Metabolomics of Plasma in XLH Patients with Arterial Hypertension: New Insights into the Underlying Mechanisms
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Study Participants
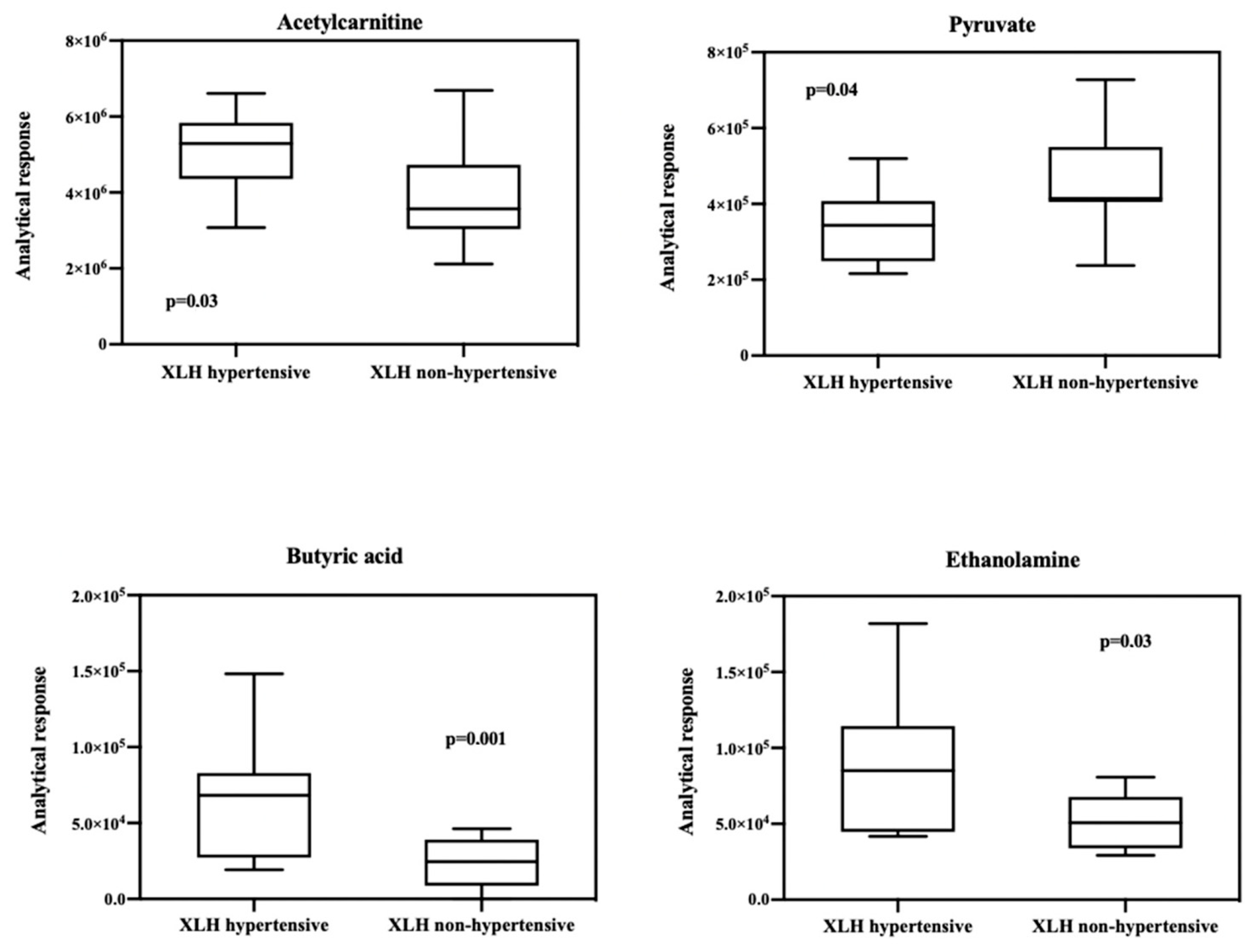
2.2. Biochemical Analyses
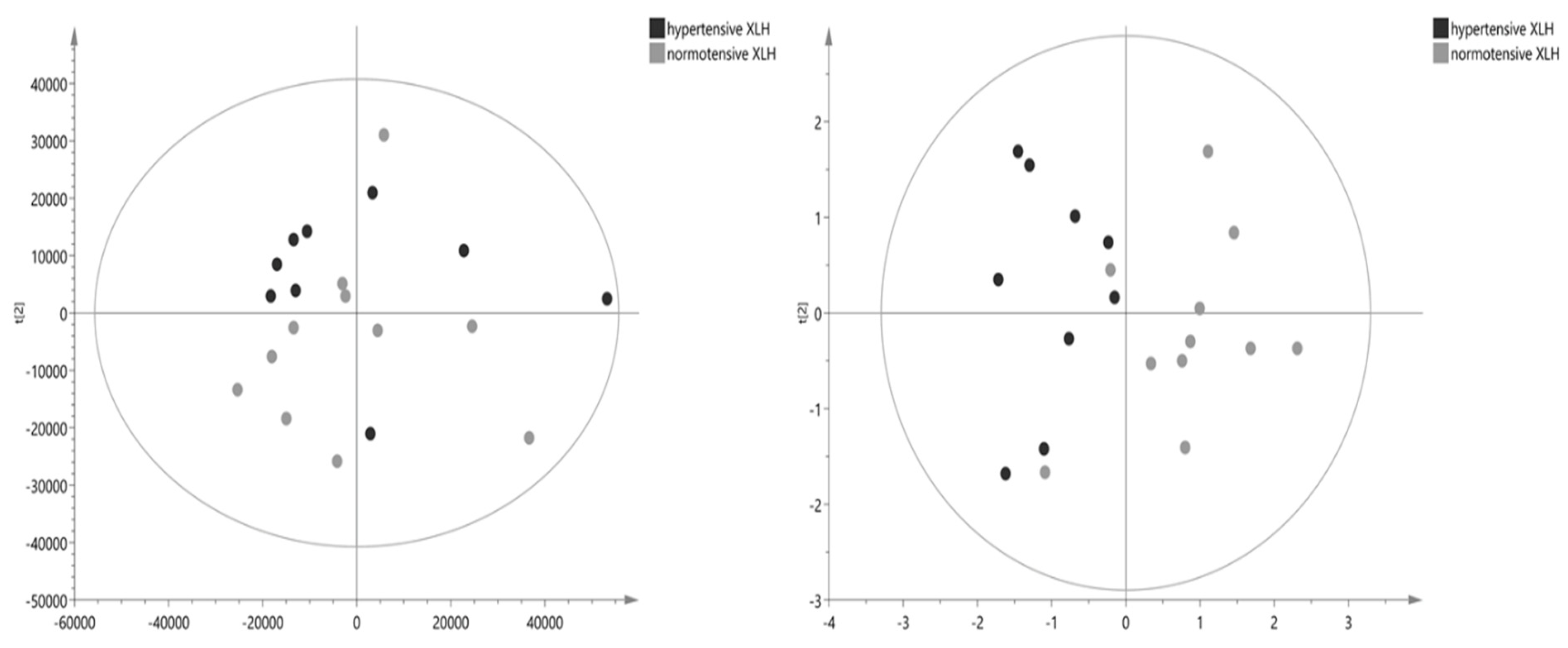
2.3. Metabolomic Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Measurement of Blood Pressure and Covariables
4.3. Blood Sample Collection and Preparation
4.4. Untargeted Metabolomics Based on UPLC-Q-ToF Mass Spectrometry
4.5. Data Processing and Acquisition
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beck-Nielsen, S.S.; Mughal, Z.; Haffner, D.; Nilsson, O.; Levtchenko, E.; Ariceta, G.; de Lucas Collantes, C.; Schnabel, D.; Jandhyala, R.; Mäkitie, O. FGF23 and its role in X-linked hypophosphatemia-related morbidity. Orphanet. J. Rare Dis. 2019, 14, 58. [Google Scholar] [CrossRef]
- European Commissions. Rare Diseases. Public Health–Eur Comm 2016. Available online: https://ec.europa.eu/health/non_conmunicable_diaseases/rare_diaseases_en (accessed on 27 March 2019).
- González-Lamuño, D.; Lorente Rodríguez, A.; Luis Yanes, M.I.; Marín-Del Barrio, S.; Martínez Díaz-Guerra, G.; Peris, P. Clinical practice recommendations for the diagnosis and treatment of X-linked hypophosphatemia: A consensus based on the ADAPTE method. Med. Clin. 2022, 159, 152.e1–152.e12. [Google Scholar] [CrossRef]
- Nakamura, Y.; Takagi, M.; Takeda, R.; Miyai, K.; Hasegawa, Y. Hypertension is a characteristic complication of X-linked hypophosphatemia. Endocr. J. 2017, 64, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Alon, U.S.; Monzavi, R.; Lilien, M.; Rasoulpour, M.; Geffner, M.E.; Yadin, O. Hypertension in hypophosphatemic rickets-role of secondary hyperparathyroidism. Pediatr. Nephrol. 2003, 18, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Andrukhova, O.; Slavic, S.; Smorodchenko, A.; Zeitz, U.; Shalhoub, V.; Lanske, B.; Pohl, E.E.; Erben, R.G. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol. Med. 2014, 6, 744–759. [Google Scholar] [CrossRef] [PubMed]
- McMullan, C.J.; Borgi, L.; Curhan, G.C.; Fisher, N.; Forman, J.P. The effect of vitamin D on renin-angiotensin system activation and blood pressure: A randomized control trial. J. Hypertens. 2017, 35, 822–829. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Ameta, K.; Gupta, A.; Kumar, S.; Sethi, R.; Kumar, D.; Mahdi, A.A. Essential hypertension: A filtered serum based metabolomics study. Sci. Rep. 2017, 7, 2153. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324, Erratum in: Hypertension 2018, 71, e136–e139. Erratum in: Hypertension 2018, 72, e33. [Google Scholar] [CrossRef]
- Sun, Z. Aging, arterial stiffness, and hypertension. Hypertension 2015, 65, 252–256. [Google Scholar] [CrossRef]
- Dietrich, S.; Floegel, A.; Weikert, C.; Prehn, C.; Adamski, J.; Pischon, T.; Boeing, H.; Drogan, D. Identification of serum metabolites associated with incident hypertension in the European prospective investigation into cancer and Nutrition-Potsdam study. Hypertension 2016, 68, 471–477. [Google Scholar] [CrossRef]
- Arnett, D.K.; Claas, S.A. Omics of blood pressure and hypertension. Circ. Res. 2018, 122, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- He, W.J.; Li, C.; Mi, X.; Shi, M.; Gu, X.; Bazzano, L.A.; Razavi, A.C.; Nierenberg, J.L.; Dorans, K.; He, H.; et al. An untargeted metabolomics study of blood pressure: Findings from the bogalusa heart study. J. Hypertens. 2020, 38, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Vaz, F.M.; Wanders, R.J. Carnitine biosynthesis in mammals. Biochem. J. 2002, 361, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, D.; Jiang, Y. Function, Detection and Alteration of Acylcarnitine Metabolism in Hepatocellular Carcinoma. Metabolites 2019, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.L.; McDonald, D.A.; Borum, P.R. Acylcarnitines: Role in brain. Prog. Lipid Res. 2010, 49, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Zheng, Y.; Ruiz-Canela, M.; Hruby, A.; Martínez-González, M.A.; Clish, C.B.; Corella, D.; Estruch, R.; Ros, E.; Fitó, M.; et al. Plasma Acylcarnitines and Risk of Cardiovascular Disease: Effect of Mediterranean Diet Interventions. Am. J. Clin. Nutr. 2016, 103, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhao, M.; Yang, L.; Liu, X.; Pacifico, L.; Chiesa, C.; Xi, B. Identification of Potential Metabolic Markers of Hypertension in Chinese Children. Int. J. Hypertens. 2021, 2021, 6691734. [Google Scholar] [CrossRef]
- Aitken-Buck, H.M.; Krause, J.; Zeller, T.; Jones, P.P.; Lamberts, R.R. Long-Chain Acylcarnitines and Cardiac Excitation-Contraction Coupling: Links to Arrhythmias. Front. Physiol. 2020, 11, 577856. [Google Scholar] [CrossRef]
- Hua, S.; Clish, C.; Scott, J.; Hanna, D.; Haberlen, S.; Shah, S.; Hodis, H.; Landy, A.; Post, W.; Anastos, K.; et al. Abstract P201: Associations of Plasma Acylcarnitines With Incident Carotid Artery Plaque in Individuals With or at Risk of HIV Infection. Circulation 2018, 137, AP201. [Google Scholar] [CrossRef]
- Kosacka, J.; Berger, C.; Ceglarek, U.; Hoffmann, A.; Blüher, M.; Klöting, N. Ramipril Reduces Acylcarnitines and Distinctly Increases Angiotensin-Converting Enzyme 2 Expression in Lungs of Rats. Metabolites 2022, 12, 293. [Google Scholar] [CrossRef] [PubMed]
- Natali, A.; Santoro, D.; Palombo, C.; Cerri, M.; Ghione, S.; Ferrannini, E. Impaired insulin action on skeletal muscle metabolism in essential hypertension. Hypertension 1991, 17, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, M.; Nakatomi, Y.; Tamaki, K.; Ishitsuka, T.; Kawasaki, T.; Omae, T. Cerebrospinal fluid lactate and pyruvate concentrations in patients with malignant hypertension. J. Neurol. 1984, 231, 71–74. [Google Scholar] [CrossRef]
- Xu, W.; Liu, D.; Lin, D. Comparison of mechanisms of endothelial cell protections between high-density lipo-protein and apolipoprotein A-I mimetic peptide. Front. Pharmacol. 2019, 10, 817. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Takahashi, Y.; Mano, T.; Sakata, Y.; Nishikawa, N.; Yoshida, J.; Oishi, Y.; Hori, M.; Miwa, T.; Inoue, S.; et al. N-methyl-ethanolamine attenuates cardiac fibrosis and improves diastolic function: Inhibition of phospholipase D as a possible mechanism. Eur. Heart J. 2004, 25, 1221–1229. [Google Scholar] [CrossRef]
- Chen, X.F.; Chen, X.; Tang, X. Short-chain fatty acid, acylation and cardiovascular diseases. Clin. Sci. 2020, 134, 657–676. [Google Scholar] [CrossRef]
- Huc, T.; Nowinski, A.; Drapala, A.; Konopelski, P.; Ufnal, M. Indole and indoxyl sulfate, gut bacteria metabolites of tryptophan, change arterial blood pressure via peripheral and central mechanisms in rats. Pharmacol. Res. 2018, 130, 172–179. [Google Scholar] [CrossRef]
- Wretlind, A. Effect of tributyrin on circulation and respiration. Acta Physiol. Scand. 1957, 40, 59–74. [Google Scholar] [CrossRef]
- Onyszkiewicz, M.; Gawrys-Kopczynska, M.; Konopelski, P.; Aleksandrowicz, M.; Sawicka, A.; Koźniewska, E.; Samborowska, E.; Ufnal, M. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflug. Arch.–Eur. J. Physiol. 2019, 471, 1441–1453. [Google Scholar] [CrossRef]
- Cao, H.; Zhu, Y.; Hu, G.; Zhang, Q.; Zheng, L. Gut microbiome and metabolites, the future direction of diagnosis and treatment of atherosclerosis. Pharmacol. Res. 2023, 187, 106586. [Google Scholar] [CrossRef]
- Broseta, J.J. Different approaches to improve cohort identification using electronic health records: X-linked hypophosphatemia as an example. Intractable Rare Dis. Res. 2021, 10, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 19–62. [Google Scholar] [CrossRef]
- García-Cañaveras, J.C.; Lancho, O.; Ducker, G.S.; Ghergurovich, J.M.; Xu, X.; da Silva-Diz, V.; Minuzzo, S.; Indraccolo, S.; Kim, H.; Herranz, D.; et al. SHMT inhibition is effective and synergizes with methotrexate in T-cell acute lymphoblastic leukemia. Leukemia 2021, 35, 377–388. [Google Scholar] [CrossRef] [PubMed]

| Variable | Overall (n = 20) | XLH Hypertensive (n = 9) | XLH Non-Hypertensive (n = 11) |
|---|---|---|---|
| Age (years) | 44 (33.2–54.7) | 49 (39–57) | 43 (27–47) |
| Male sex, n (%) | 10 (50) | 6 (66) | 4 (40) |
| Weight (kg) | 73 (50.7–85.5) | 81.5 (50.2–93.7) | 71 (50–79.7) |
| Height (cm) | 158 (150–170.5) | 159.5 (152.2–182.7) | 155.5 (150–163) |
| BMI (Kg/m2) | 27.5 (22–32.2) | 30 (22.5–32.7) | 25 (22–32) |
| Diagnosis in childhood, n (%) | 10 (50) | 5 (55.5) | 5 (50) |
| Office SBP (mmHg) | 124.75 ± 17.66 | 139.77 ± 10.67 | 112.45 ± 11.50 |
| Office DBP (mmHg) | 71.5 ± 7.72 | 75.66 ± 8.93 | 68.09 ± 4.59 |
| Familiar history of hypertension n (%) | 7 (35) | 3 (33.3) | 4 (40) |
| Nephrocalcinosis n (%) | 3 (15) | 2 (22.2) | 1 (10) |
| Hyperparathyroidism n (%) | 7 (35) | 4 (44.4) | 3 (30) |
| Non-steroidal anti-inflammatories n (%) | 4 (20) | 1 (11.1) | 3 (30) |
| Creatinine (mg/dL), mean ± SD | 0.91 ± 0.29 | 1.08 ± 0.31 | 0.78 ± 0.19 |
| GFR (mL/min/1.73 m2), mean ± SD | 92.80 ± 23 | 79.77 ± 19.75 | 103.45 ± 20.51 |
| Uric acid (mg/dL), mean ± SD | 5.69 ± 1.52 | 6.45 ± 1.61 | 5.07 ± 1.18 |
| Total cholesterol(mg/dL), mean ± SD | 197 ± 28.36 | 206.88 ± 32.06 | 189 ± 23.39 |
| LDL (mg/dL), mean ± SD | 119 ± 24.33 | 133.33 ± 21.92 | 107.27 ± 20.12 |
| HDL (mg/dL), mean ± SD | 55.3 ± 14.91 | 50.22 ± 16.57 | 59.45 ± 12.68 |
| Triglycerides (mg/dL), mean ± SD | 115.94 ± 50.38 | 122.62 ± 58.63 | 111.09 ± 45.84 |
| Hemoglobin (g/dL), mean ± SD | 14.64 ± 1.63 | 14.9 ± 1.29 | 14.26 ± 1.91 |
| Alkaline phosphatase (UI/L), mean ± SD | 97.85 ± 43.68 | 112 ± 53.78 | 86.27 ±31.37 |
| Phosphorus (mg/dL), mean ± SD | 2.14 ± 0.49 | 2.08 ± 0.38 | 2.18 ± 0.58 |
| Calcium (mg/dL), mean ± SD | 9.46 ± 0.72 | 9.51 ± 0.54 | 9.42 ± 0.86 |
| Intact FGF23 (pg/mL), mean ± SD | 211.96 ± 248.58 | 288.28 ± 346.29 | 149.52 ± 109.28 |
| Vitamin D (ng/mL), mean ± SD | 27.97 ± 9.44 | 25.87 ± 6.37 | 29.5 ± 11.22 |
| PTH (pg/mL), mean ± SD | 74.88 ± 48.87 | 83.34 ± 38.80 | 67.96 ± 56.71 |
| TmP/GFR (mg/dL), mean ± SD | 2.12 ± 0.48 | 2.08 ± 0.28 | 2.07 ± 0.53 |
| Compound | Formula | m/z | Rt | Adduction | Class |
|---|---|---|---|---|---|
| glycine | C2H5NO2 | 74.024834 | 12.671 | [M−H]− | Amino acid/peptides/analogues |
| Hypotaurine | C2H7NO2S | 108.012642 | 11.876 | [M−H]− | Sulfinic acid and derivatives |
| taurine | C2H7NO3S | 126.021965 | 9.911 | [M+H]+ | Organosulfonic acid and derivatives |
| O-Phosphoethanolamine | C2H8NO4P | 142.026367 | 13.674 | [M+H]+ | Phosphate esters |
| pyruvate | C3H4O3 | 87.008896 | 3.546 | [M−H]− | Keto acids and derivatives |
| Guanidoacetic acid | C3H7N3O2 | 118.061012 | 12.4 | [M+H]+ | Amino acid/peptides/analogues |
| serine | C3H7NO3 | 104.035446 | 12.858 | [M−H]− | Amino acid/peptides/analogues |
| succinate | C4H6O4 | 117.019478 | 12.021 | [M−H]− | Carboxylic acid and derivatives |
| 4-aminobutyrate | C4H9NO2 | 104.070511 | 11.303 | [M+H]+ | Amino acid/peptides/analogues |
| D-2-Aminobutyric acid | C4H9NO2 | 104.070511 | 11.303 | [M+H]+ | Amino acid/peptides/analogues |
| dimethylglycine | C4H9NO2 | 102.056160 | 11.155 | [M−H]− | Amino acid/peptides/analogues |
| glutamine | C5H10N2O3 | 147.076279 | 12.909 | [M+H]+ | Amino acid/peptides/analogues |
| 2-Hydroxyvaleric acid | C5H10O3 | 117.055817 | 3.579 | [M−H]− | Fatty and conjugated acids |
| methionine | C5H11NO2S | 148.043945 | 9.137 | [M−H]− | Amino acid/peptides/analogues |
| ornithine | C5H12N2O2 | 131.082657 | 16.205 | [M−H]− | Amino acid/peptides/analogues |
| Phosphorylcholine | C5H14NO4P | 184.073257 | 13.76 | [M+H]+ | Quaternary ammonium salts |
| hypoxanthine | C5H4N4O | 135.031296 | 6.754 | [M−H]− | Purines and derivatives |
| 2-hydroxyglutarate | C5H8O5 | 147.030060 | 10.955 | [M−H]− | Short chain hydroxy acids and derivatives |
| lysine | C6H14N2O2 | 145.098297 | 17.035 | [M−H]− | Amino acid/peptides/analogues |
| N′-Methylnicotinamide | C7H8N2O | 137.070877 | 13.417 | [M+H]+ | Pyridines and derivatives |
| Guaiacol | C7H8O2 | 123.045341 | 2.574 | [M−H]− | Phenols |
| 2-Hydroxyoctanoic acid | C8H16O3 | 159.102707 | 2.447 | [M−H]− | Fatty and conjugated acids |
| 2-Phenylbutyric acid | C10H12O2 | 163.076599 | 2.439 | [M−H]− | Benzene and derivatives |
| Indole-3-acetaldehyde | C10H9NO | 158.061234 | 2.544 | [M−H]− | Indoles and derivatives |
| DL-Indole-3-lactic acid | C11H11NO3 | 204.066666 | 2.485 | [M−H]− | Indoles and derivatives |
| tryptophan | C11H12N2O2 | 205.097092 | 7.689 | [M+H]+ | Indoles and derivatives |
| Undecanoic acid | C11H22O2 | 185.154770 | 2.226 | [M−H]− | Fatty and conjugated acids |
| trans-3-Indoleacrylic acid | C11H9NO2 | 188.07074 | 3.044 | [M+H]+ | Indoles and derivatives |
| Indole-3-pyruvic acid | C11H9NO3 | 202.051147 | 2.215 | [M−H]− | Indoles and derivatives |
| Butyric acid | C4H8O2 | 202.087463 | 2.495 | [M−H]− | Indoles and derivatives |
| Myristic acid | C14H28O2 | 227.201828 | 2.147 | [M−H]− | Fatty and conjugated acids |
| Linoleic acid | C18H32O2 | 279.233337 | 2.101 | [M−H]− | Lineolic acids and derivatives |
| Stearic acid | C18H36O2 | 283.264160 | 2.082 | [M−H]− | Fatty and conjugated acids |
| Arachidic acid | C20H40O2 | 311.295898 | 2.048 | [M−H]− | Fatty and conjugated acids |
| Chenodeoxycholic acid | C24H40O4 | 393.299957 | 2.996 | [M+H]+ | Bile acids, alcohols and derivatives |
| Glycodeoxycholic acid | C26H43NO5 | 450.321655 | 3.096 | [M+H]+ | Bile acids, alcohols and derivatives |
| Glycocholic acid | C26H43NO6 | 464.302124 | 6.826 | [M−H]− | Bile acids, alcohols and derivatives |
| Taurodeoxycholic acid | C26H45NO6S | 498.290253 | 2.389 | [M−H]− | Bile acids, alcohols and derivatives |
| Glycolic acid | C2H4O3 | 75.008797 | 8.434 | [M−H]− | Hydroxyacids and derivatives |
| acetylphosphate | C2H5O5P | 140.994659 | 12.582 | [M+H]+ | Phosphate esters |
| lactate | C3H6O3 | 89.024582 | 7.071 | [M−H]− | Hydroxyacids and derivatives |
| glycerate | C3H6O4 | 105.019455 | 9.939 | [M−H]− | Carbohydrates and conjugates |
| alanine | C3H7NO2 | 90.054924 | 12.178 | [M+H]+ | Amino acid/peptides/analogues |
| b-alanine | C3H7NO2 | 90.054924 | 12.809 | [M+H]+ | Amino acid/peptides/analogues |
| sarcosine | C3H7NO2 | 90.054924 | 12.178 | [M+H]+ | Amino acid/peptides/analogues |
| Trimethylamine N-oxide | C3H9NO | 76.075676 | 12.071 | [M+H]+ | Organonitrogenic compounds |
| Ethanolamine | C2H7N0 | 106.086212 | 11.132 | [M+H]+ | Organonitrogenic compounds |
| acetoacetate | C4H6O3 | 101.024567 | 5.873 | [M−H]− | Keto acids and derivatives |
| 2-Ketobutyric acid | C4H6O3 | 101.024574 | 2.401 | [M−H]− | Keto acids and derivatives |
| N-Acetylglycine | C4H7NO3 | 116.035461 | 8.303 | [M−H]− | Amino acid/peptides/analogues |
| aspartate | C4H7NO4 | 134.044907 | 13.404 | [M+H]+ | Amino acid/peptides/analogues |
| asparagine | C4H8N2O3 | 133.060822 | 13.126 | [M+H]+ | Amino acid/peptides/analogues |
| 3-hydroxylbutyrate | C4H8O3 | 103.040222 | 7.334 | [M−H]− | Hydroxyacids and derivatives |
| (R)-3-Hydroxybutanoic acid | C4H8O3 | 103.040222 | 7.700 | [M−H]− | Hydroxyacids and derivatives |
| 2-Hydroxybutyric acid | C4H8O3 | 103.040237 | 5.802 | [M−H]− | Hydroxyacids and derivatives |
| creatine | C4H9N3O2 | 130.062256 | 12.102 | [M−H]− | Amino acid/peptides/analogues |
| L-Homoserine | C4H9NO3 | 120.065437 | 12.502 | [M+H]+ | Amino acid/peptides/analogues |
| threonine | C4H9NO3 | 120.065437 | 12.502 | [M+H]+ | Amino acid/peptides/analogues |
| 3-Hydroxyisovaleric acid | C5H10O3 | 117.055817 | 5.689 | [M−H]− | Amino acid/peptides/analogues |
| DL-Valine | C5H11NO2 | 116.071785 | 10.032 | [M−H]− | Amino acid/peptides/analogues |
| betaine | C5H11NO2 | 116.071785 | 10.032 | [M−H]− | Amino acid/peptides/analogues |
| Adonitol | C5H12O5 | 151.061417 | 8.769 | [M−H]− | Carbohydrates and conjugates |
| Choline | C5H13NO | 104.106903 | 12.645 | [M+H]+ | Organonitrogenic compounds |
| Uric acid | C5H4N4O3 | 167.021011 | 8.487 | [M−H]− | Purines and derivatives |
| Pyroglutamic acid | C5H7NO3 | 128.035507 | 8.453 | [M−H]− | Amino acid/peptides/analogues |
| 2-Oxoisopentanoic acid | C5H8O3 | 115.040176 | 2.285 | [M−H]− | Keto acids and derivatives |
| Methylsuccinic acid | C5H8O4 | 131.035095 | 10.675 | [M−H]− | Fatty and conjugated acids |
| proline | C5H9NO2 | 116.070442 | 10.857 | [M+H]+ | Amino acid/peptides/analogues |
| N-Propionylglycine | C5H9NO3 | 132.065613 | 12.203 | [M+H]+ | Amino acid/peptides/analogues |
| Aminolevulinic acid | C5H9NO3 | 130.051025 | 12.026 | [M−H]− | Amino acid/peptides/analogues |
| L-Hydroxyproline | C5H9NO3 | 130.051056 | 7.712 | [M−H]− | Amino acid/peptides/analogues |
| N-Methyl-D-aspartic acid | C5H9NO4 | 148.060257 | 12.906 | [M+H]+ | Amino acid/peptides/analogues |
| glutamate | C5H9NO4 | 146.045898 | 12.746 | [M−H]− | Amino acid/peptides/analogues |
| 2-Methyl-3-ketovaleric acid | C6H10O3 | 129.055695 | 2.195 | [M−H]− | Keto acids and derivatives |
| Adipic acid | C6H10O4 | 145.050690 | 11.644 | [M−H]− | Fatty and conjugated acids |
| DL-Pipecolic acid | C6H11NO2 | 130.086411 | 10.533 | [M+H]+ | Amino acid/peptides/analogues |
| 4-Methylvaleric acid | C6H12O2 | 115.076584 | 2.533 | [M−H]− | Fatty and conjugated acids |
| L-Rhamnose | C6H12O5 | 163.061142 | 8.018 | [M−H]− | Carbohydrates and conjugates |
| myo-inositol | C6H12O6 | 179.056076 | 13.264 | [M−H]− | Alcohols and polyols |
| Fructose | C6H12O6 | 179.056091 | 10.639 | [M−H]− | Carbohydrates and conjugates |
| Mannose | C6H12O6 | 179.056091 | 10.639 | [M−H]− | Carbohydrates and conjugates |
| citrulline | C6H13N3O3 | 174.088364 | 13.056 | [M−H]− | Amino acid/peptides/analogues |
| leucine | C6H13NO2 | 130.087402 | 8.878 | [M−H]− | Amino acid/peptides/analogues |
| arginine | C6H14N4O2 | 173.104385 | 16.941 | [M−H]− | Amino acid/peptides/analogues |
| Aconitic acid | C6H6O6 | 173.009048 | 13.093 | [M−H]− | Carboxylic acid and derivatives |
| 3-Methylglutaconic Acid | C6H8O4 | 143.035065 | 10.646 | [M−H]− | Fatty and conjugated acids |
| citrate/isocitrate | C6H8O7 | 191.019623 | 13.506 | [M−H]− | Carboxylic acid and derivatives |
| 3-Methyl-Histidine | C7H11N3O2 | 168.077744 | 12.867 | [M−H]− | Amino acid/peptides/analogues |
| Homocitrulline | C7H15N3O3 | 190.1185 | 13.066 | [M+H]+ | Amino acid/peptides/analogues |
| gamma-Butyrobetaine | C7H15NO2 | 146.117508 | 13.111 | [M+H]+ | Fatty and conjugated acids |
| carnitine | C7H15NO3 | 162.11232 | 12.578 | [M+H]+ | Quaternary amine |
| Homoarginine | C7H16N4O2 | 189.134613 | 17.538 | [M+H]+ | Amino acid/peptides/analogues |
| Benzoic acid | C7H6O2 | 121.029625 | 10.036 | [M−H]− | Benzene and derivatives |
| 3-Hydroxybenzoic acid | C7H6O3 | 137.024445 | 2.072 | [M−H]− | Benzene and derivatives |
| 2-6-Dihydroxybenzoic acid | C7H6O4 | 153.019501 | 1.822 | [M−H]− | Benzene and derivatives |
| N-Acetyl-L-arginine | C8H16N4O3 | 217.129395 | 12.865 | [M+H]+ | Amino acid/peptides/analogues |
| DL-2-Aminooctanoic acid | C8H17NO2 | 160.133209 | 6.675 | [M+H]+ | Amino acid/peptides/analogues |
| Glycerophosphocholine | C8H20NO6P | 258.109863 | 13.14 | [M+H]+ | Glycerophospholipids |
| 3-Indoxyl sulfate | C8H7NO4S | 212.002319 | 1.863 | [M−H]− | Sulfuric acids and derivatives |
| 2-Phenylpropionic acid | C9H10O2 | 149.060959 | 2.475 | [M−H]− | Phenylpropanoids |
| phenylalanine | C9H11NO2 | 164.071762 | 8.015 | [M−H]− | Amino acid/peptides/analogues |
| tyrosine | C9H11NO3 | 180.066513 | 9.686 | [M−H]− | Amino acid/peptides/analogues |
| Acetylcarnitine | C9H17NO4 | 204.123001 | 10.376 | [M+H]+ | Fatty acid esters |
| 4-Hydroxyphenylpyruvic acid | C9H8O4 | 179.035172 | 2.368 | [M−H]− | Benzene and derivatives |
| 3-Methylindole | C9H9N | 132.080872 | 3.064 | [M+H]+ | Indoles and derivatives |
| Hydroxyphenylformamidoacetic acid | C9H9NO4 | 194.045883 | 6.769 | [M−H]− | Phenol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Romero, L.C.; Broseta, J.J.; Roca-Marugán, M.; Muñoz-Castañeda, J.R.; Lahoz, A.; Hernández-Jaras, J. Metabolomics of Plasma in XLH Patients with Arterial Hypertension: New Insights into the Underlying Mechanisms. Int. J. Mol. Sci. 2024, 25, 3545. https://doi.org/10.3390/ijms25063545
López-Romero LC, Broseta JJ, Roca-Marugán M, Muñoz-Castañeda JR, Lahoz A, Hernández-Jaras J. Metabolomics of Plasma in XLH Patients with Arterial Hypertension: New Insights into the Underlying Mechanisms. International Journal of Molecular Sciences. 2024; 25(6):3545. https://doi.org/10.3390/ijms25063545
Chicago/Turabian StyleLópez-Romero, Luis Carlos, José Jesús Broseta, Marta Roca-Marugán, Juan R. Muñoz-Castañeda, Agustín Lahoz, and Julio Hernández-Jaras. 2024. "Metabolomics of Plasma in XLH Patients with Arterial Hypertension: New Insights into the Underlying Mechanisms" International Journal of Molecular Sciences 25, no. 6: 3545. https://doi.org/10.3390/ijms25063545





