Dibenzocyclooctadiene Lignans from Schisandra chinensis with Anti-Inflammatory Effects
Abstract
:1. Introduction
2. Result and Discussion
2.1. Isolation of Compounds
2.2. Antioxidant Activity of Lignans
2.3. Anti-Inflammatory Activity
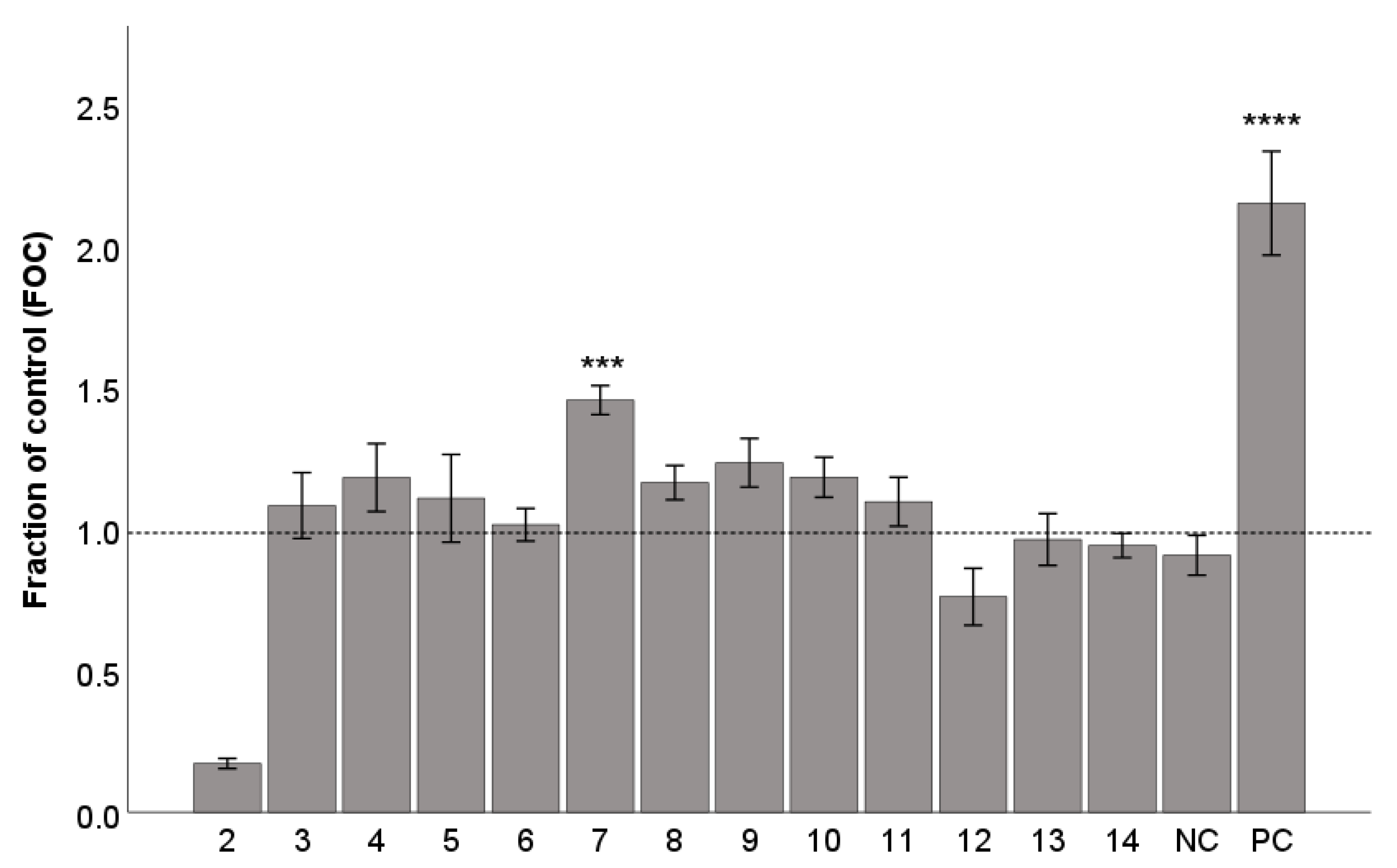
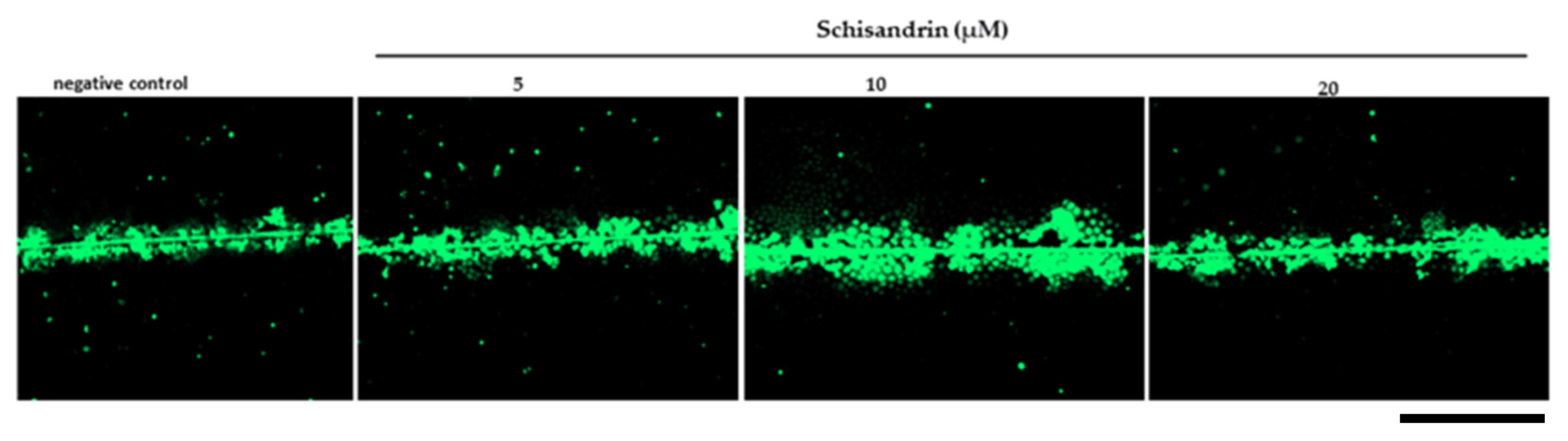
2.4. Gap Junction Intercellular Communication (GJIC)
3. Material and Methods
3.1. Plant Material
3.2. General Analytical Apparatus and Conditions
3.3. Extraction and Isolation
Isolation of Compound 1
3.4. Antiproliferative Activity
3.5. Cellular Antioxidant Activity (CAA) Assay
3.6. Detection of the Activation of NF-κB
3.7. The Scrape Loading-Dye Transfer (SLDT) Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, M. La Médicine Chinoise par les Plantes, 1st ed.; Le Corps a Vivre Series; Éditions Tchou: Paris, France, 1976; Volume 274. [Google Scholar]
- Panossian, A.; Wikman, G. Pharmacology of Schisandra chinensis Bail.: An overview of Russian research and uses in medicine. J. Ethnopharmacol. 2008, 118, 183–212. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Schisandra chinensis, Five Flavor Berry, Traditional Chinese Medicine and Super Fruit from North Eastern China. Pharmacogn. Commun. 2021, 11, 13–21. [Google Scholar] [CrossRef]
- Hancke, J.L.; Burgos, R.A.; Ahumada, F. Schisandra chinensis (Turcz.) Baill. Fitoterapia 1999, 70, 451–471. [Google Scholar] [CrossRef]
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as medicinal plant species a review on the bioactive components, pharmacological properties, analytical and biotechnical studies. Phytochem. Rev. 2017, 16, 195–218. [Google Scholar] [CrossRef]
- Van der Valk, J.M.A.; Leon, C.J.; Nesbitt, M. Microscopic authentication of Chinese materia medica (CMM). A UK market study of seeds and fruits. J. Herb. Med. 2017, 173, 40–51. [Google Scholar] [CrossRef]
- Slanina, J. Biological and pharmacological activity of lignans. Chem. Listy 2000, 94, 111–116. [Google Scholar]
- Opletal, L.; Sovová, H.; Bártlová, M. Dibenzo[a, c]cyclooctadiene lignans of the genus Schisandra: Importance, isolation and determination. J. Chromatogr. B 2004, 812, 357–371. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Bernatoniene, J. Antioxidant effects of Schisandra chinensis fruits and their active constituents. Antioxidants 2021, 10, 620. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Kim, T.; Kim, H.; Kim, S. Evaluation of proximate composition, bioactive lignans and volatile composition of Schisandra chinensis fruits from Inje and Mungyeong, Republic of Korea. J. Appl. Pharm. Sci. 2016, 6, 001–008. [Google Scholar] [CrossRef]
- Zhou, Y.; Men, L.; Sun, Y.; Wei, M.; Fan, X. Pharmacodynamic effects and molecular mechanisms of lignans from Schisandra chinensis Turcz. (Baill.), a current review. Eur. J. Pharmacol. 2021, 892, 173796. [Google Scholar] [CrossRef]
- Young, I.S.; Woodside, J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef]
- Black, H.S. A synopsis of the associations of oxidative stress, ROS, and antioxidants with diabetes mellitus. Antioxidants 2022, 11, 2003. [Google Scholar] [CrossRef]
- Choi, Y.W.; Takamatsu, S.; Khan, S.I.; Srinivas, P.V.; Ferreira, D.; Zhao, J.; Khan, I.A. Schisandrene, a dibenzocyclooctadiene lignan from Schisandra chinensis: Structure- antioxidant activity relationships of dibenzocyclooctadiene lignans. J. Nat. Prod. 2006, 69, 356–359. [Google Scholar] [CrossRef]
- Liu, H.; Liu, G.T. Antioxidant activity of dibenzocyclooctadiene lignans isolated from Schisandraceae. Planta Med. 1992, 58, 311–313. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, M.K.; Koo, K.A.; Kim, S.H.; Sung, S.H.; Lee, N.G.; Markelonis, G.J.; Oh, T.H.; Yang, J.H.; Kim, Y.C. Dibenzocyclooctadiene lignans from Schisandra chinensis protect primary cultures of rat cortical cells from glutamate-induced toxicity. J. Neurosci. Res. 2004, 76, 397–405. [Google Scholar] [CrossRef]
- Stankov, S.V. Definition of inflammation, causes of inflammation and possible anti-inflammatory strategies. Open Inflamm. J. 2012, 5, 1–9. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Luo, G.; Cheng, B.C.Y.; Zhao, H.; Fu, X.Q.; Xie, R.; Zhang, S.F.; Pan, S.Y.; Zhang, Y. Schisandra chinensis lignans suppresses the production of inflammatory mediators regulated by NF-κB, AP-1, and IRF3 in lipopolysaccharide-stimulated RAW264.7 cells. Molecules 2018, 23, 3319. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, Y.; Mizoguchi, Y.; Morisawa, S.; Takeda, S.; Aburada, M.; Hosoya, E. Effect of gomisin A (TJN-101) on arachidonic acid cascade in macrophages. Jap. J. Pharmacol. 1990, 52, 331–336. [Google Scholar] [CrossRef]
- Hervé, J.C.; Derangeon, M. Gap-junction-mediated cell to cell communication. Cell Tissue Res. 2013, 352, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Di Pietro, L.; Caraci, F. Gap Junction and Connexins in Microglia-Related Oxidative Stress and Neuroinflammation: Perspectives for drug Discovery. Biomolecules 2023, 13, 505. [Google Scholar] [CrossRef]
- Hansson, E.; Skiöldebrand, E. Low-grade inflammation causes gap junction-coupled cell dysfunction throughout the body which can lead to spread of systemic inflammation. Scan. J. Pain 2019, 19, 639–649. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Ikeya, Y.; Taguchi, H.; Yosioka, I.; Kobayashi, H. The constituents of Schizandra chinensis Baill. I. Isolation and structure determination of five new lignans, gomisin A, B, C, F and G, and the absolute structure of schisandrin. Chem. Pharm. Bull. 1979, 27, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Ikeya, Y.; Taguchi, H.; Yosioka, I.; Kobayashi, H. The constituents of Schizandra chinensis Baill. VIII. The structures of two new lignans, tiogloylgomisin P and angeloylgomisin P. Chem. Pharm. Bull. 1980, 28, 3357–3361. [Google Scholar] [CrossRef]
- Ikeya, Y.; Taguchi, H.; Yosioka, I.; Kobayashi, H. The constituents of Schizandra chinensis Baill. V. The structures of four new lignans, gomisin N, gomisin O, epigomisin O and gomisin E, and transformation of gomisin N to deangeloylgomisin B. Chem. Pharm. Bull. 1979, 27, 2695–2709. [Google Scholar] [CrossRef]
- Chen, Y.B.; Chang, M.T.; Lo, Y.W.; Ho, C.J.; Kuo, Y.C.; Chien, C.T.; Chen, S.Y.; Liou, S.S.; Kuo, Y.H.; Shen, Y.C. Oxygenated lignans from the fruits of Schisandra arisanensis. J. Nat. Prod. 2009, 72, 1663–1668. [Google Scholar] [CrossRef]
- Ikeya, Y.; Miki, E.; Okada, M.; Mitsuhashi, H.; Chai, J.G. Benzoylgomisin Q and benzoylgomisin P, two new lignans from Schisandra sphenanthera Rehd. et Wils. Chem. Pharm. Bull. 1990, 35, 1408–1411. [Google Scholar] [CrossRef]
- Šmejkal, K.; Šlapetová, T.; Krmenčík, P.; Kubínová, R.; Suchý, P.; Dall’Acqua, S.; Innocenti, G.; Vančo, J.; Kalvarová, K.; Dvorská, M.; et al. Evaluation of the antiradical activity of Schisandra chinensis lignans using different experimental models. Molecules 2000, 15, 1223–1231. [Google Scholar] [CrossRef]
- Blasa, M.; Angelino, D.; Gennari, L.; Ninfali, P. The cellular antioxidant activity in red blood cells (CAA-RBC): A new approach to bioavailability and synergy of phytochemicals and botanical extracts. Food Chem. 2011, 125, 685–691. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Jeong, J.B.; Jeong, H.J. Schisandra chinensis inhibits oxidative DNA damage and lipid peroxidation via antioxidant activity. Korean J. Plant Res. 2009, 22, 195–202. [Google Scholar]
- Wang, M.C.; Lai, Y.C.; Chang, C.L. High throughput screening and antioxidant assay of dibenzo[a, c]cyclooctadiene lignans in modified-ultrasonic and supercritical fluid extracts of Schisandra chinensis Baill by liquid chromatography–mass spectrometry and a free radical-scavenging method. J. Sep. Sci. 2008, 31, 1322–1332. [Google Scholar] [CrossRef]
- Min, X.; Zhao, L.; Shi, Y.; Wang, J.; Lv, H.; Song, X.; Zhao, Q.; Zhao, Q.; Jing, R.; Hu, J. Gomisin J attenuates cerebral ischemia/reperfusion injury by inducing anti-apoptotic, anti-inflammatory, and antioxidant effects in rats. Bioengineered 2022, 13, 6908–6918. [Google Scholar] [CrossRef]
- Yoshikawa, A.; Saito, Y.; Maruyama, K. Lignan compounds and 4,4′-dihydroxybiphenyl protect C2C12 cells against damage from oxidative stress. Biochem. Biophys. Res. Commun. 2006, 344, 394–399. [Google Scholar] [CrossRef]
- Ngo, T.C.; Mai, T.V.T.; Pham, T.T.; Jeremic, S.; Markovic, Z.; Hyunh, L.K.; Dao, D.Q. Natural acridones and coumarins as free radical scavengers. Mechanistic and kinetic studies. Chem. Phys. Lett. 2020, 746, 137312. [Google Scholar] [CrossRef]
- Li, X.; Zhao, B.; Liu, G.; Xin, W. Scavenging effects on active oxygen radicals by schizandrins with different structures and configurations. Free Radic. Biol. Med. 1990, 9, 99–104. [Google Scholar] [PubMed]
- Takanche, J.S.; Kim, J.E.; Han, S.H.; Yi, H.K. Effect of gomisin A on osteoblast differentiation in high glucose-mediated oxidative stress. Phytomedicine 2020, 66, 153107. [Google Scholar] [CrossRef] [PubMed]
- You, Y.L.; Lee, J.Y.; Choi, H.S. Schisandra chinensis derived gomisin C suppreses lipid accumulation by JAK2-STAT signaling in adipocyte. Food Sci. Biotechnol. 2023, 32, 1225–1233. [Google Scholar] [CrossRef]
- Choi, Y.H. Schisandrin A prevents oxidative stress induced DNA damaged and apoptosis by attenuating ROS generation in C2Cl2 cells. Biomed. Pharmacother. 2018, 106, 902–909. [Google Scholar] [CrossRef]
- Ding, M.; Shu, P.; Gao, S.; Wang, F.; Gao, Y.; Chen, Y.; Deng, W.; He, G.; Hu, Z.; Li, T. Schisandrin B protects human keratinocyte-derived HaCaT cells from tert-butyl hydroperoxide-induced oxidative damage through activating the Nrf2 signaling pathway. Int. J. Mol. Med. 2018, 42, 3571–3581. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Kim, Y.H.; Bae, D.S.; Um, B.H.; Pan, C.H.; Kim, C.Y.; Lee, H.J.; Lee, J.K.M. Anti-inflammatory effects of gomisin N, gomisin J, and schisandrin C isolated from the fruit of Schisandra chinensis. Biosci. Biotechnol. Biochem. 2010, 74, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Yang, Z.; Yao, X.; Wang, H.; Han, N.; Liu, Z.; Wang, Y.; Yang, J.; Yin, J. Dibenzocyclooctadiene lignans from Schisandra chinensis and their inhibitory activity on NO production in lipopolysaccharide-activated microglia cells. Phytochemistry 2014, 104, 72. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Qin, X.; Shi, M.; Qin, Z.; Meng, Y.; Qin, Z.; Guo, S. Schisandrin B inhibits LPS-induced inflammatory response in human umbilical vein endothelial cells by activating Nrf2. Int. Immunopharmacol. 2017, 49, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Pang, S.; Yang, N.; Meng, H.; Liu, J.; Zhou, N.; Zhang, M.; Xu, Z.; Gao, W.; Chen, B.; et al. Beneficial effects of schisandrin B on the cardiac function in mice model of myocardial infarction. PLoS ONE 2013, 8, e79418. [Google Scholar] [CrossRef] [PubMed]
- Kortesoja, M.; Karhu, E.; Olafsdottir, E.S.; Olafsdottir, E.S.; Freysdottir, J.; Hanski, L. Impact of dibenzocyclooctadiene lignans from Schisandra chinensis on the redox status and activation of human innate immune system cells. Free Radic. Biol. Med. 2019, 131, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Wang, Z.; Yu, T.; Lu, Q.; Chen, H. Suppression of MAPK and NF-κB pathways by schisandrin B contributes to attenuation of DSS-induced mice model of inflammatory bowel disease. Pharmazie 2015, 70, 598–603. [Google Scholar]
- Guo, M.; An, F.; Yu, H.; Wei, X.; Hong, M.; Lu, Y. Comparative effects of schisandrin A, B, and C on Propionibacterium acnes-induced, NLRP3 inflammasome activation-mediated IL-1β secretion and pyroptosis. Biomed. Pharmacother. 2017, 96, 129–136. [Google Scholar] [CrossRef]
- Liu, N.; Zheng, J.X.; Zhuang, Y.S.; Zhou, Z.K.; Zhao, J.H.; Yang, L. Anti-inflammatory effects of schisandrin B on LPS-stimulated BV2 microglia via activating PPAR-γ. Inflammation 2017, 40, 1006–1011. [Google Scholar] [CrossRef]
- Kwon, D.H.; Cha, H.J.; Choi, E.O.; Leem, S.H.; Kim, G.Y.; Moon, S.K.; Chang, Y.C.; Yun, S.J.; Hwang, H.J.; Kim, B.W.; et al. Schisandrin A suppresses lipopolysaccharide-induced inflammation and oxidative stress in RAW 264.7 macrophages by suppressing the NF-κB, MAPKs and PI3K/Akt pathways and activating Nrf2/HO-1 signaling. Int. J. Mol. Med. 2018, 41, 264–274. [Google Scholar] [CrossRef]
- Tu, C.; Huang, X.; Xiao, Y.; Song, M.; Ma, Y.; Yan, J.; You, H.; Wu, H. Schisandrin A inhibits the IL-1β induced inflammation and cartilage degradation via suppression of MAPK and NF-κB signal pathways in rat chondrocytes. Front. Pharmacol. 2019, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, Y.; Qian, H.Y.; Yoshigai, E.; Okumura, T.; Ikeya, Y.; Nishizawa, M. Gomisin N in the herbal drug gomishi (Schisandra chinensis) suppresses inducible nitric oxide synthase gene via C/EBPβ and NF-κB in rat hepatocytes. Nitric Oxide 2013, 28, 47–56. [Google Scholar] [CrossRef]
- Szopa, A.; Dziurka, M.; Warzecha, A.; Kubica, P.; Klimek-Szczykutowicz, M.; Ekiert, H. Targeted lignan profiling and anti-inflammatory properties of Schisandra rubriflora and Schisandra chinensis extracts. Molecules 2018, 23, 3103. [Google Scholar] [CrossRef] [PubMed]
- Araki, R.; Hiraki, Y.; Nishida, S.; Inatomi, Y.; Yabe, T. Gomisin N ameliorates lipopolysaccharide-induced depressive-like behaviors by attenuating inflammation in the hypothalamic paraventricular nucleus and central nucleus of the amygdala in mice. J. Pharmacol. Sci. 2016, 132, 138–144. [Google Scholar] [CrossRef]
- Park, S.Y.; Bae, Y.S.; Ko, M.J.; Lee, S.J.; Choi, Y.W. Comparison of anti-inflammatory potential of four different dibenzocyclooctadiene lignans in microglia; action via activation of PKA and Nrf-2 signaling and inhibition of MAPK/STAT/NF-κB pathways. Mol. Nutr. Food Res. 2014, 58, 738–748. [Google Scholar] [CrossRef]
- Dydowiczova, A.; Brozman, O.; Babica, P.; Sovadinová, I. Improved multiparametric scrape loading-dye transfer assay for a simultaneous high-throughput analysis of gap junctional intercellular assay for a simultaneous high-throughput analysis of gap junctional intercellular communication, cell density and viability. Sci. Rep. 2020, 10, 730. [Google Scholar] [PubMed]
- Sun, H.; Liu, G.T. Chemopreventive effect of dimethyl dicarboxylate biphenyl on malignant transformation of WB- F344 rat liver epithelial cells. Acta Pharmacol. Sin. 2005, 26, 1339–1344. [Google Scholar] [CrossRef]
- Dobrowolska, A.; Regulska-Ilow, B. The legitimacy of using dietary supplement dicloside seicosolariciresinol (SDG) from flaxseed in cancer. Rocz. Panstw. Zakl. Hig. 2021, 72, 9–20. [Google Scholar] [CrossRef]
- Šmejkal, K.; Šlapetová, T.; Krmenčík, P.; Babula, P.; Dall’Acqua, S.; Innocenti, G.; Vančo, J.; Casarin, E.; Carrara, M.; Kalvarová, K.; et al. Evaluation of cytotoxic activity of Schisandra chinensis lignans. Planta Med. 2010, 76, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Malaník, M.; Treml, J.; Leláková, V.; Nykodýmová, D.; Oravec, M.; Marek, J.; Šmejkal, K. Anti-Inflammatory and Antioxidant Properties of Chemical Constituents of Broussonetia Papyrifera. Bioorg. Chem. 2020, 104, 104298. [Google Scholar] [CrossRef] [PubMed]
- Defeijter, A.W.; Ray, J.S.; Weghorst, C.M.; Klaunig, J.E.; Goodman, J.I.; Chang, C.C.; Ruch, R.J.; Trosko, J.E. Infection of Rat-Liver Epithelial-Cells with V-Ha-Ras-Correlation between Oncogene Expression, Gap Junctional Communication, and Tumorigenicity. Mol. Carcinog. 1990, 3, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Nomata, K.; Chang, C.C.; Ruch, R.J.; Trosko, J.E. Cooperative effects of v-myc and c-Ha-ras oncogenes on gap junctional intercellular communication and tumorigenicity in rat liver epithelial cells. Cancer Lett. 1998, 128, 145–154. [Google Scholar] [CrossRef]
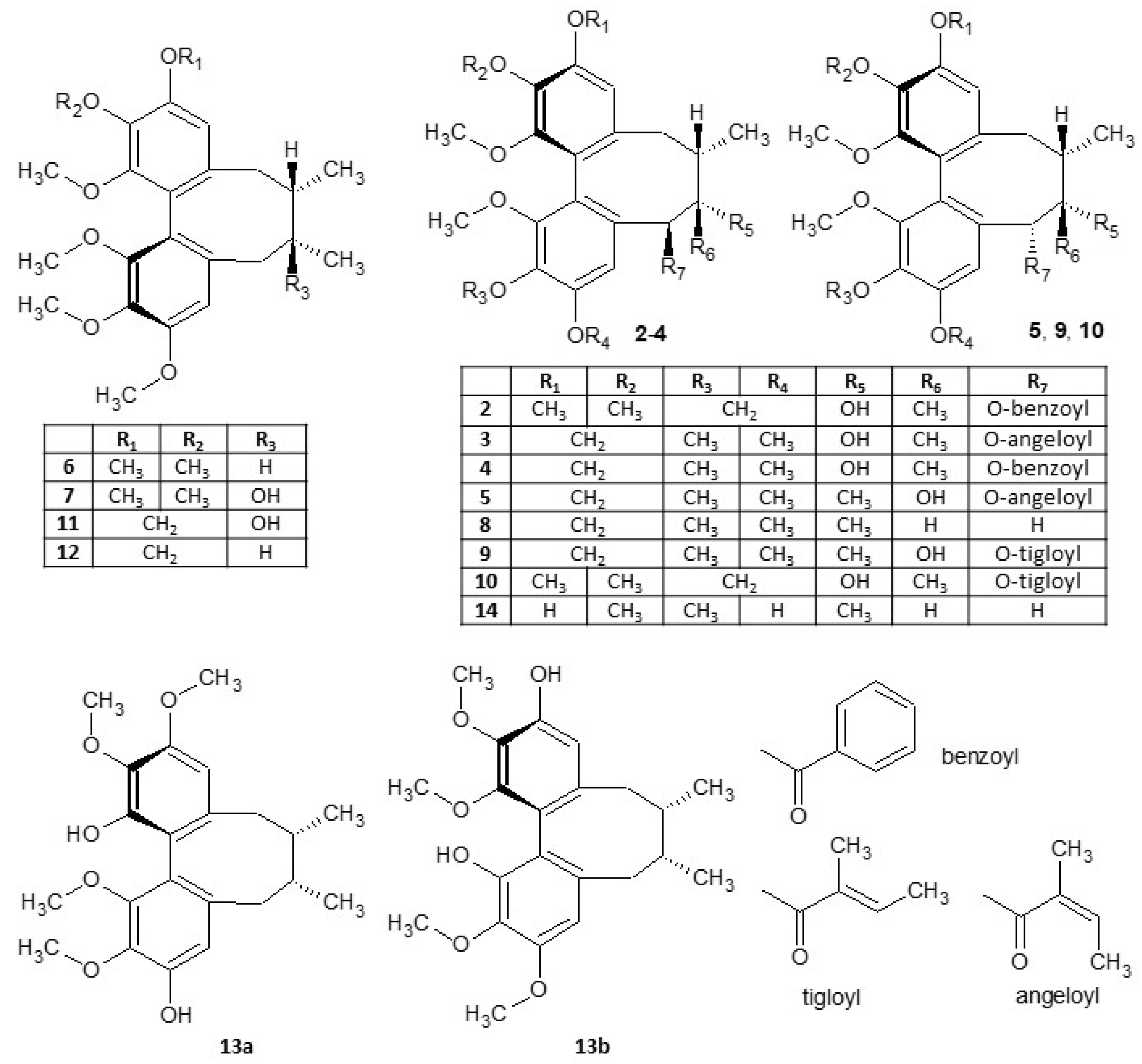
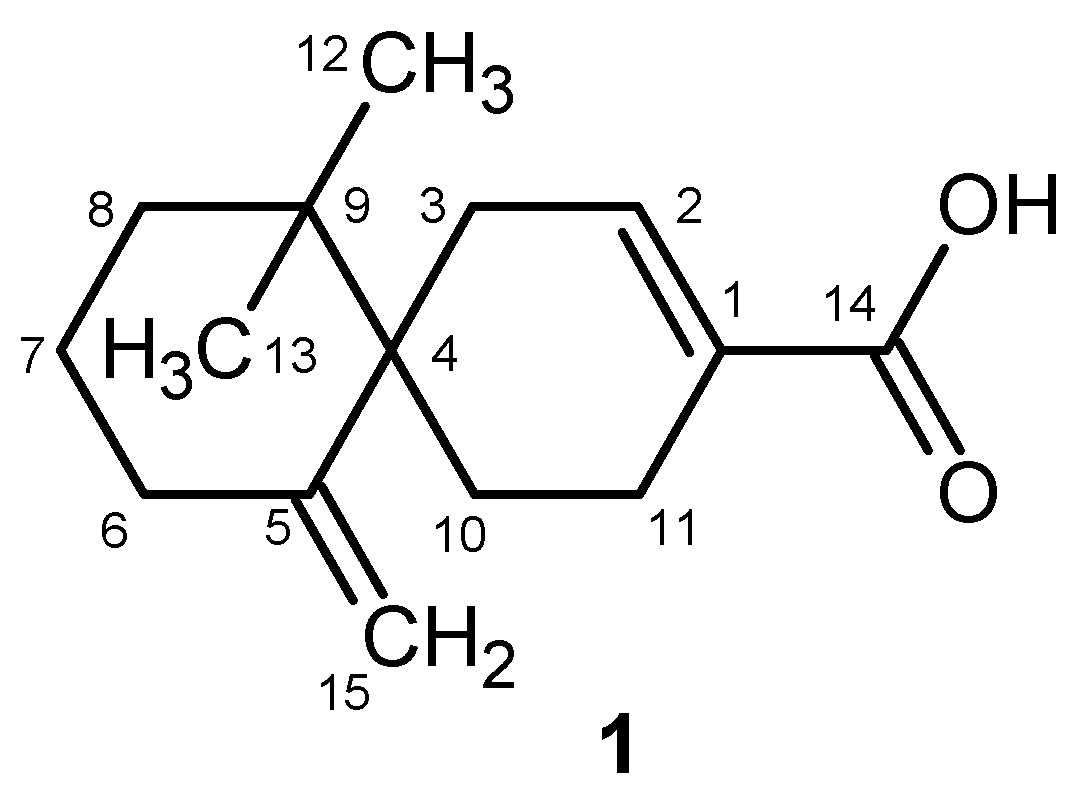


| Position | δH | δC |
|---|---|---|
| 1 | - | 138.4 |
| 2 | 6.96, s | 140.3 |
| 3 | 2.15–2.26, m | 31.6 |
| 4 | - | 44.6 |
| 5 | - | 148.0 |
| 6 | 1.75–1.95, m | 30.9 |
| 7 | 2.29, m | 29.6 |
| 8 | 1.41–2.16, m | 25.4 |
| 9 | - | 36.7 |
| 10 | 1.80–1.96, m | 23.4 |
| 11 | 2.34–2.36, m | 39.0 |
| 12 | 0.91, s | 24.0 |
| 13 | 0.87, s | 22.1 |
| 14 | - | 175.0 |
| 15 | 4.41–4.84, d, J = 2.2 Hz | 110.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybnikář, M.; Malaník, M.; Šmejkal, K.; Švajdlenka, E.; Shpet, P.; Babica, P.; Dall’Acqua, S.; Smištík, O.; Jurček, O.; Treml, J. Dibenzocyclooctadiene Lignans from Schisandra chinensis with Anti-Inflammatory Effects. Int. J. Mol. Sci. 2024, 25, 3465. https://doi.org/10.3390/ijms25063465
Rybnikář M, Malaník M, Šmejkal K, Švajdlenka E, Shpet P, Babica P, Dall’Acqua S, Smištík O, Jurček O, Treml J. Dibenzocyclooctadiene Lignans from Schisandra chinensis with Anti-Inflammatory Effects. International Journal of Molecular Sciences. 2024; 25(6):3465. https://doi.org/10.3390/ijms25063465
Chicago/Turabian StyleRybnikář, Michal, Milan Malaník, Karel Šmejkal, Emil Švajdlenka, Polina Shpet, Pavel Babica, Stefano Dall’Acqua, Ondřej Smištík, Ondřej Jurček, and Jakub Treml. 2024. "Dibenzocyclooctadiene Lignans from Schisandra chinensis with Anti-Inflammatory Effects" International Journal of Molecular Sciences 25, no. 6: 3465. https://doi.org/10.3390/ijms25063465









