; Wu, K. Akkermansia muciniphila Alters Gut Microbiota and Immune System to Improve Cardiovascular Diseases in Murine Model. Front. Microbiol. 2022, 13, 6920. [Google Scholar] [CrossRef]
- Zheng, M.; Han, R.; Yuan, Y.; Xing, Y.; Zhang, W.; Sun, Z.; Liu, Y.; Li, J.; Mao, T. The Role of Akkermansia muciniphila in Inflammatory Bowel Disease: Current Knowledge and Perspectives. Front. Immunol. 2023, 13, 1089600. [Google Scholar] [CrossRef]
- Xue, L.; Zhao, Y.; Wang, H.; Li, Z.; Wu, T.; Liu, R.; Sui, W.; Zhang, M. The Effects of Live and Pasteurized Akkermansia muciniphila on DSS-Induced Ulcerative Colitis, Gut Microbiota, and Metabolomics in Mice. Food Funct. 2023, 14, 4632–4646. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, N.; Tan, H.-Y.; Li, S.; Zhang, C.; Feng, Y. Function of Akkermansia muciniphila in Obesity: Interactions with Lipid Metabolism, Immune Response and Gut Systems. Front. Microbiol. 2020, 11, 219. [Google Scholar] [CrossRef]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia—A New Functional Genus with Potential Probiotic Properties? Gut Microbes 2021, 13, 1875796. [Google Scholar] [CrossRef]
- Hall, A.B.; Yassour, M.; Sauk, J.; Garner, A.; Jiang, X.; Arthur, T.; Lagoudas, G.K.; Vatanen, T.; Fornelos, N.; Wilson, R.; et al. A Novel Ruminococcus Gnavus Clade Enriched in Inflammatory Bowel Disease Patients. Genome Med. 2017, 9, 103. [Google Scholar] [CrossRef]
- Ćesić, D.; Lugović Mihić, L.; Ozretić, P.; Lojkić, I.; Buljan, M.; Šitum, M.; Zovak, M.; Vidović, D.; Mijić, A.; Galić, N.; et al. Association of Gut Lachnospiraceae and Chronic Spontaneous Urticaria. Life 2023, 13, 1280. [Google Scholar] [CrossRef]
- Lobionda, S.; Sittipo, P.; Kwon, H.Y.; Lee, Y.K. The Role of Gut Microbiota in Intestinal Inflammation with Respect to Diet and Extrinsic Stressors. Microorganisms 2019, 7, 271. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Zhang, K.; Zhu, L.; Zhong, Y.; Xu, L.; Lang, C.; Chen, J.; Yan, F.; Li, J.; Qiu, J.; Chen, Y.; et al. Prodrug Integrated Envelope on Probiotics to Enhance Target Therapy for Ulcerative Colitis. Adv. Sci. 2023, 10, 2205422. [Google Scholar] [CrossRef]
- Xia, T.; Duan, W.; Zhang, Z.; Li, S.; Zhao, Y.; Geng, B.; Zheng, Y.; Yu, J.; Wang, M. Polyphenol-Rich Vinegar Extract Regulates Intestinal Microbiota and Immunity and Prevents Alcohol-Induced Inflammation in Mice. Food Res. Int. 2021, 140, 110064. [Google Scholar] [CrossRef]
- Puupponen-Pimia, R.; Nohynek, L.; Meier, C.; Kahkonen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.-M. Antimicrobial Properties of Phenolic Compounds from Berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef]
- Nowak, B.; Poźniak, B.; Popłoński, J.; Bobak, Ł.; Matuszewska, A.; Kwiatkowska, J.; Dziewiszek, W.; Huszcza, E.; Szeląg, A. Pharmacokinetics of Xanthohumol in Rats of Both Sexes after Oral and Intravenous Administration of Pure Xanthohumol and Prenylflavonoid Extract. Adv. Clin. Exp. Med. 2020, 29, 1101–1109. [Google Scholar] [CrossRef]
- Fernández, J.; de la Fuente, V.G.; García, M.T.F.; Sánchez, J.G.; Redondo, B.I.; Villar, C.J.; Lombó, F. A Diet Based on Cured Acorn-Fed Ham with Oleic Acid Content Promotes Anti-Inflammatory Gut Microbiota and Prevents Ulcerative Colitis in an Animal Model. Lipids Health Dis. 2020, 19, 28. [Google Scholar] [CrossRef]

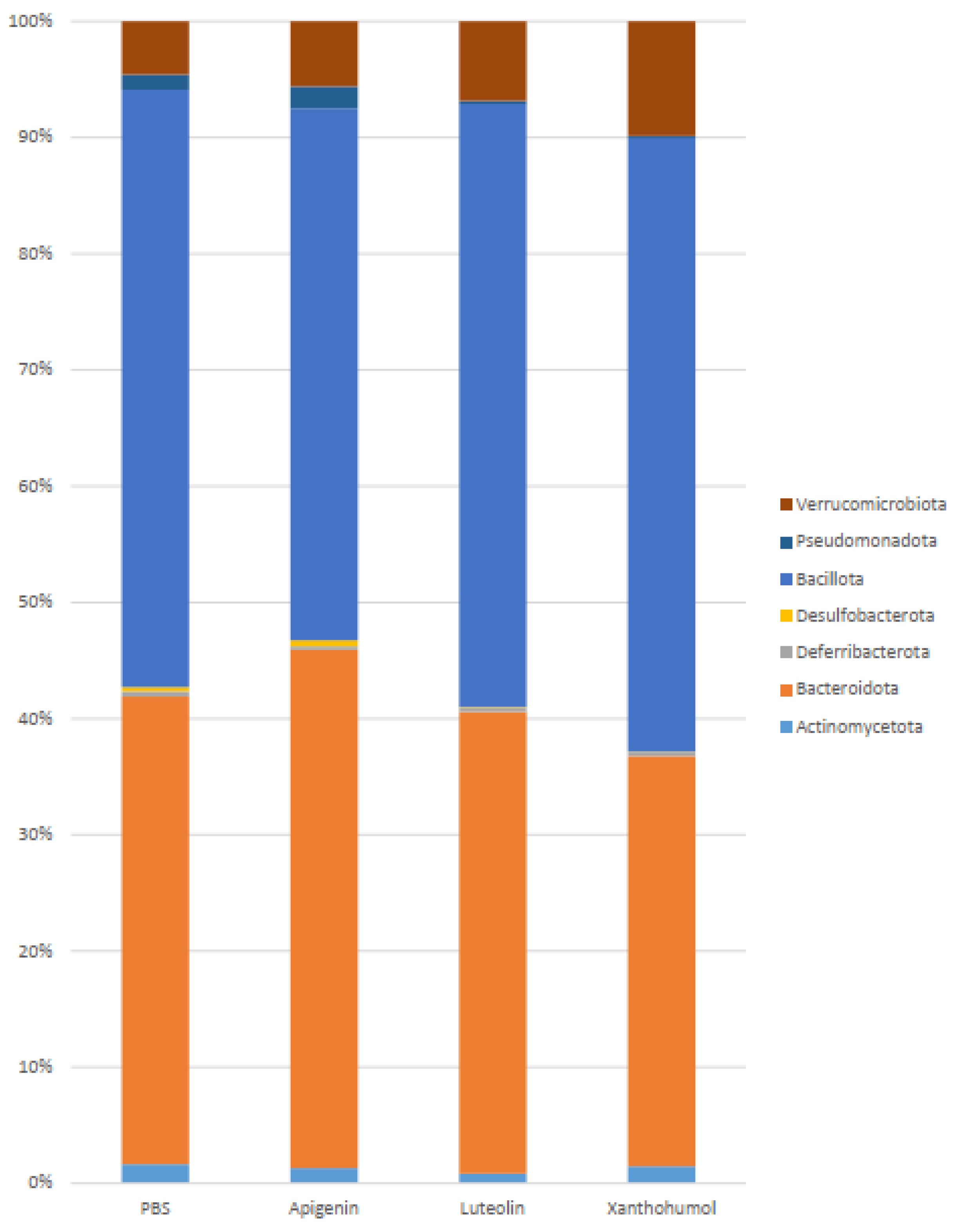

| Phylum | PBS | Apigenin | Luteolin | Xanthohumol | PBS vs. API | PBS vs. LUT | PBS vs. XAN |
|---|---|---|---|---|---|---|---|
| Actinomycetota | 1.65 | 1.31 | 0.83 | 1.45 | |||
| Bacteroidota | 40.05 | 44.32 | 39.40 | 34.99 | * | ||
| Deferribacterota | 0.46 | 0.29 | 0.33 | 0.35 | |||
| Desulfobacterota | 0.36 | 0.53 | 0.07 | 0.03 | * | ||
| Bacillota | 51.20 | 45.49 | 51.48 | 52.32 | * | ||
| Pseudomonadota | 1.28 | 1.87 | 0.23 | 0.17 | ** | *** | |
| Verrucomicrobiota | 4.56 | 5.57 | 6.80 | 9.77 | * |
| Family | PBS | Apigenin | Luteolin | Xanthohumol | PBS vs. API | PBS vs. LUT | PBS vs. XAN |
|---|---|---|---|---|---|---|---|
| Bifidobacteriaceae | 0.52 | 0.43 | 0.03 | 0.35 | ** | ||
| Atopobiaceae | 0.42 | 0.43 | 0.007 | 0.18 | ** | ||
| Eggerthellaceae | 0.68 | 0.43 | 0.79 | 0.89 | |||
| Bacteroidaceae | 12.66 | 16.23 | 10.59 | 15.25 | |||
| Muribaculaceae | 14.15 | 13.16 | 17.56 | 12.16 | |||
| Prevotellaceae | 1.73 | 2.48 | 2.38 | 3.83 | * | ||
| Rikenellaceae | 7.76 | 5.71 | 6.39 | 0.46 | **** | ||
| Tannerellaceae | 3.74 | 6.51 | 2.33 | 3.22 | |||
| Deferribacteraceae | 0.46 | 0.29 | 0.33 | 0.35 | |||
| Desulfovibrionaceae | 0.36 | 0.53 | 0.07 | 0.03 | * | ||
| Bacillaceae | 0.45 | 0.29 | 0.37 | 0.13 | |||
| Erysipelatoclostridiaceae | 0.79 | 1.09 | 1.20 | 0.57 | |||
| Erysipelotrichaceae | 2.08 | 0.53 | 0.38 | 0.36 | * | ** | ** |
| Lactobacillaceae | 7.08 | 5.77 | 8.04 | 9.31 | |||
| Streptococcaceae | 0.16 | 0.02 | 0.003 | 0.02 | * | *** | * |
| Staphylococcaceae | 0.35 | 0.05 | 0 | 0.003 | ** | *** | **** |
| Christensenellaceae | 0.36 | 0.10 | 0.08 | 1.07 | * | * | |
| Clostridia UCG-014 | 1.57 | 1.30 | 3.07 | 5.10 | ** | **** | |
| Clostridiaceae | 0.4 | 0.43 | 0.11 | 0.12 | *** | ** | |
| Lachnospiraceae | 14.96 | 15.65 | 23.25 | 19.75 | ** | ||
| Monoglobaceae | 1.08 | 0.59 | 0.71 | 1.41 | |||
| Oscillospiraceae | 4.63 | 6.21 | 4.92 | 3.89 | |||
| Ruminococcaceae | 12.28 | 9.39 | 7.38 | 7.93 | |||
| Eubacterium | 0.75 | 0.58 | 0.93 | 0.97 | |||
| Peptococcaceae | 0.39 | 0.63 | 0.35 | 0.17 | ** | ||
| Anaerovoracaceae | 0.33 | 0.29 | 0.13 | 0.11 | ** | *** | |
| Peptostreptococcaceae | 2.88 | 1.96 | 0.21 | 0.86 | **** | * | |
| Sutterellaceae | 0.54 | 0.45 | 0.11 | 0.08 | ** | **** | |
| Enterobacteriaceae | 0.68 | 1.30 | 0.05 | 0.03 | ** | ** | |
| Akkermansiaceae | 4.56 | 5.57 | 6.80 | 9.77 | * |
| Genus Species | PBS | Apigenin | Luteolin | Xanthohumol | PBS vs. API | PBS vs. LUT | PBS vs. XAN |
|---|---|---|---|---|---|---|---|
| Bifidobacterium | 0.52 | 0.43 | 0.03 | 0.35 | ** | ||
| B. animalis | 0.41 | 0.41 | 0.03 | 0.28 | * | ||
| Adlercreutzia | 0.40 | 0.22 | 0.17 | 0.24 | * | ||
| Enterorhabdus | 0.17 | 0.15 | 0.55 | 0.46 | **** | *** | |
| Bacteroides | 12.66 | 16.23 | 10.59 | 15.25 | |||
| B. dorei | 0.87 | 1.59 | 0.36 | 1.57 | * | ** | * |
| B. thetaiotaomicron | 1.31 | 2.09 | 1.12 | 2.32 | * | ||
| Alistipes | 7.66 | 5.67 | 6.37 | 0.39 | **** | ||
| Bilophila | 0.36 | 0.53 | 0.07 | 0.03 | * | ||
| Turicibacter | 0.85 | 0.35 | 0.10 | 0.22 | **** | * | |
| Streptococcus | 0.15 | 0.02 | 0.003 | 0.016 | ** | * | |
| Staphylococcus | 0.35 | 0.05 | 0 | 0.003 | *** | *** | **** |
| Clostridia_UCG-014 | 1.57 | 1.30 | 3.07 | 5.10 | ** | **** | |
| Clostridium sensu stricto 1 | 0.40 | 0.43 | 0.11 | 0.12 | *** | ** | |
| Blautia | 0.07 | 0.30 | 0.37 | 0.19 | * | * | |
| Uncharacterized | 0.01 | 0.28 | 0.33 | 0.15 | ** | ** | * |
| Lachnospiraceae NK4A136 | 6.19 | 7.32 | 15.48 | 12.27 | * | ||
| Ruminococcus | 6.19 | 3.47 | 1.48 | 2.93 | ** | ||
| Peptococacceae uncultured | 0.36 | 0.53 | 0.31 | 0.17 | *** | ||
| Romboutsia | 2.88 | 1.96 | 0.21 | 0.86 | **** | * | |
| Parasutterella | 0.54 | 0.45 | 0.11 | 0.08 | ** | **** | |
| Escherichia-Shigella | 0.68 | 1.30 | 0.05 | 0.03 | ** | ** | |
| Akkermansia | 4.56 | 5.57 | 6.80 | 9.77 | * | ||
| A. muciniphila | 4.56 | 5.57 | 6.80 | 9.77 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magadán-Corpas, P.; Pérez-Valero, Á.; Ye, S.; Sordon, S.; Huszcza, E.; Popłoński, J.; Villar, C.J.; Lombó, F. Gut Microbiota and Inflammation Modulation in a Rat Model for Ulcerative Colitis after the Intraperitoneal Administration of Apigenin, Luteolin, and Xanthohumol. Int. J. Mol. Sci. 2024, 25, 3236. https://doi.org/10.3390/ijms25063236
Magadán-Corpas P, Pérez-Valero Á, Ye S, Sordon S, Huszcza E, Popłoński J, Villar CJ, Lombó F. Gut Microbiota and Inflammation Modulation in a Rat Model for Ulcerative Colitis after the Intraperitoneal Administration of Apigenin, Luteolin, and Xanthohumol. International Journal of Molecular Sciences. 2024; 25(6):3236. https://doi.org/10.3390/ijms25063236
Chicago/Turabian StyleMagadán-Corpas, Patricia, Álvaro Pérez-Valero, Suhui Ye, Sandra Sordon, Ewa Huszcza, Jarosław Popłoński, Claudio J. Villar, and Felipe Lombó. 2024. "Gut Microbiota and Inflammation Modulation in a Rat Model for Ulcerative Colitis after the Intraperitoneal Administration of Apigenin, Luteolin, and Xanthohumol" International Journal of Molecular Sciences 25, no. 6: 3236. https://doi.org/10.3390/ijms25063236





