Synergistic Effect of a Combination of Proteasome and Ribonucleotide Reductase Inhibitors in a Biochemical Model of the Yeast Saccharomyces cerevisiae and a Glioblastoma Cell Line
Abstract
:1. Introduction
2. Results
2.1. RNR Subunit Levels Are Elevated in Strains with Impaired Proteasome Function
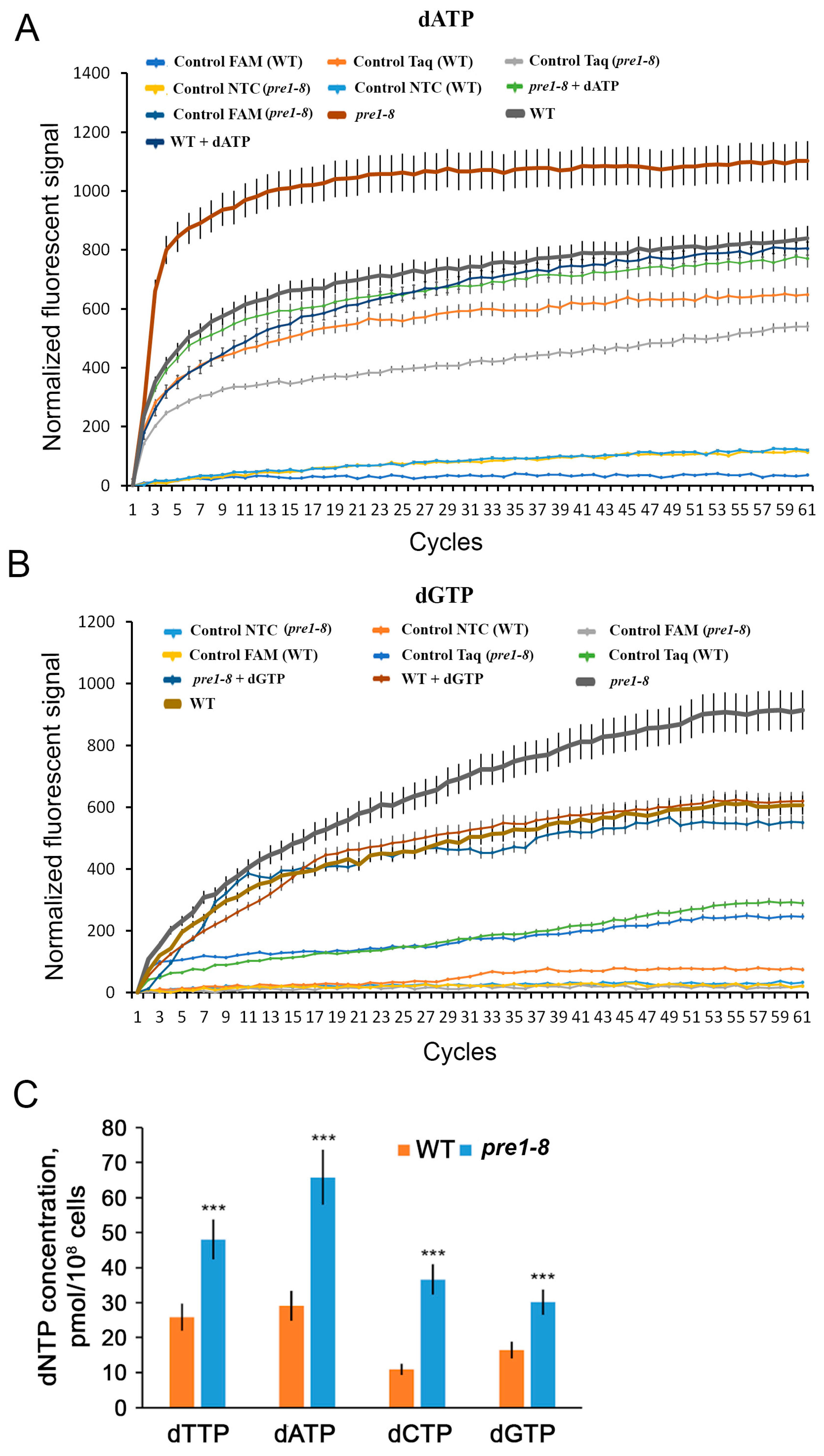
2.2. RNR Activity Is Elevated When Proteasome Function Is Impaired
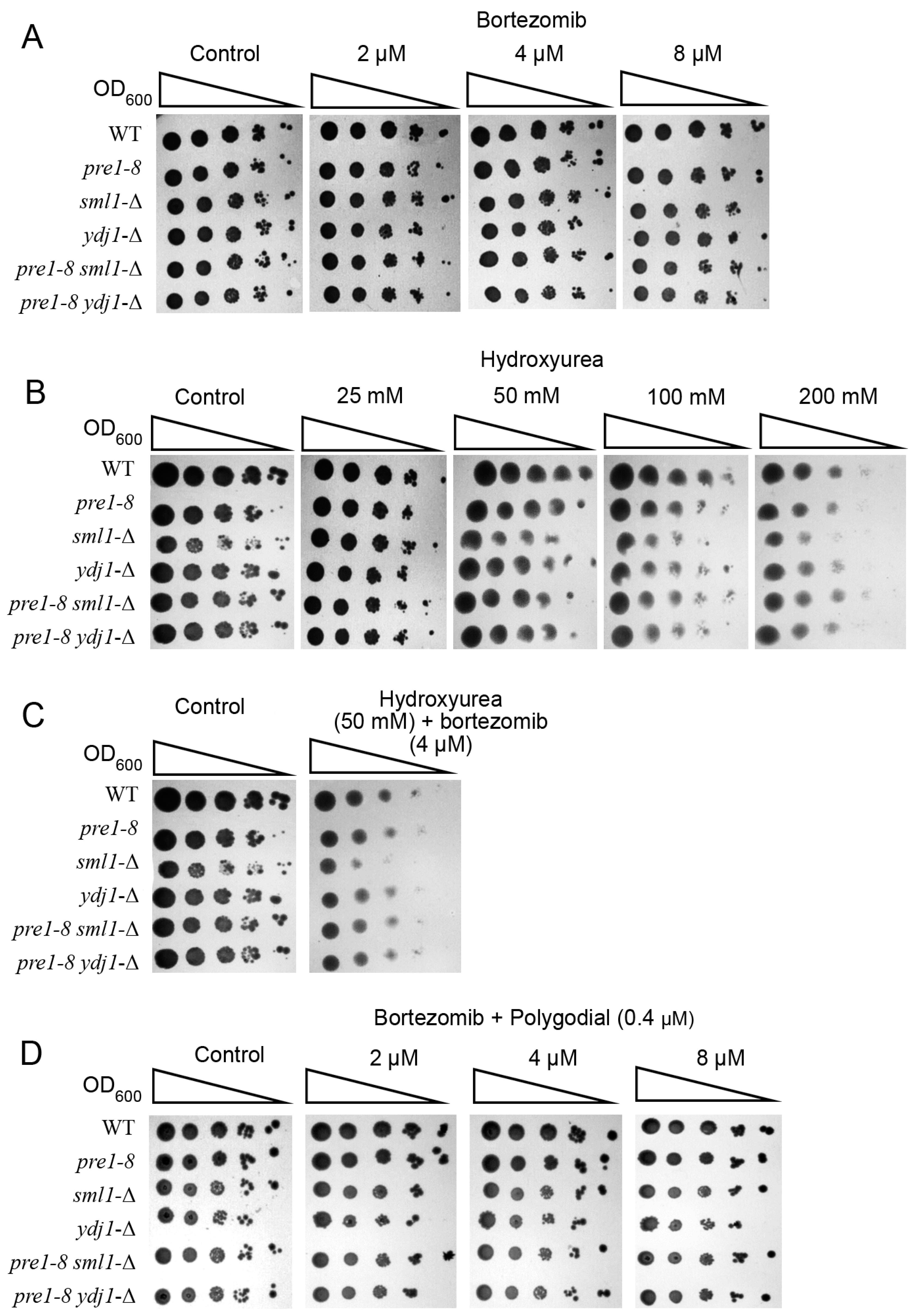
2.3. Inhibitors of Proteasome and RNR Exhibit Synergistic Effects in Yeast
2.4. Inhibition of Proteasome and RNR Suppresses Glioblastoma Cell Growth
3. Discussion
4. Materials and Methods
4.1. Strains and Cell Lines
4.2. Real Time PCR
4.3. Determination of dNTP Levels
4.4. Determination of Yeast Cultures Resistance to Proteasome and Ribonucleotide Reductase Inhibitors
4.5. Western Blot Analysis
4.6. Cell Viability Assay
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bard, J.A.M.; Goodall, E.A.; Greene, E.R.; Jonsson, E.; Dong, K.C.; Martin, A. Structure and function of the 26S proteasome. Annu. Rev. Biochem. 2018, 87, 697–724. [Google Scholar] [CrossRef] [PubMed]
- Morozov, A.V.; Karpov, V.L. Proteasomes and several aspects of their heterogeneity relevant to cancer. Front. Oncol. 2019, 9, 761. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, F.; Sohrabi, B.; Mostafavi, E.; Pazoki-Toroudi, H.; Webster, T.J. Ubiquitin-proteasome system and the role of its inhibitors in cancer therapy. Open Biol. 2021, 11, 200390. [Google Scholar] [CrossRef] [PubMed]
- Ito, S. Proteasome inhibitors for the treatment of multiple myeloma. Cancers 2020, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.J.; Savani, M.; Huang, N.; Epner, E.M. Mantle cell lymphoma management trends and novel agents: Where are we going? Ther. Adv. Hematol. 2022, 13, 20406207221080743. [Google Scholar] [CrossRef] [PubMed]
- Sin, C.F.; Man, P.M. The role of proteasome inhibitors in treating acute lymphoblastic leukaemia. Front. Oncol. 2021, 11, 802832. [Google Scholar] [CrossRef] [PubMed]
- Van Stiphout, C.M.; Luu, A.K.; Viloria-Petit, A.M. Proteasome inhibitors and their potential applicability in osteosarcoma treatment. Cancers 2022, 14, 4544. [Google Scholar] [CrossRef] [PubMed]
- Rashid, F.; Niklison-Chirou, M.V. Proteasome inhibition-a new target for brain tumours. Cell Death Discov. 2019, 5, 147. [Google Scholar] [CrossRef] [PubMed]
- Gozdz, A. Proteasome inhibitors against glioblastoma-overview of molecular mechanisms of cytotoxicity, progress in clinical trials, and perspective for use in personalized medicine. Curr. Oncol. 2023, 30, 9676–9688. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee Sh, U. Glioblastoma multiforme: A review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar]
- Karachi, A.; Dastmalchi, F.; Mitchell, D.A.; Rahman, M. Temozolomide for immunomodulation in the treatment of glioblastoma. Neuro Oncol. 2018, 20, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Boylan, J.; Byers, E.; Kelly, D.F. The Glioblastoma landscape: Hallmarks of disease, therapeutic resistance, and treatment opportunities. Med. Res. Arch. 2023, 11. [Google Scholar] [CrossRef]
- Delgado-Lopez, P.D.; Corrales-Garcia, E.M. Survival in glioblastoma: A review on the impact of treatment modalities. Clin. Transl. Oncol. 2016, 18, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Ibragimova, M.K.; Tsyganov, M.M.; Litviakov, N.V. Natural and chemotherapy-induced clonal evolution of tumors. Biochemistry 2017, 82, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, K.; Bhumika; Das, A. Combinatorial drug therapy in cancer—New insights. Life Sci. 2020, 258, 118134. [Google Scholar] [CrossRef] [PubMed]
- Djamgoz, M.B.A. Combinatorial therapy of cancer: Possible advantages of involving modulators of ionic mechanisms. Cancers 2022, 14, 2703. [Google Scholar] [CrossRef] [PubMed]
- Carrick, S.; Parker, S.; Thornton, C.E.; Ghersi, D.; Simes, J.; Wilcken, N. Single agent versus combination chemotherapy for metastatic breast cancer. Cochrane Database Syst. Rev. 2009, 2009, CD003372. [Google Scholar] [PubMed]
- Shiraishi, S.; Tada, K.; Nakamura, H.; Makino, K.; Kochi, M.; Saya, H.; Kuratsu, J.; Ushio, Y. Influence of p53 mutations on prognosis of patients with glioblastoma. Cancer 2002, 95, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Park, S.; Pentek, C.; Liebman, S.W. Tumor suppressor protein p53 expressed in yeast can remain diffuse, form a prion, or form unstable liquid-like droplets. iScience 2021, 24, 102000. [Google Scholar] [CrossRef]
- Zorniak, M.; Clark, P.A.; Umlauf, B.J.; Cho, Y.; Shusta, E.V.; Kuo, J.S. Yeast display biopanning identifies human antibodies targeting glioblastoma stem-like cells. Sci. Rep. 2017, 7, 15840. [Google Scholar] [CrossRef]
- Karpov, D.S.; Spasskaya, D.S.; Tutyaeva, V.V.; Mironov, A.S.; Karpov, V.L. Proteasome inhibition enhances resistance to DNA damage via upregulation of Rpn4-dependent DNA repair genes. FEBS Lett. 2013, 587, 3108–3114. [Google Scholar] [CrossRef] [PubMed]
- Spasskaya, D.S.; Nadolinskaia, N.I.; Tutyaeva, V.V.; Lysov, Y.P.; Karpov, V.L.; Karpov, D.S. Yeast Rpn4 links the proteasome and DNA repair via RAD52 regulation. Int. J. Mol. Sci. 2020, 21, 8097. [Google Scholar] [CrossRef] [PubMed]
- Spasskaya, D.S.; Kulagin, K.A.; Grineva, E.N.; Osipova, P.J.; Poddubko, S.V.; Bubis, J.A.; Kazakova, E.M.; Kusainova, T.T.; Gorshkov, V.A.; Kjeldsen, F.; et al. Yeast ribonucleotide reductase is a direct target of the proteasome and provides hyper resistance to the carcinogen 4-NQO. J. Fungi 2023, 9, 351. [Google Scholar] [CrossRef] [PubMed]
- Greene, B.L.; Kang, G.; Cui, C.; Bennati, M.; Nocera, D.G.; Drennan, C.L.; Stubbe, J. Ribonucleotide reductases: Structure, chemistry, and metabolism suggest new therapeutic targets. Annu. Rev. Biochem. 2020, 89, 45–75. [Google Scholar] [CrossRef] [PubMed]
- Tsaponina, O.; Barsoum, E.; Astrom, S.U.; Chabes, A. Ixr1 is required for the expression of the ribonucleotide reductase Rnr1 and maintenance of dNTP pools. PLoS Genet. 2011, 7, e1002061. [Google Scholar] [CrossRef] [PubMed]
- Tringe, S.G.; Willis, J.; Liberatore, K.L.; Ruby, S.W. The WTM genes in budding yeast amplify expression of the stress-inducible gene RNR3. Genetics 2006, 174, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Kotova, I.; Chabes, A.L.; Lobov, S.; Thelander, L.; Bjorklund, S. Sequences downstream of the transcription initiation site are important for proper initiation and regulation of mouse ribonucleotide reductase R2 gene transcription. Eur. J. Biochem. 2003, 270, 1791–1801. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Hjortsberg, K.; Thelander, L. Two YY-1-binding proximal elements regulate the promoter strength of the TATA-less mouse ribonucleotide reductase R1 gene. J. Biol. Chem. 1998, 273, 29816–29821. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Luo, Y.; Warncke, K.; Sun, Y.; Yu, D.S.; Fu, H.; Behera, M.; Ramalingam, S.S.; Doetsch, P.W.; Duong, D.M.; et al. Acetylation regulates ribonucleotide reductase activity and cancer cell growth. Nat. Commun. 2019, 10, 3213. [Google Scholar] [CrossRef]
- Shu, Z.; Li, Z.; Huang, H.; Chen, Y.; Fan, J.; Yu, L.; Wu, Z.; Tian, L.; Qi, Q.; Peng, S.; et al. Cell-cycle-dependent phosphorylation of RRM1 ensures efficient DNA replication and regulates cancer vulnerability to ATR inhibition. Oncogene 2020, 39, 5721–5733. [Google Scholar] [CrossRef]
- Sanvisens, N.; Romero, A.M.; An, X.; Zhang, C.; de Llanos, R.; Martinez-Pastor, M.T.; Bano, M.C.; Huang, M.; Puig, S. Yeast Dun1 kinase regulates ribonucleotide reductase inhibitor Sml1 in response to iron deficiency. Mol. Cell Biol. 2014, 34, 3259–3271. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, M. Dif1 controls subcellular localization of ribonucleotide reductase by mediating nuclear import of the R2 subunit. Mol. Cell Biol. 2008, 28, 7156–7167. [Google Scholar] [CrossRef] [PubMed]
- Chabes, A.; Georgieva, B.; Domkin, V.; Zhao, X.; Rothstein, R.; Thelander, L. Survival of DNA damage in yeast directly depends on increased dNTP levels allowed by relaxed feedback inhibition of ribonucleotide reductase. Cell 2003, 112, 391–401. [Google Scholar] [CrossRef]
- D’Angiolella, V.; Donato, V.; Forrester, F.M.; Jeong, Y.T.; Pellacani, C.; Kudo, Y.; Saraf, A.; Florens, L.; Washburn, M.P.; Pagano, M. Cyclin F-mediated degradation of ribonucleotide reductase M2 controls genome integrity and DNA repair. Cell 2012, 149, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Chen, Z.; Bepler, G. Ubiquitination and degradation of ribonucleotide reductase M1 by the polycomb group proteins RNF2 and Bmi1 and cellular response to gemcitabine. PLoS ONE 2014, 9, e91186. [Google Scholar] [CrossRef]
- Zhao, X.; Rothstein, R. The Dun1 checkpoint kinase phosphorylates and regulates the ribonucleotide reductase inhibitor Sml1. Proc. Natl. Acad. Sci. USA 2002, 99, 3746–3751. [Google Scholar] [CrossRef]
- Lee, Y.D.; Wang, J.; Stubbe, J.; Elledge, S.J. Dif1 is a DNA-damage-regulated facilitator of nuclear import for ribonucleotide reductase. Mol. Cell 2008, 32, 70–80. [Google Scholar] [CrossRef]
- Chen, Y.R.; Tsou, B.; Hu, S.; Ma, H.; Liu, X.; Yen, Y.; Ann, D.K. Autophagy induction causes a synthetic lethal sensitization to ribonucleotide reductase inhibition in breast cancer cells. Oncotarget 2016, 7, 1984–1999. [Google Scholar] [CrossRef]
- Kulsh, J. Biochemistry-not oncogenes-may demystify and defeat cancer. Oncol. Ther. 2023, 11, 153–169. [Google Scholar] [CrossRef]
- Brown, A.; Pan, Q.; Fan, L.; Indersie, E.; Tian, C.; Timchenko, N.; Li, L.; Hansen, B.S.; Tan, H.; Lu, M.; et al. Ribonucleotide reductase subunit switching in hepatoblastoma drug response and relapse. Commun. Biol. 2023, 6, 249. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Y.; Xu, Z.; Lu, Y.; Song, D.; Gao, L.; Yu, D.; Li, B.; Chen, G.; Zhang, H.; et al. Preclinical validation and phase I trial of 4-hydroxysalicylanilide, targeting ribonucleotide reductase mediated dNTP synthesis in multiple myeloma. J. Biomed. Sci. 2022, 29, 32. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Ensbey, K.S.; Offenhauser, C.; D’Souza, R.C.J.; Cullen, J.K.; Stringer, B.W.; Quek, H.; Bruce, Z.C.; Kijas, A.; Cianfanelli, V.; et al. Simultaneous targeting of DNA replication and homologous recombination in glioblastoma with a polyether ionophore. Neuro Oncol. 2020, 22, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Corrales-Guerrero, S.; Cui, T.; Castro-Aceituno, V.; Yang, L.; Nair, S.; Feng, H.; Venere, M.; Yoon, S.; DeWees, T.; Shen, C.; et al. Inhibition of RRM2 radiosensitizes glioblastoma and uncovers synthetic lethality in combination with targeting CHK1. Cancer Lett. 2023, 570, 216308. [Google Scholar] [CrossRef] [PubMed]
- Perrault, E.N.; Shireman, J.M.; Ali, E.S.; Lin, P.; Preddy, I.; Park, C.; Budhiraja, S.; Baisiwala, S.; Dixit, K.; James, C.D.; et al. Ribonucleotide reductase regulatory subunit M2 drives glioblastoma TMZ resistance through modulation of dNTP production. Sci. Adv. 2023, 9, eade7236. [Google Scholar] [CrossRef] [PubMed]
- Kapranov, A.B.; Kuryatova, M.V.; Preobrazhenskaya, O.V.; Tutyaeva, V.V.; Stucka, R.; Feldmann, H.; Karpov, V.L. Isolation and identification of PACE-binding protein Rpn4, a new transcriptional activator regulating 26S-proteasomal and other genes. Mol. Biol. 2001, 35, 356–364. [Google Scholar] [CrossRef]
- Mannhaupt, G.; Schnall, R.; Karpov, V.; Vetter, I.; Feldmann, H. Rpn4 acts as a transcription factor by binding to PACE, a nonamer box found upstream of 26S proteasomal and other genes in yeast. FEBS Lett. 1999, 450, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Varshavsky, A. RPN4 is a ligand, substrate and transcriptional regulator of the 26S proteasome: A negative feedback circuit. Proc. Natl. Acad. Sci. USA 2001, 98, 3056–3061. [Google Scholar] [CrossRef]
- Subramaniam, R.; Lamb, N.A.; Hwang, Y.; Johengen, L.; Surtees, J.A. Extracting and measuring dNTP pools in Saccharomyces cerevisiae. Methods Mol. Biol. 2019, 1999, 103–127. [Google Scholar]
- Tran, N.H.T.; Trinh, K.T.L.; Lee, J.H.; Yoon, W.J.; Ju, H. Fluorescence enhancement using bimetal surface plasmon-coupled emission from 5-carboxyfluorescein (FAM). Micromachines 2018, 9, 460. [Google Scholar] [CrossRef]
- Patterson, K.N.; Romero-Reyes, M.A.; Heemstra, J.M. Fluorescence quenching of xanthene dyes during amide bond formation using DMTMM. ACS Omega 2022, 7, 33046–33053. [Google Scholar] [CrossRef]
- Huff, S.E.; Winter, J.M.; Dealwis, C.G. Inhibitors of the cancer target ribonucleotide reductase, past and present. Biomolecules 2022, 12, 815. [Google Scholar] [CrossRef] [PubMed]
- Kozalak, G.; Butun, I.; Toyran, E.; Kosar, A. Review on bortezomib resistance in multiple myeloma and potential role of emerging technologies. Pharmaceuticals 2023, 16, 111. [Google Scholar] [CrossRef] [PubMed]
- Hakansson, P.; Hofer, A.; Thelander, L. Regulation of mammalian ribonucleotide reduction and dNTP pools after DNA damage and in resting cells. J. Biol. Chem. 2006, 281, 7834–7841. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.L.; Tippery, N.P. Systematic quantification of gene interactions by phenotypic array analysis. Genome Biol. 2004, 5, R49. [Google Scholar] [CrossRef] [PubMed]
- Sluder, I.T.; Nitika; Knighton, L.E.; Truman, A.W. The Hsp70 co-chaperone Ydj1/HDJ2 regulates ribonucleotide reductase activity. PLoS Genet. 2018, 14, e1007462. [Google Scholar] [CrossRef] [PubMed]
- Andreson, B.L.; Gupta, A.; Georgieva, B.P.; Rothstein, R. The ribonucleotide reductase inhibitor, Sml1, is sequentially phosphorylated, ubiquitylated and degraded in response to DNA damage. Nucleic Acids Res. 2010, 38, 6490–6501. [Google Scholar] [CrossRef] [PubMed]
- Chabes, A.L.; Pfleger, C.M.; Kirschner, M.W.; Thelander, L. Mouse ribonucleotide reductase R2 protein: A new target for anaphase-promoting complex-Cdh1-mediated proteolysis. Proc. Natl. Acad. Sci. USA 2003, 100, 3925–3929. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Contreras, A.J.; Specks, J.; Barlow, J.H.; Ambrogio, C.; Desler, C.; Vikingsson, S.; Rodrigo-Perez, S.; Green, H.; Rasmussen, L.J.; Murga, M.; et al. Increased Rrm2 gene dosage reduces fragile site breakage and prolongs survival of ATR mutant mice. Genes. Dev. 2015, 29, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Zhou, Z.; Chang, Y.; Liu, Y.; Shen, Y.; Li, Q.; Zhang, L. Ribonucleotide reductase M2 (RRM2): Regulation, function and targeting strategy in human cancer. Genes. Dis. 2024, 11, 218–233. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, D.; Aleksandrovich, K.D.; Ye, J.; Wang, L.; Chen, X.; Gao, M.; Wang, X.; Yan, T.; Yang, H.; et al. RRM2 mediates the anti-tumor effect of the natural product pectolinarigenin on glioblastoma through promoting CDK1 protein degradation by increasing autophagic flux. Front. Oncol. 2022, 12, 887294. [Google Scholar] [CrossRef]
- Arima, Y.; Nishigori, C.; Takeuchi, T.; Oka, S.; Morimoto, K.; Utani, A.; Miyachi, Y. 4-Nitroquinoline 1-oxide forms 8-hydroxydeoxyguanosine in human fibroblasts through reactive oxygen species. Toxicol. Sci. 2006, 91, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Kurepa, J.; Toh, E.A.; Smalle, J.A. 26S proteasome regulatory particle mutants have increased oxidative stress tolerance. Plant J. 2008, 53, 102–114. [Google Scholar] [CrossRef]
- Choi, W.H.; de Poot, S.A.; Lee, J.H.; Kim, J.H.; Han, D.H.; Kim, Y.K.; Finley, D.; Lee, M.J. Open-gate mutants of the mammalian proteasome show enhanced ubiquitin-conjugate degradation. Nat. Commun. 2016, 7, 10963. [Google Scholar] [CrossRef] [PubMed]
- Karpov, D.S.; Spasskaya, D.S.; Nadolinskaia, N.I.; Tutyaeva, V.V.; Lysov, Y.P.; Karpov, V.L. Deregulation of the 19S proteasome complex increases yeast resistance to 4-NQO and oxidative stress via upregulation of Rpn4- and proteasome-dependent stress responsive genes. FEMS Yeast Res. 2019, 19, foz002. [Google Scholar] [CrossRef] [PubMed]
- Karpuz, B.; ÇAkir, Ö. Effect of proteasome inhibitor MG132 on the expression of oxidative metabolism related genes in tomato. Food Sci. Technol. 2022, 42, e52420. [Google Scholar] [CrossRef]
- Chernov, A.N.; Alaverdian, D.A.; Galimova, E.S.; Renieri, A.; Frullanti, E.; Meloni, I.; Shamova, O.V. The phenomenon of multidrug resistance in glioblastomas. Hematol. Oncol. Stem Cell Ther. 2022, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Goffeau, A. Yeast ATP-binding cassette transporters conferring multidrug resistance. Annu. Rev. Microbiol. 2012, 66, 39–63. [Google Scholar] [CrossRef]
- Fleming, J.A.; Lightcap, E.S.; Sadis, S.; Thoroddsen, V.; Bulawa, C.E.; Blackman, R.K. Complementary whole-genome technologies reveal the cellular response to proteasome inhibition by PS-341. Proc. Natl. Acad. Sci. USA 2002, 99, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- San Miguel, J.F.; Schlag, R.; Khuageva, N.K.; Dimopoulos, M.A.; Shpilberg, O.; Kropff, M.; Spicka, I.; Petrucci, M.T.; Palumbo, A.; Samoilova, O.S.; et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N. Engl. J. Med. 2008, 359, 906–917. [Google Scholar] [CrossRef]
- Caponigro, F.; Lacombe, D.; Twelves, C.; Bauer, J.; Govaerts, A.S.; Marreaud, S.; Milano, A.; Anthoney, A. An EORTC phase I study of Bortezomib in combination with oxaliplatin, leucovorin and 5-fluorouracil in patients with advanced colorectal cancer. Eur. J. Cancer 2009, 45, 48–55. [Google Scholar] [CrossRef]
- Zhan, Y.; Jiang, L.; Jin, X.; Ying, S.; Wu, Z.; Wang, L.; Yu, W.; Tong, J.; Zhang, L.; Lou, Y.; et al. Inhibiting RRM2 to enhance the anticancer activity of chemotherapy. Biomed. Pharmacother. 2021, 133, 110996. [Google Scholar] [CrossRef] [PubMed]
- Wakisaka, N.; Yoshizaki, T.; Raab-Traub, N.; Pagano, J.S. Ribonucleotide reductase inhibitors enhance cidofovir-induced apoptosis in EBV-positive nasopharyngeal carcinoma xenografts. Int. J. Cancer 2005, 116, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Menolfi, D.; Lee, B.J.; Zhang, H.; Jiang, W.; Bowen, N.E.; Wang, Y.; Zhao, J.; Holmes, A.; Gershik, S.; Rabadan, R.; et al. ATR kinase supports normal proliferation in the early S phase by preventing replication resource exhaustion. Nat. Commun. 2023, 14, 3618. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.M.; Icyuz, M.; Pound, I.; William, D.; Guo, J.; McKinney, B.A.; Niederweis, M.; Rodgers, J.; Hartman, J.I. A humanized yeast phenomic model of deoxycytidine kinase to predict genetic buffering of nucleoside analog cytotoxicity. Genes 2019, 10, 770. [Google Scholar] [CrossRef] [PubMed]
- Madaan, K.; Kaushik, D.; Verma, T. Hydroxyurea: A key player in cancer chemotherapy. Expert. Rev. Anticancer. Ther. 2012, 12, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Ismalaj, T.; Sackett, D.L. An inexpensive replacement for dry ice in the laboratory. Anal. Biochem. 2015, 474, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Kushnirov, V.V. Rapid and reliable protein extraction from yeast. Yeast 2000, 16, 857–860. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]

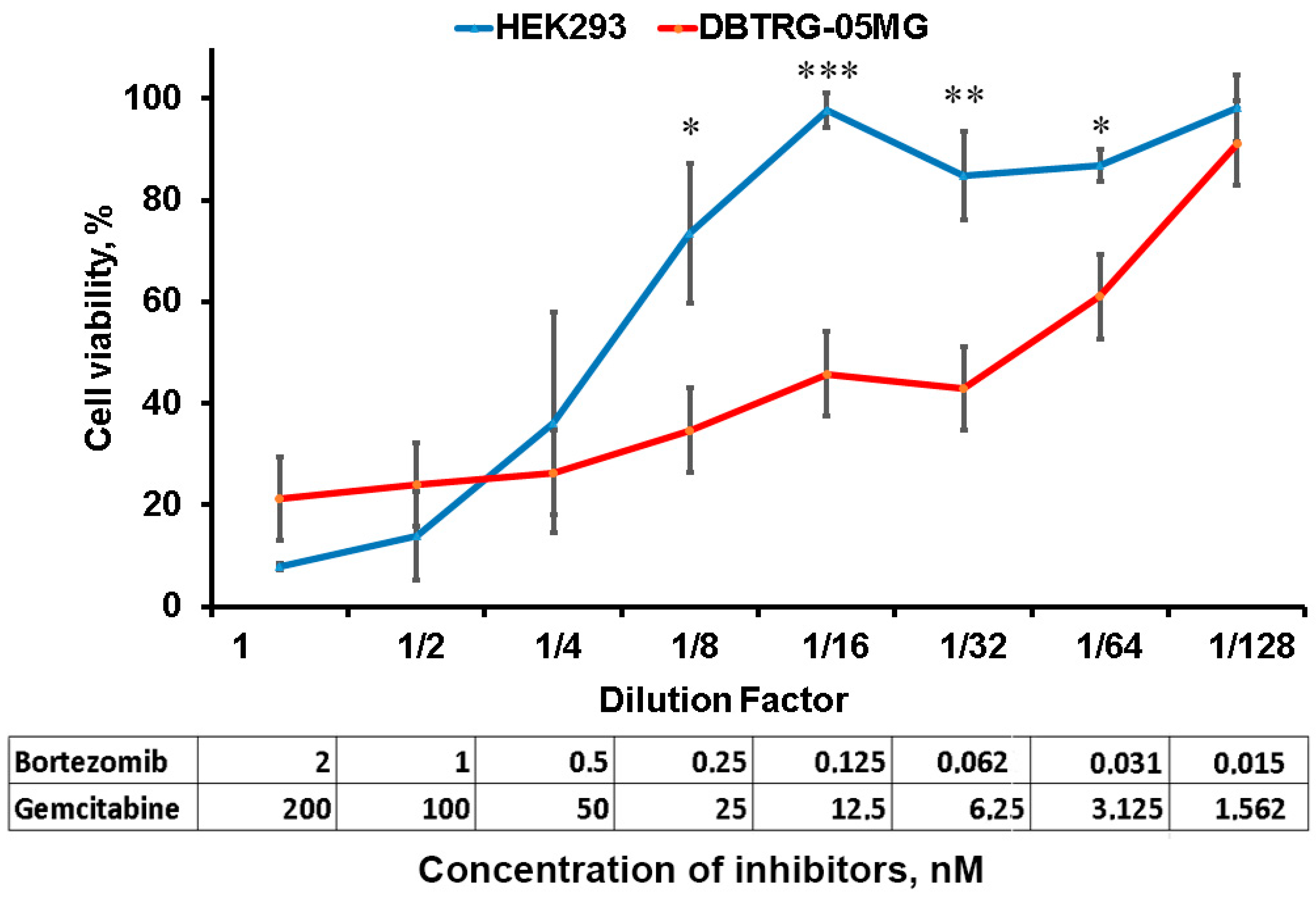
| Cell Line | IC50 Bortezomib | IC50 Gemcitabine |
|---|---|---|
| HEK293 | 10.3 nM | 1 µM |
| DBTRG-05MG | 15.2 nM | 24.4 µM |
| Name | Nucleotide Sequence 5′ → 3′ | Purpose | Reference |
|---|---|---|---|
| Primer- NDP1 | CCGCCTCCACCGCC | Determination of dNTP levels | [48] |
| Probe- FAM-dTTP | 6FAM/AGGACCGAG/ZEN/GCAAGAGCGAGCGA/BHQ | ||
| Template- DT1-dTTP | TCGCTCGCTCTTGCCTCGGTCCTTTATTTGGCGGTGGAGGCGG | ||
| Template- DT2-dTTP | TCGCTCGCTCTTGCCTCGGTCCTTTATTTATTTGGCGGTGGAGGCGG | ||
| Probe- FAM-dATP | 6FAM/TGGTCCGTG/ZEN/GCTTGTGCGTGCGT/BHQ | ||
| Template- DT1-dATP | ACGCACGCACAAGCCACGGACCAAATAAAGGCGGTGGAGGCGG | ||
| Template- DT2-dATP | ACGCACGCACAAGCCACGGACCAAATAAATAAAGGCGGTGGAGGCGG | ||
| Probe- FAM-dGTP | 6FAM/ACCATTCAC/ZEN/CTCACACTCACTCC/BHQ | ||
| Template- DT1-dGTP | GGAGTGAGTGTGAGGTGAATGGTTTCTTTGGCGGTGGAGGCGG | ||
| Template- DT2-dGTP | GGAGTGAGTGTGAGGTGAATGGTTTCTTTCTTTGGCGGTGGAGGCGG | ||
| Probe- FAM-dCTP | 6FAM/AGGATTGAG/ZEN/GTAAGAGTGAGTGG/BHQ | ||
| Template- DT1-dCTP | CCACTCACTCTTACCTCAATCCTTTGTTTGGCGGTGGAGGCGG | ||
| Template- DT2-dCTP | CCACTCACTCTTACCTCAATCCTTTGTTTGTTTGGCGGTGGAGGCGG | ||
| RNR1-ex-top | GTTGCTGATATTTCCAACTTG | Real-time PCR | [23] |
| RNR1-ex-bot | CTATCTAGAGATGGAATAGTTG | ||
| RNR2-ex-top | TGAAAAAGAGAGGTATGATG | ||
| RNR2-ex-bot | GTCTGGTTTGTTCTTCAAATG | ||
| ACT1-ex-top | CCTTCTGTTTTGGGTTTGGAATC | ||
| ACT1-ex-bot | TGGAGCCAAAGCGGTGATTTCCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulagin, K.A.; Starodubova, E.S.; Osipova, P.J.; Lipatova, A.V.; Cherdantsev, I.A.; Poddubko, S.V.; Karpov, V.L.; Karpov, D.S. Synergistic Effect of a Combination of Proteasome and Ribonucleotide Reductase Inhibitors in a Biochemical Model of the Yeast Saccharomyces cerevisiae and a Glioblastoma Cell Line. Int. J. Mol. Sci. 2024, 25, 3977. https://doi.org/10.3390/ijms25073977
Kulagin KA, Starodubova ES, Osipova PJ, Lipatova AV, Cherdantsev IA, Poddubko SV, Karpov VL, Karpov DS. Synergistic Effect of a Combination of Proteasome and Ribonucleotide Reductase Inhibitors in a Biochemical Model of the Yeast Saccharomyces cerevisiae and a Glioblastoma Cell Line. International Journal of Molecular Sciences. 2024; 25(7):3977. https://doi.org/10.3390/ijms25073977
Chicago/Turabian StyleKulagin, Kirill A., Elizaveta S. Starodubova, Pamila J. Osipova, Anastasia V. Lipatova, Igor A. Cherdantsev, Svetlana V. Poddubko, Vadim L. Karpov, and Dmitry S. Karpov. 2024. "Synergistic Effect of a Combination of Proteasome and Ribonucleotide Reductase Inhibitors in a Biochemical Model of the Yeast Saccharomyces cerevisiae and a Glioblastoma Cell Line" International Journal of Molecular Sciences 25, no. 7: 3977. https://doi.org/10.3390/ijms25073977






