Synthetic Microbial Community Members Interact to Metabolize Caproic Acid to Inhibit Potato Dry Rot Disease
Abstract
:1. Introduction
2. Results and Discussion
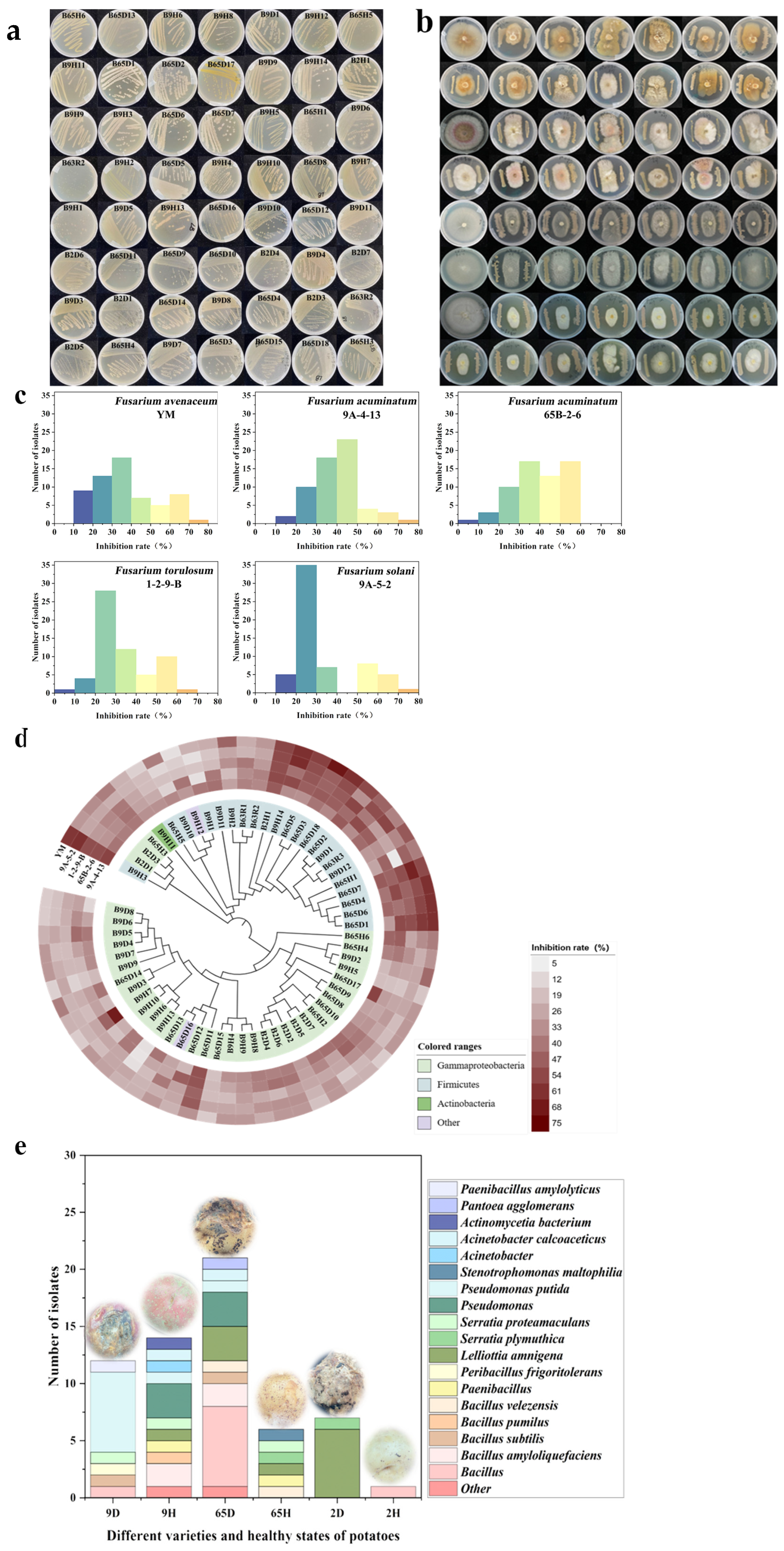
2.1. Isolation and Identification of Strains That Inhibit Fusarium spp.
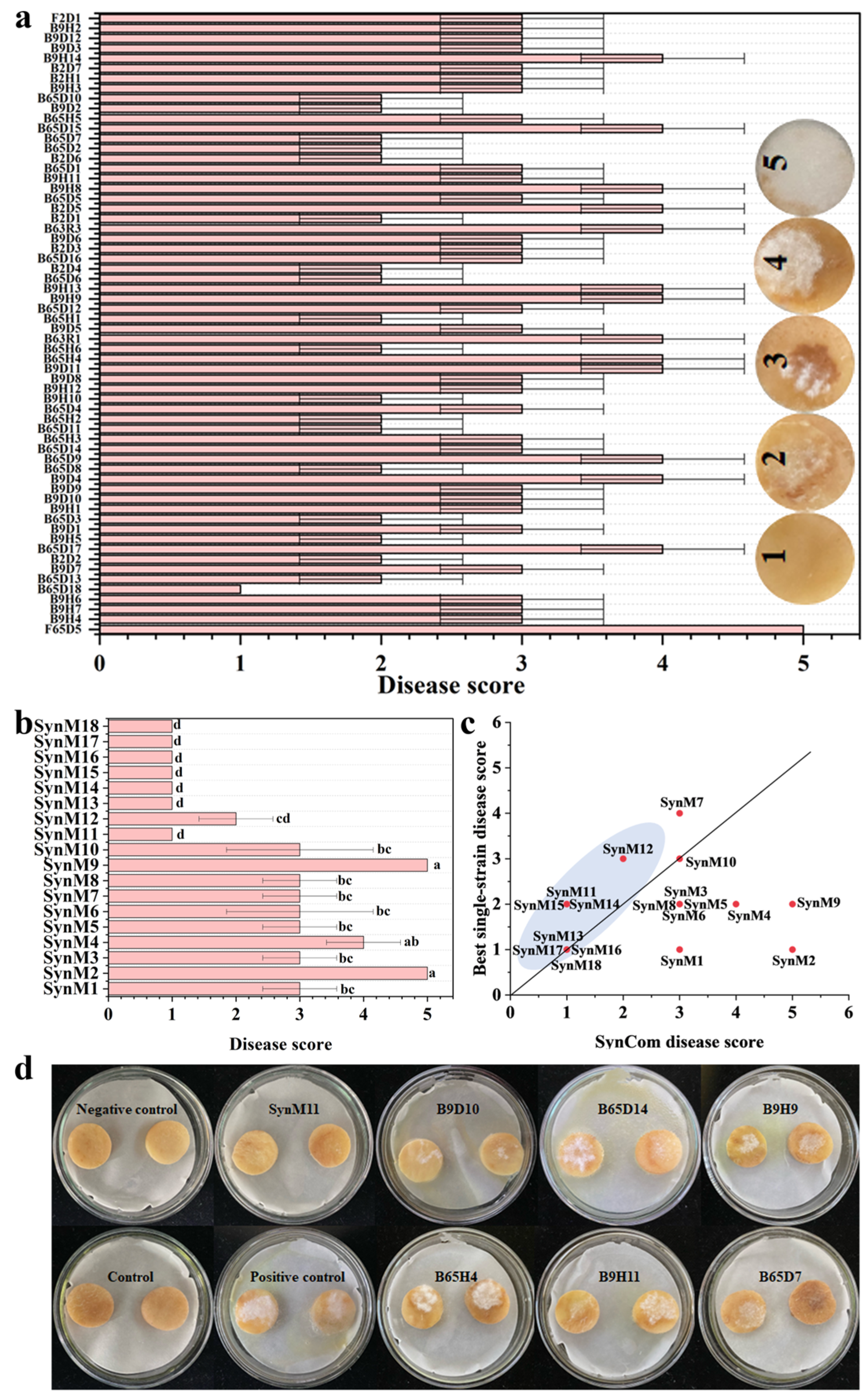
2.2. Isolation and Pathogenicity Assay of Pathogens for Dry Rot Disease
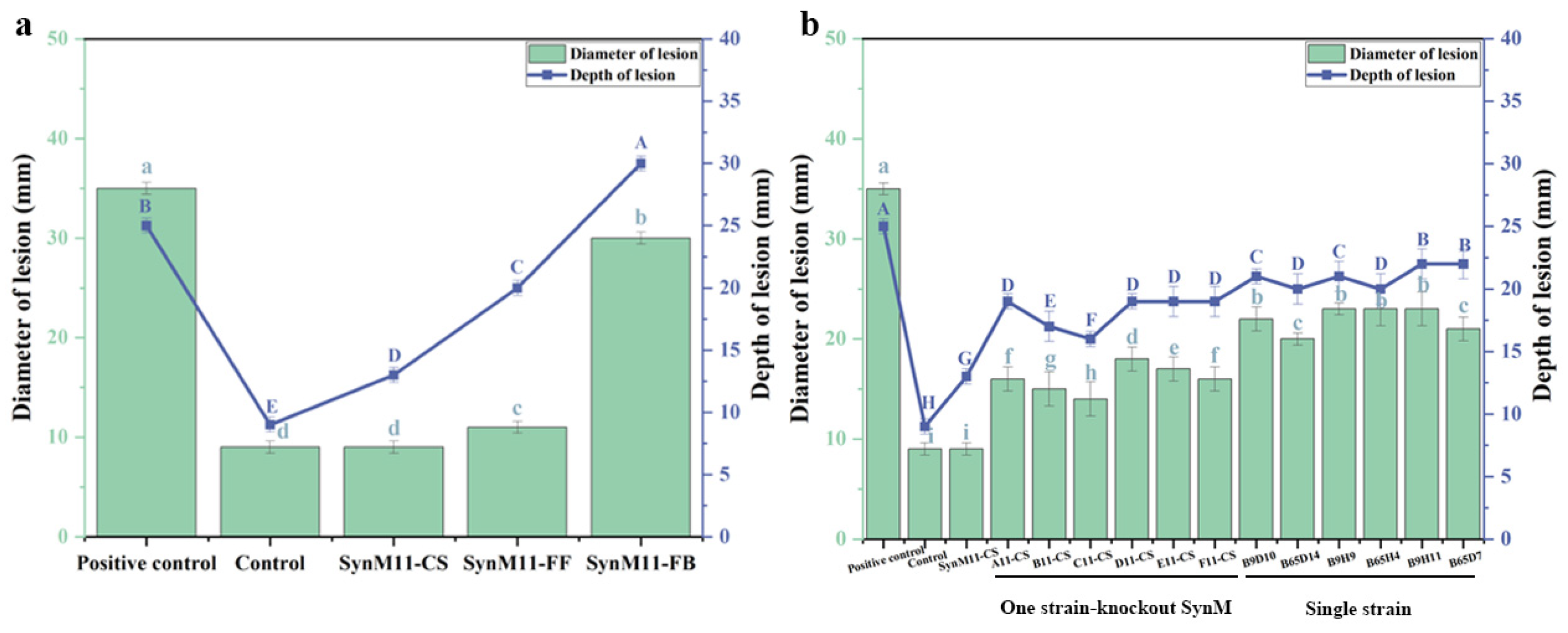
2.3. Screening of Synthetic Microbial Communities with Inhibitory Effects on Potato Dry Rot Disease
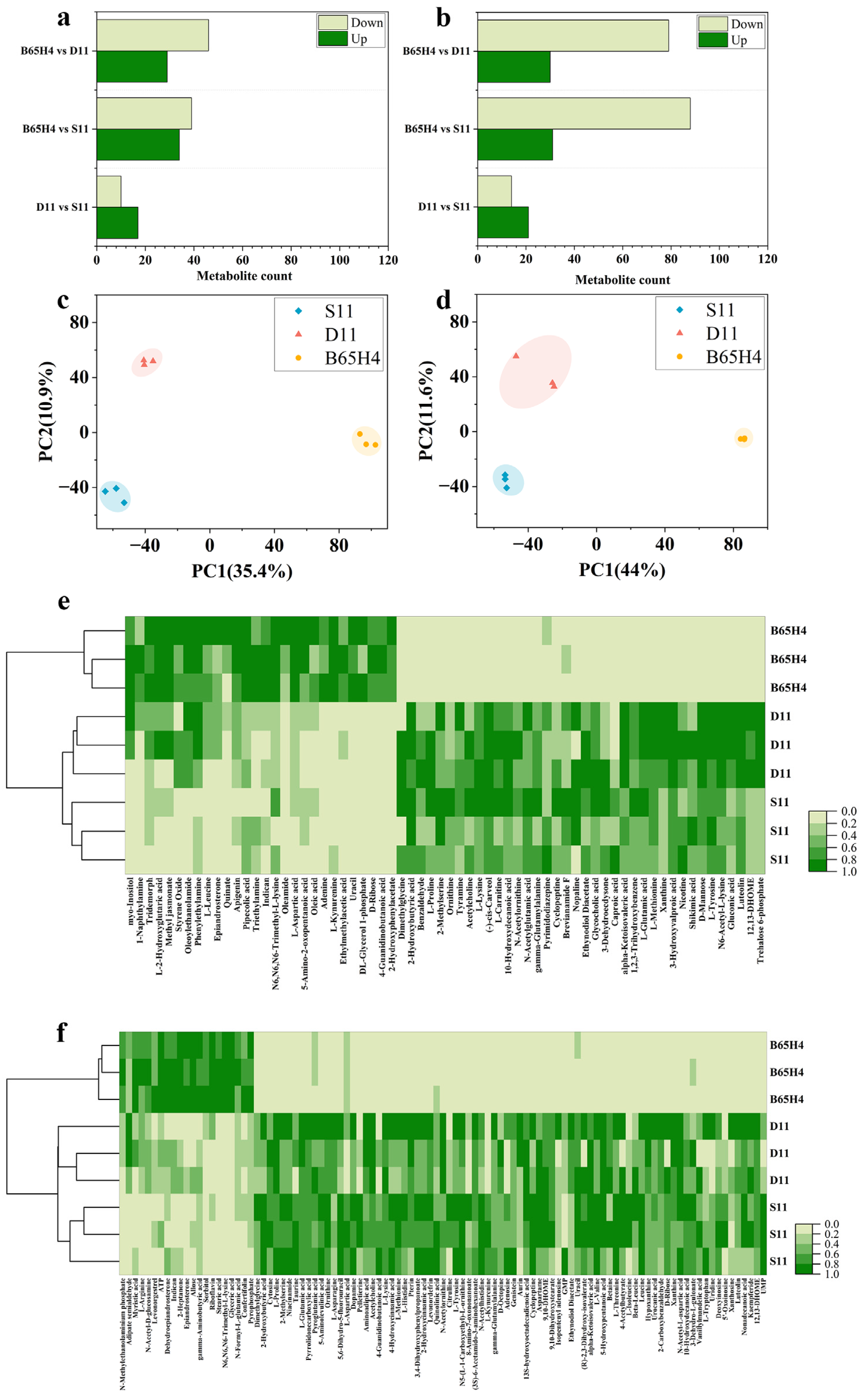
2.4. Analysis of Active Metabolites in Synthetic Microbial Communities with Inhibitory Activity on Dry Rot Disease
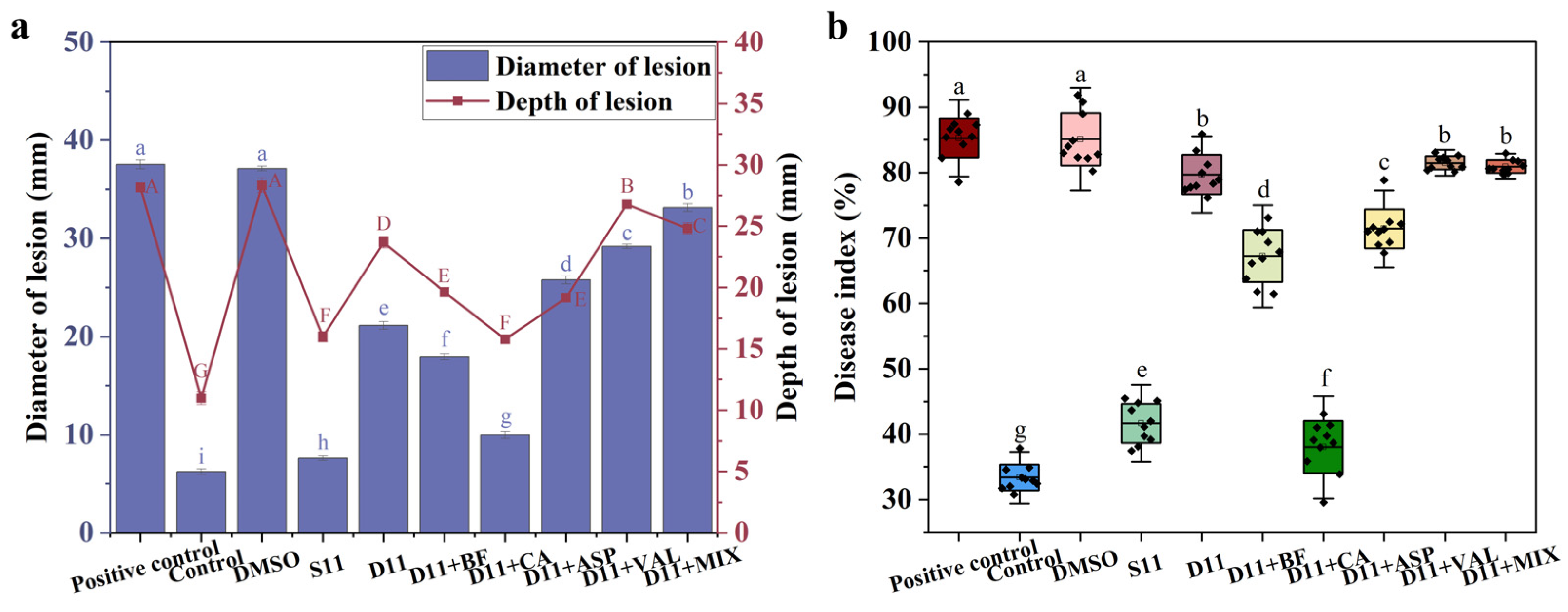
2.5. Validation of Key Metabolites in Synthetic Microbial Communities
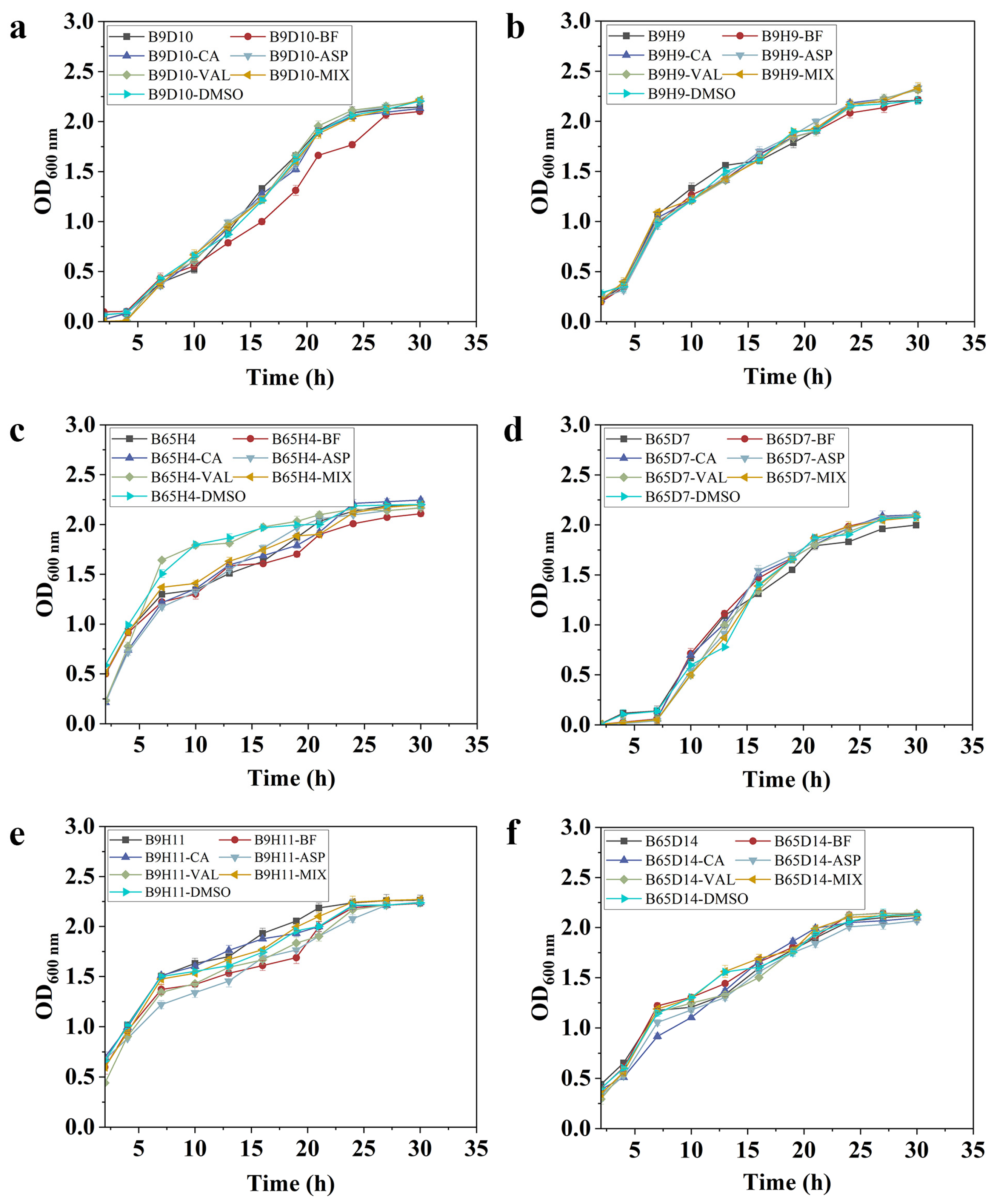
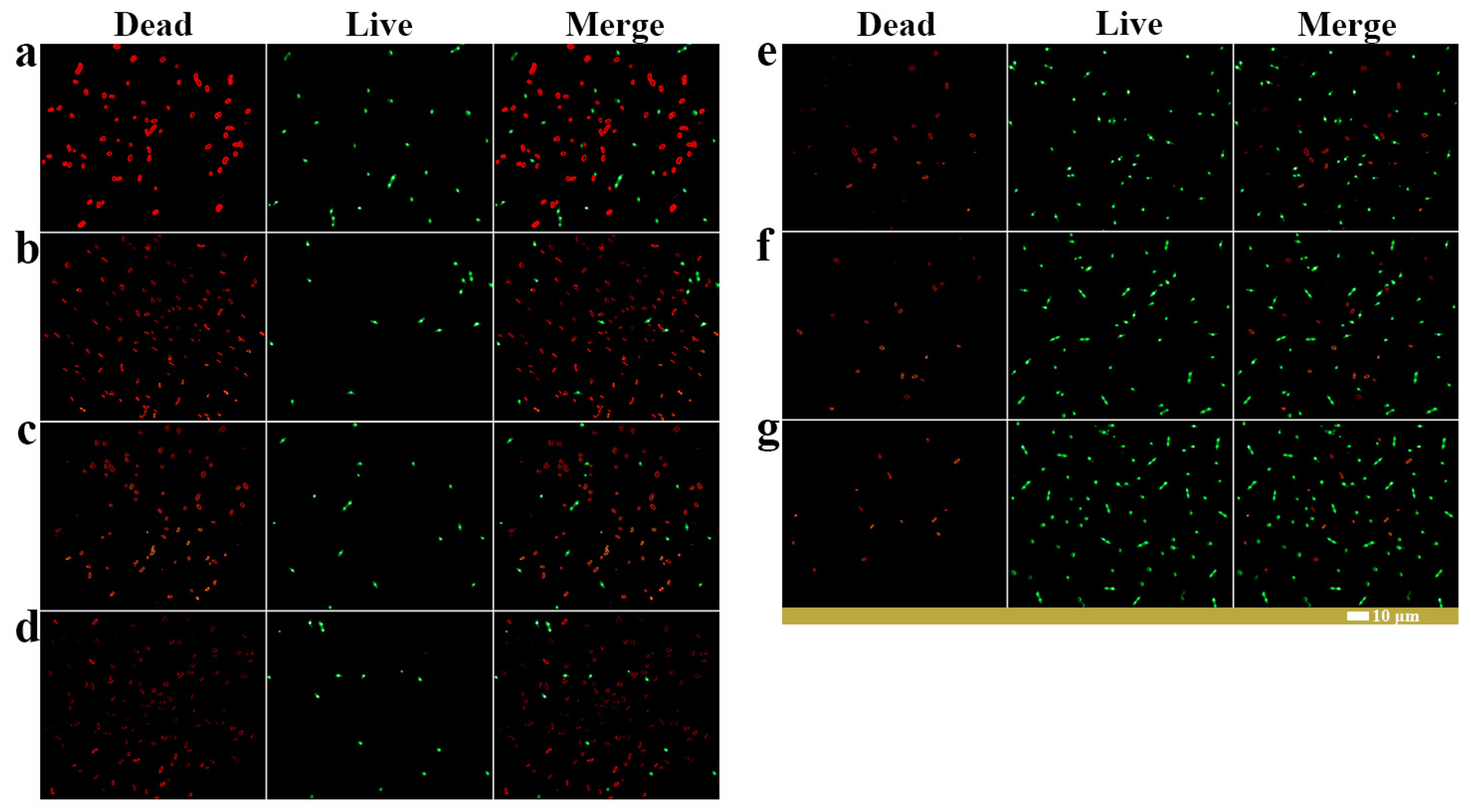
2.6. The Biocontrol Mechanism of Caproic Acid
3. Materials and Methods
3.1. Isolation of Microorganisms and Evaluation of Antifungal Activity
3.2. Identification of Microorganisms
3.3. Pathogenicity Assay of Isolated Strains
3.4. Constructing and Screening Synthetic Microbial Communities
3.5. Analysis and Validation of Active Metabolites in Synthetic Microbial Communities
3.6. Detecting the Growth of Bacterial Strains
3.7. Fungal Survival Assay
3.8. Flow Cytometric and Fluorescence Microscopy Assessment of Conidial Viability
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Biessy, A.; Filion, M. Biological control of potato common scab by plant-beneficial bacteria. Biol. Control 2021, 165, 104808. [Google Scholar] [CrossRef]
- Da Silva, H.A.O.; Teixeira, W.D.; Borges, Á.V.; Silva Junior, A.L.; Alves, K.S.; Rodrigues Junior, O.M.; de Abreu, L.M. Biocontrol of potato early blight and suppression of Alternaria grandis sporulation by Clonostachys spp. Plant Pathol. 2021, 70, 1677–1685. [Google Scholar] [CrossRef]
- Metz, N.; Hausladen, H. Trichoderma spp. As potential biological control agent against Alternaria solani in potato. Biol. Control 2021, 166, 104820. [Google Scholar] [CrossRef]
- Gleń-Karolczyk, K.; Boligłowa, E.; Antonkiewicz, J. Organic fertilization shapes the biodiversity of fungal communities associated with potato dry rot. Appl. Soil Ecol. 2018, 129, 43–51. [Google Scholar] [CrossRef]
- Ben Khedher, S.; Mejdoub-Trabelsi, B.; Tounsi, S. Biological potential of Bacillus subtilis V26 for the control of Fusarium wilt and tuber dry rot on potato caused by Fusarium species and the promotion of plant growth. Biol. Control 2020, 152, 104444. [Google Scholar] [CrossRef]
- Heltoft, P.; Brurberg, M.B.; Skogen, M.; Le, V.H.; Razzaghian, J.; Hermansen, A. Fusarium spp. Causing Dry Rot on Potatoes in Norway and Development of a Real-Time PCR Method for Detection of Fusarium coeruleum. Potato Res. 2016, 59, 67–80. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Łuniewski, S.; Kaczyński, P.; Łozowicka, B. The Influence of Humic Acids and Nitrophenols on Metabolic Compounds and Pesticide Behavior in Wheat under Biotic Stress. Agronomy 2023, 13, 1378. [Google Scholar] [CrossRef]
- Zhu, Q.; Fei, Y.-J.; Wu, Y.-B.; Luo, D.-L.; Chen, M.; Sun, K.; Zhang, W.; Dai, C.-C. Endophytic Fungus Reshapes Spikelet Microbiome to Reduce Mycotoxin Produced by Fusarium proliferatum through Altering Rice Metabolites. J. Agric. Food Chem. 2023, 71, 11350–11364. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Tong, J.; Li, P.; Huang, X.; Dong, P.; Ren, M. Chitosan is an effective inhibitor against potato dry rot caused by Fusarium oxysporum. Physiol. Mol. Plant Pathol. 2021, 113, 101601. [Google Scholar] [CrossRef]
- Lastochkina, O.; Pusenkova, L.; Garshina, D.; Yuldashev, R.; Shpirnaya, I.; Kasnak, C.; Palamutoglu, R.; Mardanshin, I.; Garipova, S.; Sobhani, M.; et al. The Effect of Endophytic Bacteria Bacillus subtilis and Salicylic Acid on Some Resistance and Quality Traits of Stored Solanum tuberosum L. Tubers Infected with Fusarium Dry Rot. Plants 2020, 9, 738. [Google Scholar] [CrossRef]
- Gabrekiristos, E.; Demiyo, T. Hot Pepper Fusarium Wilt (Fusarium oxysporum f. sp. capsici): Epidemics, Characteristic Features and Management Options. J. Agric. Sci. 2020, 12, 347. [Google Scholar] [CrossRef]
- Mejdoub-Trabelsi, B.; Ben Abdallah, R.A.; Kthiri, Z.; Daami-Remadi, W.H.A.M. Assessment of the Antifungal Activity of Non-pathogenic Potatoassociated Fungi toward Fusarium Species Causing Tuber Dry Rot Disease. J. Plant Pathol. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Lee, S.-M.; Kong, H.G.; Song, G.C.; Ryu, C.-M. Disruption of Firmicutes and Actinobacteria abundance in tomato rhizosphere causes the incidence of bacterial wilt disease. ISME J. 2020, 15, 330–347. [Google Scholar] [CrossRef] [PubMed]
- Xun, W.; Ren, Y.; Yan, H.; Ma, A.; Liu, Z.; Wang, L.; Zhang, N.; Xu, Z.; Miao, Y.; Feng, H.; et al. Sustained Inhibition of Maize Seed-Borne Fusarium Using a Bacillus-Dominated Rhizospheric Stable Core Microbiota with Unique Cooperative Patterns. Adv. Sci. 2022, 10, 2205215. [Google Scholar] [CrossRef]
- Matsumoto, H.; Fan, X.; Wang, Y.; Kusstatscher, P.; Duan, J.; Wu, S.; Chen, S.; Qiao, K.; Wang, Y.; Ma, B.; et al. Bacterial seed endophyte shapes disease resistance in rice. Nat. Plants 2021, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yu, S.; Zhao, D.; Zhang, J.; Pan, Y.; Yang, Y.; Yang, Z.; Zhu, J.; Zhao, Y.; Li, R. Inhibitory effects of non-volatiles lipopeptides and volatiles ketones metabolites secreted by Bacillus velezesis C16 against Alternaria solani. Biol. Control 2021, 152, 104421. [Google Scholar] [CrossRef]
- Feng, S.; Jin, L.; Tang, S.; Jian, Y.; Li, Z. Combination of rhizosphere bacteria isolated from resistant potato plants for biocontrol of potato late blight. Pest Manag. Sci. 2021, 78, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhuo, T.; Hu, X.; Fan, X.; Zou, H. Identification of a Pseudomonas putida as biocontrol agent for tomato bacterial wilt disease. Biol. Control 2017, 114, 45–50. [Google Scholar] [CrossRef]
- Daura-Pich, O.; Hernández, I.; Pinyol-Escala, L.; Lara, J.M.; Martínez-Servat, S.; Fernández, C.; López-García, B. No antibiotic and toxic metabolites produced by the biocontrol agent Pseudomonas putida strain B2017. FEMS Microbiol. Lett. 2020, 367, fnaa075. [Google Scholar] [CrossRef]
- Youssef, S.A.; Tartoura, K.A.; Abdelraouf, G.A. Evaluation of Trichoderma harzianum and Serratia proteamaculans effect on disease suppression, stimulation of ROS-scavenging enzymes and improving tomato growth infected by Rhizoctonia solani. Biol. Control 2016, 100, 79–86. [Google Scholar] [CrossRef]
- Gkarmiri, K.; Finlay, R.D.; Alström, S.; Thomas, E.; Cubeta, M.A.; Högberg, N. Transcriptomic changes in the plant pathogenic fungus Rhizoctonia solani AG-3 in response to the antagonistic bacteria Serratia proteamaculans and Serratia plymuthica. BMC Genom. 2015, 16, 630. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, S.; Ru, J.; Tang, B.; Zhai, Y.; Wang, Z.; Wang, L. Biological control of potato late blight by Streptomyces sp. FXP04 and potential role of secondary metabolites. Biol. Control 2022, 169, 104891. [Google Scholar] [CrossRef]
- Gajbhiye, M.H.; Kapadnis, B.P. Antifungal-activity-producing lactic acid bacteria as biocontrol agents in plants. Biocontrol Sci. Technol. 2016, 26, 1451–1470. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The Significance of Bacillus spp. in Disease Suppression and Growth Promotion of Field and Vegetable Crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Buldakov, S.; Shakleina, N.; Plekhanova, L.P. Protection of potatoes from late blight in Sakhalin. In Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022. [Google Scholar]
- Christine, M.; Vogel, D.B.P.; Schäfer, M.; Barandun, N.; Julia, A. Vorholt, Protective role of the Arabidopsis leaf microbiota against a bacterial pathogen. Nat. Microbiol. 2021, 6, 1537–1548. [Google Scholar]
- Yu, Y.Y.; Xu, J.D.; Huang, T.X.; Zhong, J.; Yu, H.; Qiu, J.P.; Guo, J.H. Combination of beneficial bacteria improves blueberry production and soil quality. Food Sci. Nutr. 2020, 8, 5776–5784. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, J.; Liu, F.; Liang, J.; Zhao, P.; Tsui, C.K.M.; Cai, L. Cross-kingdom synthetic microbiota supports tomato suppression of Fusarium wilt disease. Nat. Commun. 2022, 13, 7890. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, L.; Yan, Z.; Schneijderberg, M.; de Roij, M.; Pijnenburg, R.; Zheng, Q.; Franken, C.; Dechesne, A.; Trindade, L.M.; van Velzen, R.; et al. Synthetic bacterial community derived from a desert rhizosphere confers salt stress resilience to tomato in the presence of a soil microbiome. SME J. 2022, 16, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Prigigallo, M.I.; Cabanás, C.G.-L.; Mercado-Blanco, J.; Bubici, G. Designing a synthetic microbial community devoted to biological control: The case study of Fusarium wilt of banana. Front. Microbiol. 2022, 13, 967885. [Google Scholar] [CrossRef]
- Carlström, C.I.; Field, C.M.; Bortfeld-Miller, M.; Müller, B.; Sunagawa, S.; Vorholt, J.A. Synthetic microbiota reveal priority effects and keystone strains in the Arabidopsis phyllosphere. Nat. Ecol. Evol. 2019, 3, 1445–1454. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Nong, X.; Wang, J.; Qi, S. Brevianamides and Mycophenolic Acid Derivatives from the Deep-Sea-Derived Fungus Penicillium brevicompactum DFFSCS025. Mar. Drugs 2017, 15, 43. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Xie, F.; Ren, B.; Wang, Q.; Wang, J.; Wang, Q.; Abdel-Mageed, W.M.; Liu, M.; Han, J.; Oyeleye, A.; et al. Anti-MRSA and anti-TB metabolites from marine-derived Verrucosispora sp. MS100047. Appl. Microbiol. Biotechnol. 2016, 100, 7437–7447. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Xie, P.; Liu, H.; Liu, T.; Zhao, M.; Yang, S.; Niu, G.; Hale, L.; Singh, B.K.; Kowalchuk, G.A.; et al. Tapping the rhizosphere metabolites for the prebiotic control of soil-borne bacterial wilt disease. Nat. Commun. 2023, 14, 4497. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ruan, W.; Li, J.; Xu, H.; Wang, J.; Gao, Y.; Wang, J. Biological Control of Phytopathogenic Fungi by Fatty Acids. Mycopathologia 2008, 166, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhong, T.; Chen, K.; Du, M.; Chen, G.; Chen, X.; Wang, K.; Zalán, Z.; Takács, K.; Kan, J. Antifungal activity of volatile organic compounds produced by Pseudomonas fluorescens ZX and potential biocontrol of blue mold decay on postharvest citrus. Food Control 2020, 120, 107499. [Google Scholar] [CrossRef]
- Li, N.; Islam, M.T.; Kang, S. Secreted metabolite-mediated interactions between rhizosphere bacteria and Trichoderma biocontrol agents. PLoS ONE 2019, 14, e0227228. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Z.; Xie, J.; Hesselberg-Thomsen, V.; Tan, T.; Zheng, D.; Strube, M.L.; Dragoš, A.; Shen, Q.; Zhang, R.; et al. Bacillus velezensis stimulates resident rhizosphere Pseudomonas stutzeri for plant health through metabolic interactions. ISME J. 2021, 16, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Agirman, B.; Carsanba, E.; Settanni, L.; Erten, H. Exploring yeast-based microbial interactions: The next frontier in postharvest biocontrol. Yeast 2023, 40, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sun, C.; Guan, X.; Lian, S.; Li, B.; Wang, C. Biocontrol efficiency of Meyerozyma guilliermondii Y-1 against apple postharvest decay caused by Botry osphaeria dothidea and the possible mechanisms of action. Int. J. Food Microbiol. 2021, 338, 108957. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.-X.; Cernava, T.; Wang, H.; Zhou, Y.; Xia, T.; Cao, S.; Berg, G.; Shen, X.-X.; Wen, Z.; et al. Fusarium fruiting body microbiome member Pantoea agglomerans inhibits fungal pathogenesis by targeting lipid rafts. Nat. Microbiol. 2022, 7, 831–843. [Google Scholar] [CrossRef]
- Vanhauteghem, D.; Demeyere, K.; Callaert, N.; Boelaert, A.; Haesaert, G.; Audenaert, K.; Meyer, E. Flow Cytometry Is a Powerful Tool for Assessment of the Viability of Fungal Conidia in Metalworking Fluids. Appl. Environ. Microbiol. 2017, 83, e00938-17. [Google Scholar] [CrossRef] [PubMed]
- De Cesare, G.B.; Cristy, S.A.; Garsin, D.A.; Lorenz, M.C. Antimicrobial Peptides: A New Frontier in Antifungal Therapy. mBio 2020, 11, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Solís, D.; Zetter-Salmón, E.; Contreras-Pérez, M.; del Carmen Rocha-Granados, M.; Macías-Rodríguez, L.; Santoyo, G. Pseudomonas stutzeri E25 and Stenotrophomonas maltophilia CR71 endophytes produce antifungal volatile organic compounds and exhibit additive plant growth-promoting effects. Biocatal. Agric. Biotechnol. 2018, 13, 46–52. [Google Scholar] [CrossRef]
- Ramlawi, S.; Abusharkh, S.; Carroll, A.; McMullin, D.R.; Avis, T.J. Biological and chemical characterization of antimicrobial activity in Arthrobacter spp. isolated from dis ease-suppressive compost. J. Basic Microbiol. 2021, 61, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.R.; Fierro-Coronado, R.A.; Peñuelas-Rubio, O.; Villa-Lerma, A.G.; Plascencia-Jatomea, R.; Félix-Gastélum, R.; Maldonado-Mendoza, I.E. Rhizospheric bacteria as potential biocontrol agents against Fusarium wilt and crown and root rot diseases in tomato. Saudi J. Biol. Sci. 2021, 28, 7460–7471. [Google Scholar] [CrossRef] [PubMed]
- Sritongkam, B.; Sun, P.L.; Lo, P.H.; Shen, Y.M.; Wang, C.J.; Unartngam, J.; Chung, W.H. Novel causative agents of Fusarium solani species complex causing stem and fruit rot in cucurbit in Taiwan. J. Phytopathol. 2022, 170, 462–478. [Google Scholar] [CrossRef]
- Rabaaoui, A.; Dall’Asta, C.; Righetti, L.; Susca, A.; Logrieco, A.F.; Namsi, A.; Gdoura, R.; Werbrouck, S.P.; Moretti, A.; Masiello, M. Phylogeny and Mycotoxin Profile of Pathogenic Fusarium Species Isolated from Sudden Decline Syn drome and Leaf Wilt Symptoms on Date Palms (Phoenix dactylifera) in Tunisia. Toxins 2021, 13, 463. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Vasilev, N.; Boccard, J.; Lang, G.; Grömping, U.; Fischer, R.; Goepfert, S.; Rudaz, S.; Schillberg, S. Structured plant metabolomics for the simultaneous exploration of multiple factors. Sci. Rep. 2016, 6, 37390. [Google Scholar] [CrossRef]
- Zelena, E.; Dunn, W.B.; Broadhurst, D.; Francis-McIntyre, S.; Carroll, K.M.; Begley, P.; O’Hagan, S.; Knowles, J.D.; Halsall, A.; HUSERMETConsortium Wilson, I.D. Development of a Robust and Repeatable UPLC-MS Method for the Long-Term Metab olomic Study of Human Serum. Anal. Chem. 2009, 81, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2012, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]




| Biocontrol Group | Distribution of Strain Species (Genera) | |
|---|---|---|
| BM | Monobacteria | Distributed in 10 genera (Bacillus, Paenibacillus, Peribacillus frigoritolerans, Lelliottia amnigena, Serratia proteamaculans, Pseudomonas, Acinetobacter, Actinomycetia bacterium, Paenibacillus amylolyticus, Pantoea agglomerans) |
| SynM1 | Bacterial synthesis community (61 strains) | Distributed in 10 genera (Synthesized from all strains in BM) |
| SynM2 | Bacterial and fungal synthetic communities (62 strains) | Distributed in 11 genera (SynM1+Penicillium allii) |
| SynM3 | Bacillus synthetic community (17 strains) | Distributed in five species (Bacillus sp., Bacillus amyloliquefaciens, Bacillus subtilis, Bacillus pumilus, Bacillus velezensis) |
| SynM4 | Lelliottia amnigena synthetic community (11 strains) | Distributed in one species (Lelliottia amnigena) |
| SynM5 | Serratia synthetic community (five strains) | Distributed in two species (Serratia plymuthica, Serratia proteamaculans) |
| SynM6 | Pseudomonas synthetic community (15 strains) | Distributed in one species (Pseudomonas putida) |
| SynM7 | Acinetobacter synthetic community (three strains) | Distributed in one species (Acinetobacter calcoaceticus) |
| SynM8 | Bacterial synthesis community (16 strains) | Distributed in nine genera (Bacillus sp., Bacillus amyloliquefaciens, Paenibacillus sp., Bacillus subtilis, Bacillus pumilus, Bacillus velezensis, Peribacillus frigoritolerans, Lelliottia amnigena, Serratia plymuthica, Serratia proteamaculans, Pseudomonas sp., Pseudomonas putida, Acinetobacter sp., Acinetobacter calcoaceticus, Actinomycetia bacterium, Paenibacillus amylolyticus) |
| SynM9 | Bacterial and fungal synthetic communities (17 strains) | Distributed in 10 genera (SynM8+Penicillium allii) |
| SynM10 | Bacterial synthesis community (six strains) | Distributed in six genera (Bacillus amyloliquefaciens, Serratia proteamaculans, Pseudomonas putida, Acinetobacter calcoaceticus, Actinomycetia bacterium, Paenibacillus amylolyticus) |
| SynM11 | Bacterial synthesis community (six strains) | Distributed in six genera (Bacillus subtilis, Serratia proteamaculans, Pseudomonas putida, Acinetobacter calcoaceticus, Actinomycetia bacterium, Paenibacillus amylolyticus) |
| SynM12 | Bacterial synthesis community (six strains) | Distributed in six genera (Bacillus pumilus, Serratia proteamaculans, Pseudomonas putida, Acinetobacter calcoaceticus, Actinomycetia bacterium, Paenibacillus amylolyticus) |
| SynM13 | Bacterial synthesis community (six strains) | Distributed in six genera (Lelliottia amnigena, Bacillus velezensis, Pseudomonas sp., Serratia proteamaculans, Stenotrophomonas maltophilia, Pantoea agglomerans) |
| SynM14 | Bacterial and fungal synthetic communities (seven strains) | Distributed in seven genera (Paenibacillus amylolyticus, Pseudomonas putida, Acinetobacter calcoaceticus, Serratia proteamaculans, Actinomycetia bacterium, Bacillus subtilis, Penicillium allii) |
| SynM15 | Bacterial and fungal synthetic communities (seven strains) | Distributed in seven genera (Bacillus pumilus, Pseudomonas putida, Acinetobacter calcoaceticus, Serratia proteamaculans, Actinomycetia bacterium, Bacillus subtilis, Penicillium allii) |
| SynM16 | Bacterial and fungal synthetic communities (seven strains) | Distributed in seven genera (Lelliottia amnigena, Bacillus velezensis, Pseudomonas sp., Serratia proteamaculans, Stenotrophomonas maltophilia, Pantoea agglomerans, Penicillium allii) |
| SynM17 | Bacterial synthesis community (five strains) | Distributed in five genera (Bacillus velezensis, Pseudomonas sp., Serratia proteamaculans, Stenotrophomonas maltophilia, Pantoea agglomerans) |
| SynM18 | Bacterial and fungal synthetic communities (six strains) | Distributed in six genera (Bacillus velezensis, Pseudomonas sp., Serratia proteamaculans, Stenotrophomonas maltophilia, Pantoea agglomerans, Penicillium allii) |
| No. | Member Strains in SynM11 | Strains Name |
|---|---|---|
| 1 | B9D10 | Paenibacillus amylolyticus |
| 2 | B65D14 | Pseudomonas putida |
| 3 | B9H9 | Acinetobacter calcoaceticus |
| 4 | B65H4 | Serratia proteamaculans |
| 5 | B9H11 | Actinomycetia bacterium |
| 6 | B65D7 | Bacillus subtilis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, H.; Li, W.; Chen, H.; Meng, Y.; Wu, H.; Wang, J.; Shen, S. Synthetic Microbial Community Members Interact to Metabolize Caproic Acid to Inhibit Potato Dry Rot Disease. Int. J. Mol. Sci. 2024, 25, 4437. https://doi.org/10.3390/ijms25084437
Shi H, Li W, Chen H, Meng Y, Wu H, Wang J, Shen S. Synthetic Microbial Community Members Interact to Metabolize Caproic Acid to Inhibit Potato Dry Rot Disease. International Journal of Molecular Sciences. 2024; 25(8):4437. https://doi.org/10.3390/ijms25084437
Chicago/Turabian StyleShi, Huiqin, Wei Li, Hongyu Chen, Yao Meng, Huifang Wu, Jian Wang, and Shuo Shen. 2024. "Synthetic Microbial Community Members Interact to Metabolize Caproic Acid to Inhibit Potato Dry Rot Disease" International Journal of Molecular Sciences 25, no. 8: 4437. https://doi.org/10.3390/ijms25084437




