Anti-Neuroinflammatory Effects of a Macrocyclic Peptide-Peptoid Hybrid in Lipopolysaccharide-Stimulated BV2 Microglial Cells
Abstract
:1. Introduction
2. Results
2.1. Effects of X15856 on Cell Viability
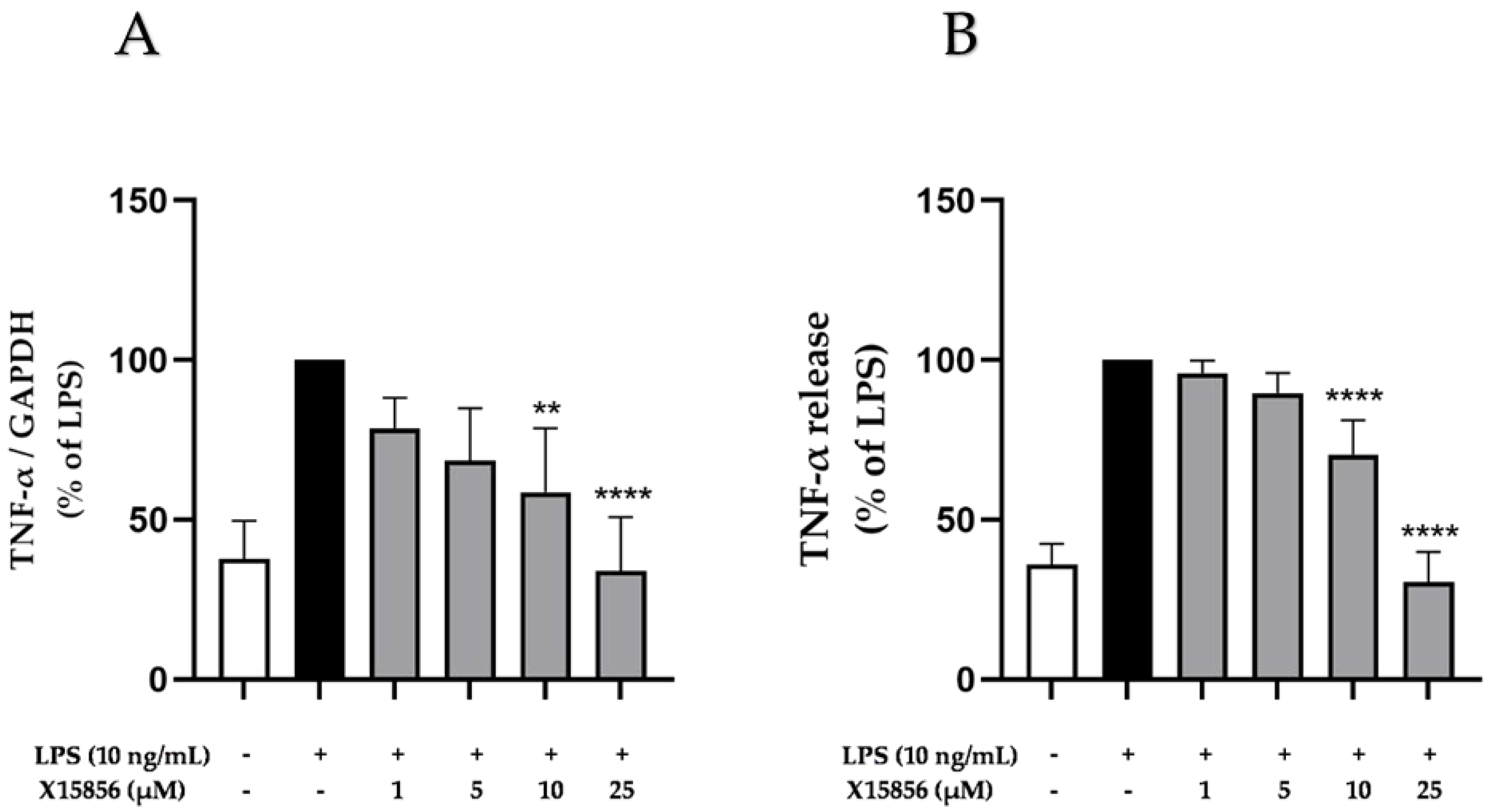
2.2. Effects of X15856 on Gene Expression and Protein Synthesis of TNF-α
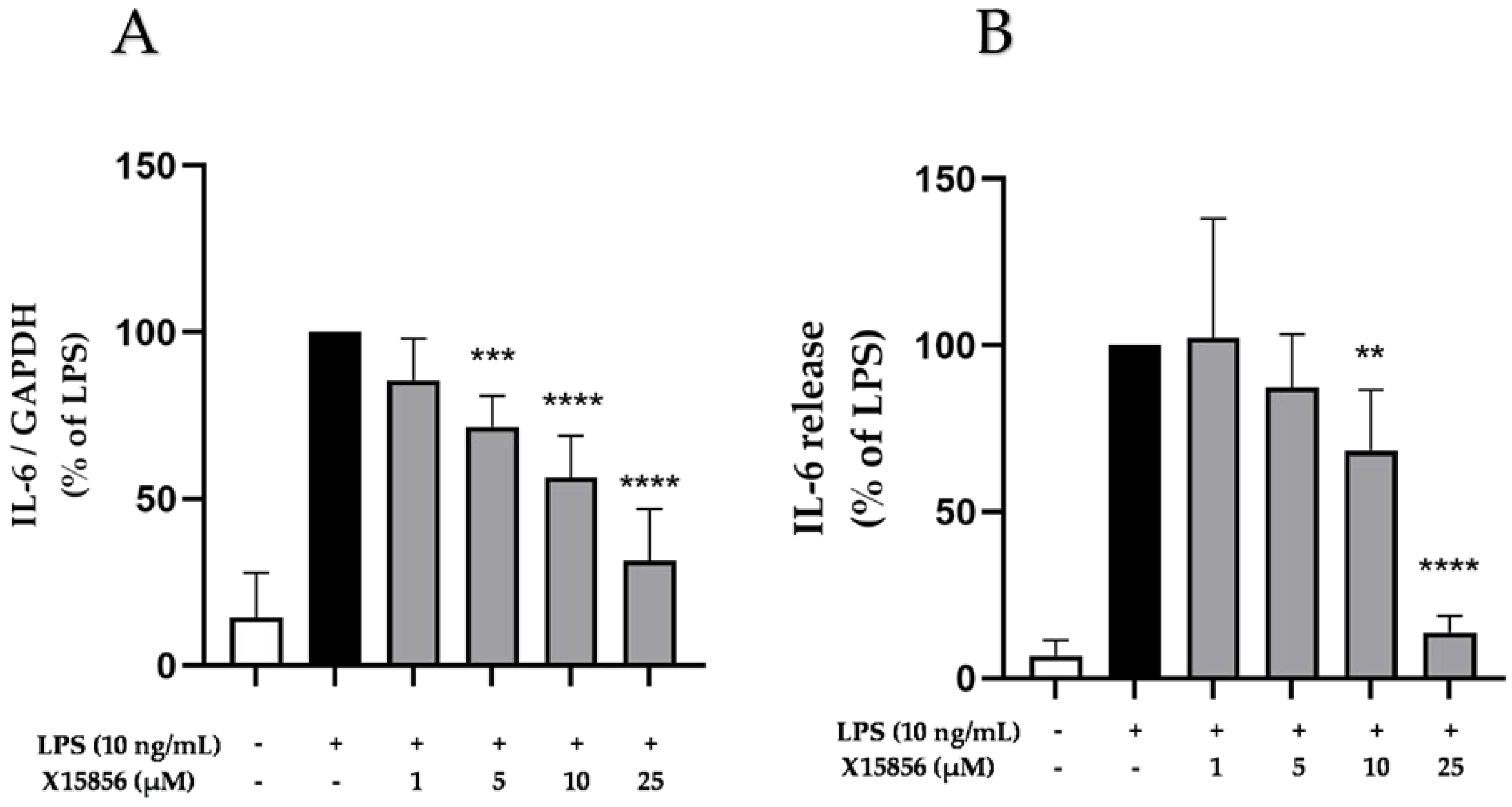
2.3. Effects of X15856 on Gene Expression and Protein Synthesis of IL-6
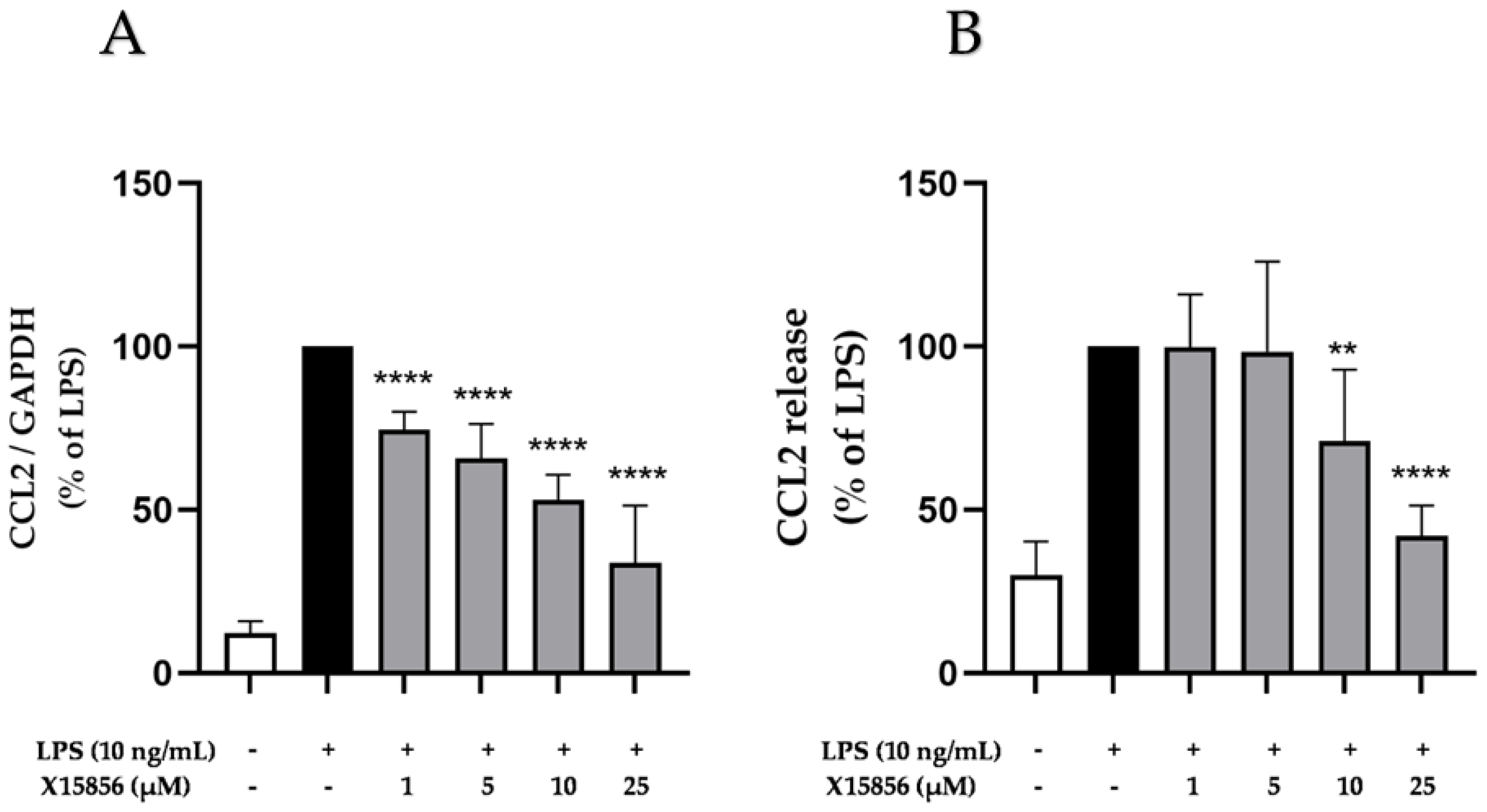
2.4. Effects of X15856 on Gene Expression and Protein Synthesis of CCL2
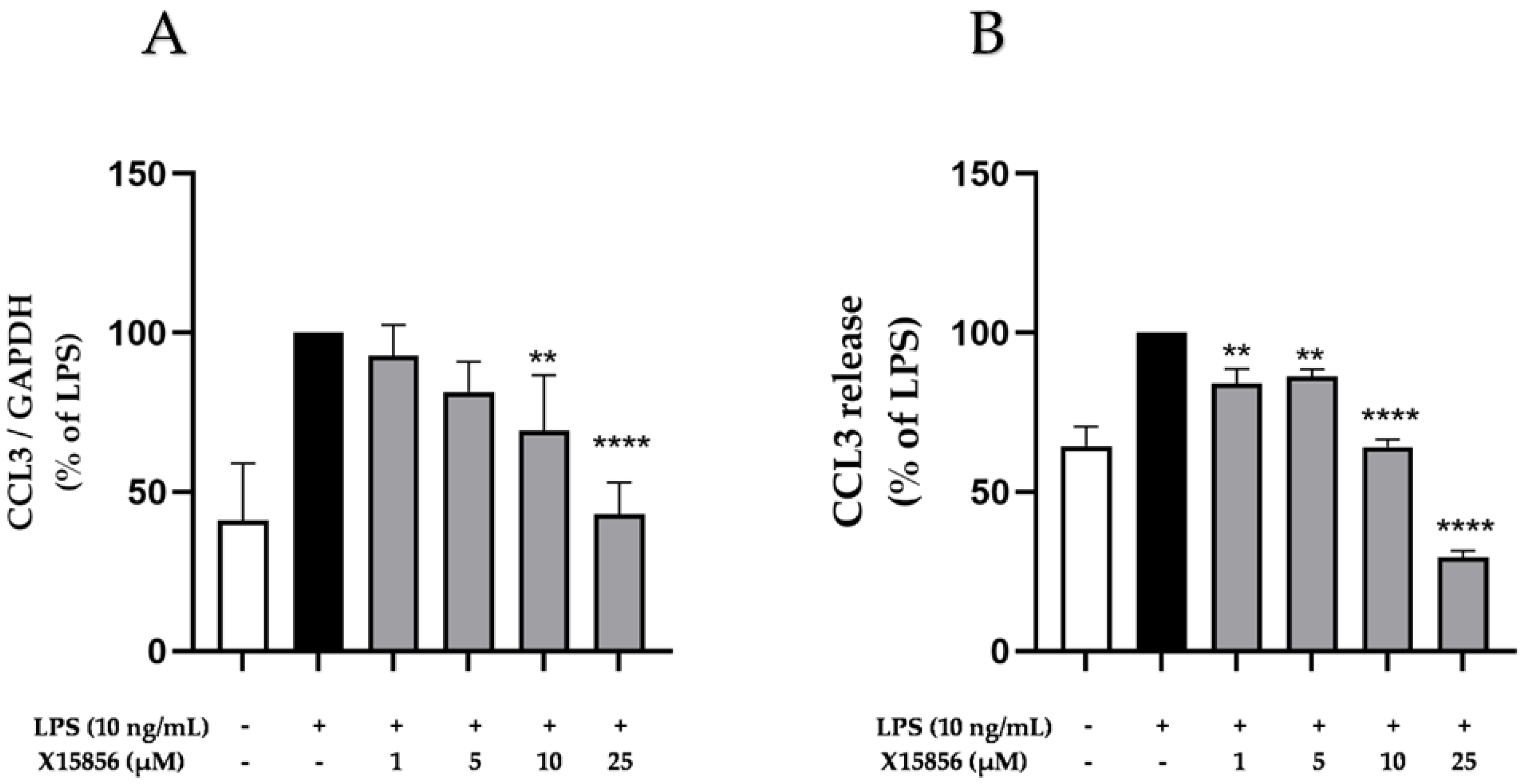
2.5. Effects of X15856 on Gene Expression and Protein Synthesis of CCL3
2.6. Effects of X15856 on Gene Expression and Protein Synthesis of CXCL2
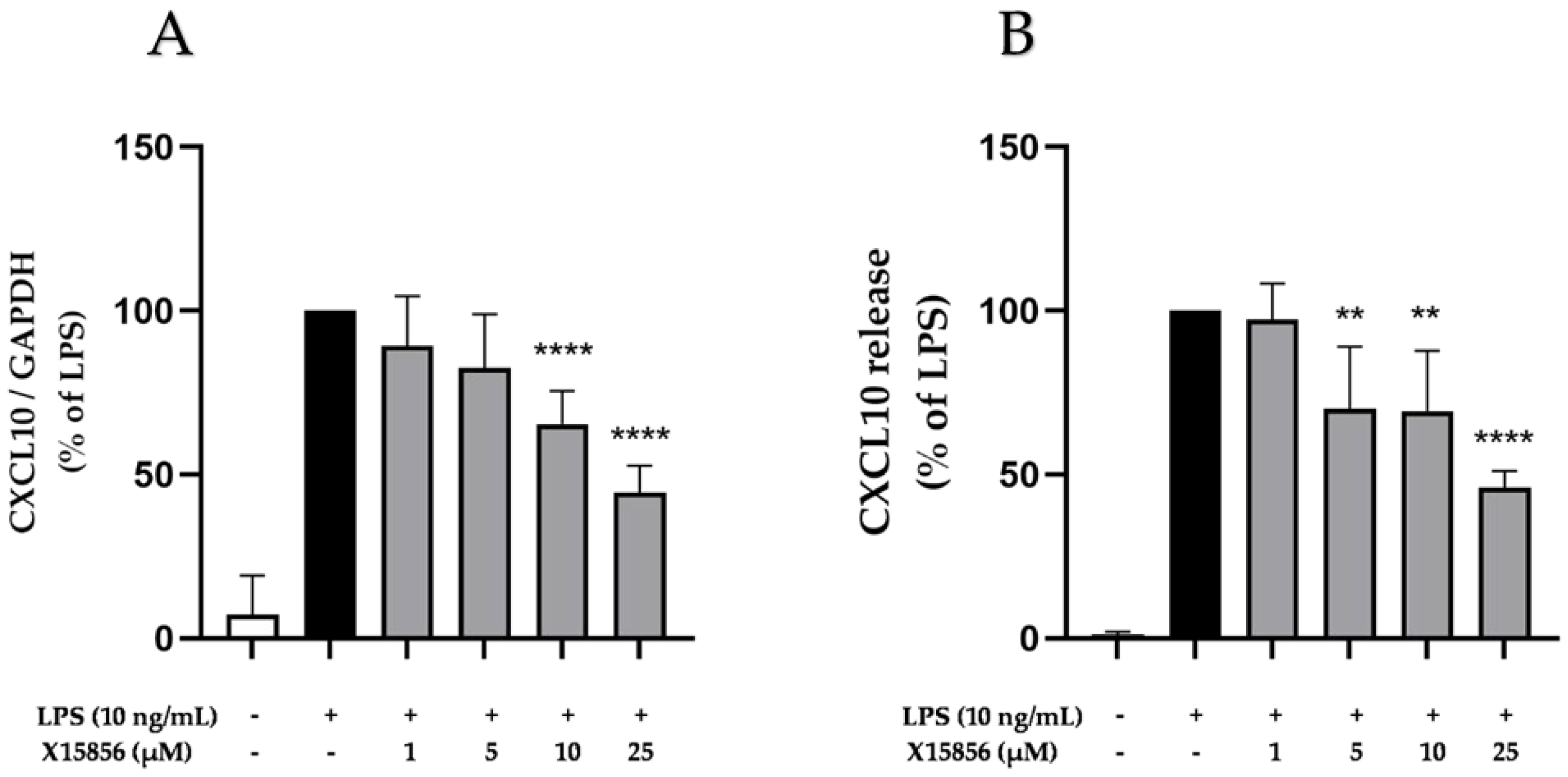
2.7. Effects of X15856 on Gene Expression and Protein Synthesis of CXCL10
2.8. Comparison of X15856 to Dexamethasone and Hydrocortisone
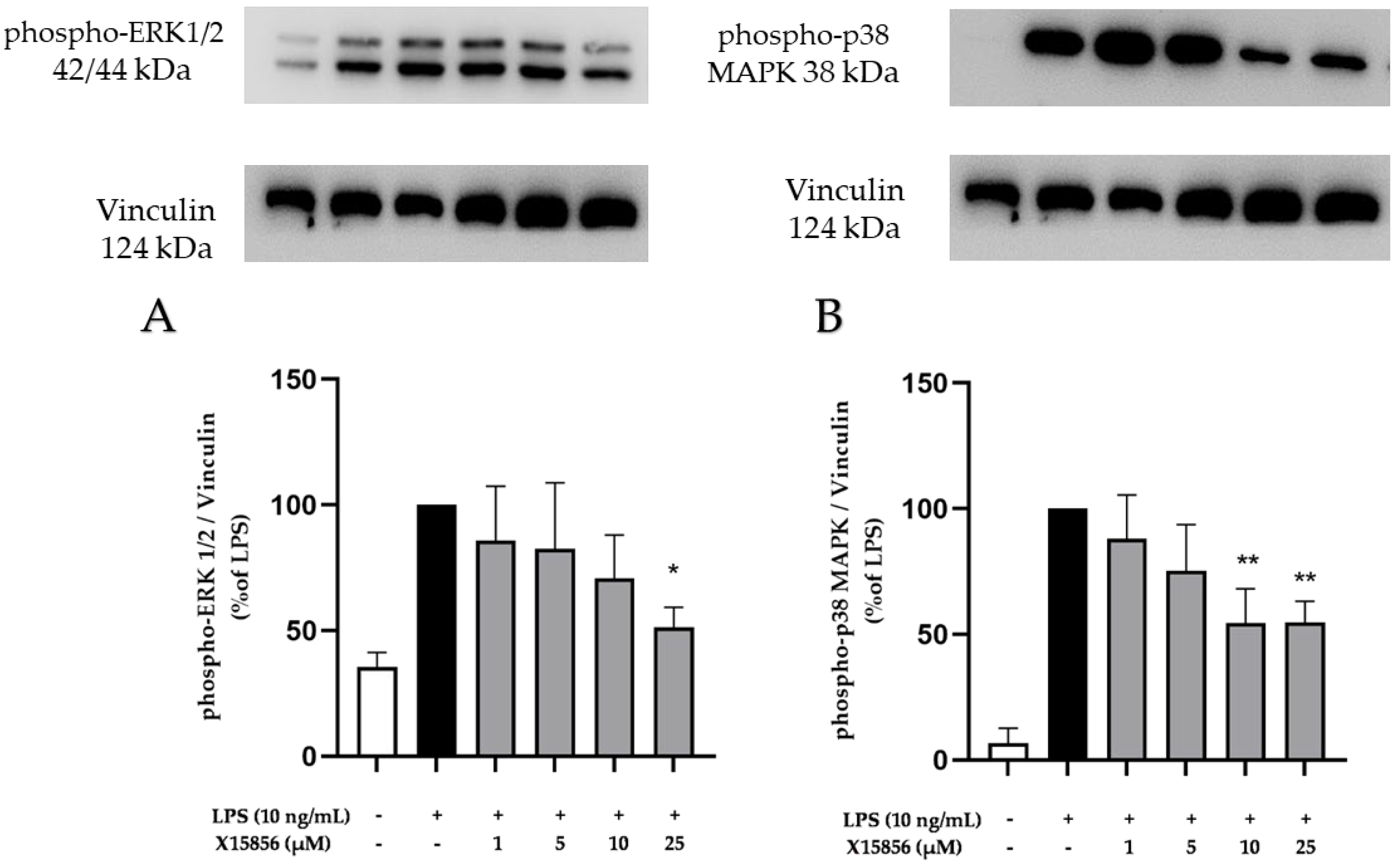
2.9. Effects of X15856 on Phosphorylation of PKCβ, p38 MAPK, ERK ½, and NF-κB
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. General Aspects
4.1.2. Synthesis of cyclo-(L-Phe-N1phpCl-N1ph-N1phpCl-N4am-L-Phe); X15856
4.1.3. Usage of X15856 and Other Chemicals
4.2. BV2 Microglial Cell Culture
4.3. Cell Viability Assay
4.4. Determination of Cytokine and Chemokine Release
4.5. RNA Isolation and Quantitative PCR
4.6. Immunoblotting
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Campos, A.I.; Mulcahy, A.; Thorp, J.G.; Wray, N.R.; Byrne, E.M.; Lind, P.A.; Medland, S.E.; Martin, N.G.; Hickie, I.B.; Rentería, M.E. Understanding genetic risk factors for common side effects of antidepressant medications. Commun. Med. 2021, 1, 45. [Google Scholar] [CrossRef]
- Hu, X.H.; Bull, S.A.; Hunkeler, E.M.; Ming, E.; Lee, J.Y.; Fireman, B.; Markson, L.E. Incidence and duration of side effects and those rated as bothersome with selective serotonin reuptake inhibitor treatment for depression: Patient report versus physician estimate. J. Clin. Psychiatry 2004, 65, 959–965. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S.; Alsuwaidan, M.; Baune, B.T.; Berk, M.; Demyttenaere, K.; Goldberg, J.F.; Gorwood, P.; Ho, R.; Kasper, S.; Kennedy, S.H.; et al. Treatment-resistant depression: Definition, prevalence, detection, management, and investigational interventions. World Psychiatry 2023, 22, 394–412. [Google Scholar] [CrossRef]
- Al-Harbi, K.S. Treatment-resistant depression: Therapeutic trends, challenges, and future directions. Patient Prefer. Adherence 2012, 6, 369–388. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, M.; Hao, W.; Wang, Y.; Zhang, T.; Liu, C. Neuroinflammation mechanisms of neuromodulation therapies for anxiety and depression. Transl. Psychiatry 2023, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Luo, Y.; Liang, X.; Tang, J.; Wang, J.; Xiao, Q.; Qi, Y.; Li, Y.; Zhu, P.; Yang, H.; et al. Beneficial effects of running exercise on hippocampal microglia and neuroinflammation in chronic unpredictable stress-induced depression model rats. Transl. Psychiatry 2021, 11, 461. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Brawek, B.; Skok, M.; Garaschuk, O. Changing Functional Signatures of Microglia along the Axis of Brain Aging. Int. J. Mol. Sci. 2021, 22, 1091. [Google Scholar] [CrossRef]
- Prinz, M.; Masuda, T.; Wheeler, M.A.; Quintana, F.J. Microglia and Central Nervous System-Associated Macrophages-From Origin to Disease Modulation. Annu. Rev. Immunol. 2021, 39, 251–277. [Google Scholar] [CrossRef]
- Liu, C.Y.; Wang, X.; Liu, C.; Zhang, H.L. Pharmacological Targeting of Microglial Activation: New Therapeutic Approach. Front. Cell. Neurosci. 2019, 13, 514. [Google Scholar] [CrossRef]
- Prinz, M.; Jung, S.; Priller, J. Microglia Biology: One Century of Evolving Concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Yirmiya, R.; Rimmerman, N.; Reshef, R. Depression as a microglial disease. Trends Neurosci. 2015, 38, 637–658. [Google Scholar] [CrossRef]
- Wang, H.; He, Y.; Sun, Z.; Ren, S.; Liu, M.; Wang, G.; Yang, J. Microglia in depression: An overview of microglia in the pathogenesis and treatment of depression. J. Neuroinflamm. 2022, 19, 132. [Google Scholar] [CrossRef]
- Deng, S.L.; Chen, J.G.; Wang, F. Microglia: A Central Player in Depression. Curr. Med. Sci. 2020, 40, 391–400. [Google Scholar] [CrossRef]
- Saliba, S.W.; Marcotegui, A.R.; Fortwängler, E.; Ditrich, J.; Perazzo, J.C.; Muñoz, E.; de Oliveira, A.C.P.; Fiebich, B.L. AM404, paracetamol metabolite, prevents prostaglandin synthesis in activated microglia by inhibiting COX activity. J. Neuroinflamm. 2017, 14, 246. [Google Scholar] [CrossRef]
- Saliba, S.W.; Gläser, F.; Deckers, A.; Keil, A.; Hurrle, T.; Apweiler, M.; Ferver, F.; Volz, N.; Endres, D.; Bräse, S.; et al. Effects of a Novel GPR55 Antagonist on the Arachidonic Acid Cascade in LPS-Activated Primary Microglial Cells. Int. J. Mol. Sci. 2021, 22, 2503. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy-from molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Herlan, C.N.; Meschkov, A.; Schepers, U.; Bräse, S. Cyclic Peptoid-Peptide Hybrids as Versatile Molecular Transporters. Front. Chem. 2021, 9, 696957. [Google Scholar] [CrossRef] [PubMed]
- Wolf, L.M.; Servossz, S.L.; Moss, M.A. Peptoids: Emerging therapeutics for neurodegeneration. J. Neurol. Neuromed. 2017, 2, 1–15. [Google Scholar] [CrossRef]
- Kwon, Y.U.; Kodadek, T. Quantitative evaluation of the relative cell permeability of peptoids and peptides. J. Am. Chem. Soc. 2007, 129, 1508–1509. [Google Scholar] [CrossRef] [PubMed]
- Corredor, M.; Bonet, R.; Moure, A.; Domingo, C.; Bujons, J.; Alfonso, I.; Pérez, Y.; Messeguer, À. Cationic Peptides and Peptidomimetics Bind Glycosaminoglycans as Potential Sema3A Pathway Inhibitors. Biophys. J. 2016, 110, 1291–1303. [Google Scholar] [CrossRef]
- Einsiedel, J.; Held, C.; Hervet, M.; Plomer, M.; Tschammer, N.; Hübner, H.; Gmeiner, P. Discovery of highly potent and neurotensin receptor 2 selective neurotensin mimetics. J. Med. Chem. 2011, 54, 2915–2923. [Google Scholar] [CrossRef]
- Bailey, M.A.; Ingram, M.J.; Naughton, D.P. A novel anti-oxidant and anti-cancer strategy: A peptoid anti-inflammatory drug conjugate with SOD mimic activity. Biochem. Biophys. Res. Commun. 2004, 317, 1155–1158. [Google Scholar] [CrossRef]
- Waugh, M.L.; Wolf, L.M.; Turner, J.P.; Phillips, L.N.; Servoss, S.L.; Moss, M.A. Modulating the RAGE-Induced Inflammatory Response: Peptoids as RAGE Antagonists. Chembiochem A Eur. J. Chem. Biol. 2023, 24, e202300503. [Google Scholar] [CrossRef]
- Fisher, A.E.; Naughton, D.P. Novel peptoids for the detection and suppression of reactive oxygen and nitrogen species. Biochem. Soc. Trans. 2003, 31, 1302–1304. [Google Scholar] [CrossRef]
- Orzáez, M.; Mondragón, L.; Marzo, I.; Sanclimens, G.; Messeguer, A.; Pérez-Payá, E.; Vicent, M.J. Conjugation of a novel Apaf-1 inhibitor to peptide-based cell-membrane transporters: Effective methods to improve inhibition of mitochondria-mediated apoptosis. Peptides 2007, 28, 958–968. [Google Scholar] [CrossRef]
- Corredor, M.; Bujons, J.; Orzáez, M.; Sancho, M.; Pérez-Payá, E.; Alfonso, I.; Messeguer, A. Optimizing the control of apoptosis by amide/triazole isosteric substitution in a constrained peptoid. Eur. J. Med. Chem. 2013, 63, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Rákosi, K.; Masaru, T.; Zarándia, M.; Telegdy, G.; Tóth, G.K. Short analogs and mimetics of human urocortin 3 display antidepressant effects in vivo. Peptides 2014, 62, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Shlik, J.; Vasar, E.; Bradwejn, J. Cholecystokinin and psychiatric disorders: Role in aetiology and potential of receptor antagonists in therapy. CNS Drugs 1997, 8, 134–152. [Google Scholar] [CrossRef] [PubMed]
- Naik, M.T.; Naik, N.; Hu, T.; Wang, S.-H.; Marshall, J. Structure-based development of new cyclic compounds targeting PSD-95 PDZ3 domain. bioRxiv 2023. [Google Scholar] [CrossRef]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A road to inflammation. Nat. Rev. Immunol. 2023, 23, 289–303. [Google Scholar] [CrossRef]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Krügel, U.; Fischer, J.; Radicke, S.; Sack, U.; Himmerich, H. Antidepressant effects of TNF-α blockade in an animal model of depression. J. Psychiatr. Res. 2013, 47, 611–616. [Google Scholar] [CrossRef]
- Curzytek, K.; Leśkiewicz, M. Targeting the CCL2-CCR2 axis in depressive disorders. Pharmacol. Rep. PR 2021, 73, 1052–1062. [Google Scholar] [CrossRef]
- Kelly, K.M.; Smith, J.A.; Mezuk, B. Depression and interleukin-6 signaling: A Mendelian Randomization study. Brain Behav. Immun. 2021, 95, 106–114. [Google Scholar] [CrossRef]
- Ting, E.Y.; Yang, A.C.; Tsai, S.J. Role of Interleukin-6 in Depressive Disorder. Int. J. Mol. Sci. 2020, 21, 2194. [Google Scholar] [CrossRef] [PubMed]
- Leighton, S.P.; Nerurkar, L.; Krishnadas, R.; Johnman, C.; Graham, G.J.; Cavanagh, J. Chemokines in depression in health and in inflammatory illness: A systematic review and meta-analysis. Mol. Psychiatry 2018, 23, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, V.M.; Stanton, E.H.; Nothdurfter, C.; Rupprecht, R.; Wetzel, C.H. The Role of Chemokines in the Pathophysiology of Major Depressive Disorder. Int. J. Mol. Sci. 2019, 20, 2283. [Google Scholar] [CrossRef]
- Qin, C.C.; Liu, Y.N.; Hu, Y.; Yang, Y.; Chen, Z. Macrophage inflammatory protein-2 as mediator of inflammation in acute liver injury. World J. Gastroenterol. 2017, 23, 3043–3052. [Google Scholar] [CrossRef]
- Pandey, G.N.; Rizavi, H.S.; Bhaumik, R.; Zhang, H. Chemokines gene expression in the prefrontal cortex of depressed suicide victims and normal control subjects. Brain Behav. Immun. 2021, 94, 266–273. [Google Scholar] [CrossRef]
- Altara, R.; Mallat, Z.; Booz, G.W.; Zouein, F.A. The CXCL10/CXCR3 Axis and Cardiac Inflammation: Implications for Immunotherapy to Treat Infectious and Noninfectious Diseases of the Heart. J. Immunol. Res. 2016, 2016, 4396368. [Google Scholar] [CrossRef] [PubMed]
- Ślusarczyk, J.; Trojan, E.; Chwastek, J.; Głombik, K.; Basta-Kaim, A. A Potential Contribution of Chemokine Network Dysfunction to the Depressive Disorders. Curr. Neuropharmacol. 2016, 14, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.L.; Dong, C.; Maestre-Mesa, J.; Licinio, J. Polymorphisms in inflammation-related genes are associated with susceptibility to major depression and antidepressant response. Mol. Psychiatry 2008, 13, 800–812. [Google Scholar] [CrossRef]
- van Zuiden, M.; Heijnen, C.J.; van de Schoot, R.; Amarouchi, K.; Maas, M.; Vermetten, E.; Geuze, E.; Kavelaars, A. Cytokine production by leukocytes of military personnel with depressive symptoms after deployment to a combat-zone: A prospective, longitudinal study. PLoS ONE 2011, 6, e29142. [Google Scholar] [CrossRef]
- Yeung, Y.T.; Aziz, F.; Guerrero-Castilla, A.; Arguelles, S. Signaling Pathways in Inflammation and Anti-inflammatory Therapies. Curr. Pharm. Des. 2018, 24, 1449–1484. [Google Scholar] [CrossRef]
- Wang, J.Q.; Mao, L. The ERK Pathway: Molecular Mechanisms and Treatment of Depression. Mol. Neurobiol. 2019, 56, 6197–6205. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Rana, T.; Alotaibi, G.H.; Shamsuzzaman, M.; Naqvi, M.; Sehgal, A.; Singh, S.; Sharma, N.; Almoshari, Y.; Abdellatif, A.A.H.; et al. Polyphenols inhibiting MAPK signalling pathway mediated oxidative stress and inflammation in depression. Biomed. Pharmacother. 2022, 146, 112545. [Google Scholar] [CrossRef]
- Merighi, S.; Nigro, M.; Travagli, A.; Gessi, S. Microglia and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 3240–3249. [Google Scholar] [CrossRef]
- Badanjak, K.; Fixemer, S.; Smajić, S.; Skupin, A.; Grünewald, A. The Contribution of Microglia to Neuroinflammation in Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 4676. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C.; Hunot, S.; Hartmann, A. Neuroinflammatory processes in Parkinson’s disease. Park. Relat. Disord. 2005, 11 (Suppl. S1), S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Koistinaho, M.; Koistinaho, J. Interactions between Alzheimer’s disease and cerebral ischemia-focus on inflammation. Brain Res. Brain Res. Rev. 2005, 48, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Cytokine, sickness behavior, and depression. Immunol. Allergy Clin. North Am. 2009, 29, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Crews, F.T. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp. Neurol. 2008, 210, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, S.F.; Caso, J.R.; Munhoz, C.D.; Sapolsky, R.M. The stressed CNS: When glucocorticoids aggravate inflammation. Neuron 2009, 64, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Yin, L.; Shi, M.; Cheng, H.; Xu, X.; Liu, Z.; Zhang, G.; Wu, Z.; Feng, G.; Zhao, G. Involvement of microglial cells in infrasonic noise-induced stress via upregulated expression of corticotrophin releasing hormone type 1 receptor. Neuroscience 2010, 167, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.G.; Miguel, Z.D.; Watkins, L.R.; Maier, S.F. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav. Immun. 2010, 24, 19–30. [Google Scholar] [CrossRef]
- Moulton, C.D.; Pickup, J.C.; Amiel, S.A.; Winkley, K.; Ismail, K. Investigating incretin-based therapies as a novel treatment for depression in type 2 diabetes: Findings from the South London Diabetes (SOUL-D) Study. Prim. Care Diabetes 2016, 10, 156–159. [Google Scholar] [CrossRef]
- Yazdani, U.; Zaman, S.; Hynan, L.S.; Brown, L.S.; Dewey, R.B., Jr.; Karp, D.; German, D.C. Blood biomarker for Parkinson disease: Peptoids. NPJ Park. Dis. 2016, 2, 16012. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.K.; Hyun, Y.J.; Lee, J.H.; Lim, H.S. Comparison of Cell Permeability of Cyclic Peptoids and Linear Peptoids. ACS Comb. Sci. 2018, 20, 237–242. [Google Scholar] [CrossRef]
- Park, S.; Kwon, Y.U. Facile solid-phase parallel synthesis of linear and cyclic peptoids for comparative studies of biological activity. ACS Comb. Sci. 2015, 17, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Diamond, G.; Molchanova, N.; Herlan, C.; Fortkort, J.A.; Lin, J.S.; Figgins, E.; Bopp, N.; Ryan, L.K.; Chung, D.; Adcock, R.S.; et al. Potent Antiviral Activity against HSV-1 and SARS-CoV-2 by Antimicrobial Peptoids. Pharmaceuticals 2021, 14, 304. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, J.A.; Hancock, A.A.; Vitt, C.R.; Knepper, S.; Buckner, S.A.; Brune, M.E.; Milicic, I.; Kerwin, J.F., Jr.; Richter, L.S.; Taylor, E.W.; et al. Pharmacologic characterization of CHIR 2279, an N-substituted glycine peptoid with high-affinity binding for alpha 1-adrenoceptors. J. Pharmacol. Exp. Ther. 1996, 277, 885–899. [Google Scholar] [PubMed]
- Driggers, E.M.; Hale, S.P.; Lee, J.; Terrett, N.K. The exploration of macrocycles for drug discovery—An underexploited structural class. Nat. Rev. Drug Discov. 2008, 7, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Shen, Q.; Cho, J.H.; Hwang, W. Entropy Hotspots for the Binding of Intrinsically Disordered Ligands to a Receptor Domain. Biophys. J. 2020, 118, 2502–2512. [Google Scholar] [CrossRef]
- Boehm, M.; Beaumont, K.; Jones, R.; Kalgutkar, A.S.; Zhang, L.; Atkinson, K.; Bai, G.; Brown, J.A.; Eng, H.; Goetz, G.H.; et al. Discovery of Potent and Orally Bioavailable Macrocyclic Peptide-Peptoid Hybrid CXCR7 Modulators. J. Med. Chem. 2017, 60, 9653–9663. [Google Scholar] [CrossRef]
- Huynh, C.; Dingemanse, J.; Meyer Zu Schwabedissen, H.E.; Sidharta, P.N. Relevance of the CXCR4/CXCR7-CXCL12 axis and its effect in pathophysiological conditions. Pharmacol. Res. 2020, 161, 105092. [Google Scholar] [CrossRef]
- Trojan, E.; Ślusarczyk, J.; Chamera, K.; Kotarska, K.; Głombik, K.; Kubera, M.; Basta-Kaim, A. The Modulatory Properties of Chronic Antidepressant Drugs Treatment on the Brain Chemokine—Chemokine Receptor Network: A Molecular Study in an Animal Model of Depression. Front. Pharmacol. 2017, 8, 779. [Google Scholar] [CrossRef]
- Chang, H.C.; Huang, P.H.; Syu, F.S.; Hsieh, C.H.; Chang, S.L.; Lu, J.; Chen, H.C. Critical involvement of atypical chemokine receptor CXCR7 in allergic airway inflammation. Immunology 2018, 154, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Hornik, T.C.; Neniskyte, U.; Brown, G.C. Inflammation induces multinucleation of Microglia via PKC inhibition of cytokinesis, generating highly phagocytic multinucleated giant cells. J. Neurochem. 2014, 128, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Nennig, S.E.; Schank, J.R. The Role of NFkB in Drug Addiction: Beyond Inflammation. Alcohol Alcohol. 2017, 52, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Langlois, A.; Chouinard, F.; Flamand, N.; Ferland, C.; Rola-Pleszczynski, M.; Laviolette, M. Crucial implication of protein kinase C (PKC)-delta, PKC-zeta, ERK-1/2, and p38 MAPK in migration of human asthmatic eosinophils. J. Leukoc. Biol. 2009, 85, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Streyczek, J.; Apweiler, M.; Sun, L.; Fiebich, B.L. Turmeric Extract (Curcuma longa) Mediates Anti-Oxidative Effects by Reduction of Nitric Oxide, iNOS Protein-, and mRNA-Synthesis in BV2 Microglial Cells. Molecules 2022, 27, 784. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Mao, R.; Cui, L.; Wang, F.; Zhou, R.; Wang, Y.; Huang, J.; Zhu, Y.; Yao, Y.; Zhao, G.; et al. PAID study design on the role of PKC activation in immune/inflammation-related depression: A randomised placebo-controlled trial protocol. Gen. Psychiatry 2021, 34, e100440. [Google Scholar] [CrossRef] [PubMed]
- Trushin, S.A.; Pennington, K.N.; Carmona, E.M.; Asin, S.; Savoy, D.N.; Billadeau, D.D.; Paya, C.V. Protein kinase Calpha (PKCalpha) acts upstream of PKCtheta to activate IkappaB kinase and NF-kappaB in T lymphocytes. Mol. Cell. Biol. 2003, 23, 7068–7081. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Sobhia, M.E. Targeting PKC-β II by Peptides and Peptidomimetics Derived from RACK 1: An In Silico Approach. Mol. Inform. 2011, 30, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Inokuchi, J.; Eto, M.; Murata, M.; Kang, J.H. Protein Kinase C (PKC) Isozymes as Diagnostic and Prognostic Biomarkers and Therapeutic Targets for Cancer. Cancers 2022, 14, 5425. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Xia, A.; Qi, X.J.M. Identification of novel peptoid agonists of fibroblast growth factor receptors using microarray-based screening. Medchemcomm 2016, 7, 1183–1189. [Google Scholar] [CrossRef]
- Kodadek, T. Chemical tools to monitor and manipulate the adaptive immune system. Chem. Biol. 2014, 21, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.E.; Maxwell, S.C.; Naughton, D.P. Catalase and superoxide dismutase mimics for the treatment of inflammatory diseases. Inorg. Chem. Commun. 2003, 6, 1205–1208. [Google Scholar] [CrossRef]
- Skovbakke, S.L.; Winther, M.; Gabl, M.; Holdfeldt, A.; Linden, S.; Wang, J.M.; Dahlgren, C.; Franzyk, H.; Forsman, H. The peptidomimetic Lau-(Lys-βNSpe)(6)-NH(2) antagonizes formyl peptide receptor 2 expressed in mouse neutrophils. Biochem. Pharmacol. 2016, 119, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.C.; Skovbakke, S.L.; Masoudi, H.; Hancock, R.E.W.; Franzyk, H. In vivo Anti-inflammatory Activity of Lipidated Peptidomimetics Pam-(Lys-βNspe)(6)-NH(2) and Lau-(Lys-βNspe)(6)-NH(2) Against PMA-Induced Acute Inflammation. Front. Immunol. 2020, 11, 2102. [Google Scholar] [CrossRef]
- Villoslada, P.; Vila, G.; Colafrancesco, V.; Moreno, B.; Fernandez-Diez, B.; Vazquez, R.; Pertsovskaya, I.; Zubizarreta, I.; Pulido-Valdeolivas, I.; Messeguer, J.; et al. Axonal and Myelin Neuroprotection by the Peptoid BN201 in Brain Inflammation. Neurotherapeutics 2019, 16, 808–827. [Google Scholar] [CrossRef] [PubMed]
- Kaltschmidt, B.; Uherek, M.; Volk, B.; Baeuerle, P.A.; Kaltschmidt, C. Transcription factor NF-kappaB is activated in primary neurons by amyloid beta peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proc. Natl. Acad. Sci. USA 1997, 94, 2642–2647. [Google Scholar] [CrossRef] [PubMed]
- Yousif, N.M.; de Oliveira, A.C.P.; Brioschi, S.; Huell, M.; Biber, K.; Fiebich, B.L. Activation of EP2 receptor suppresses poly(I: C) and LPS-mediated inflammation in primary microglia and organotypic hippocampal slice cultures: Contributing role for MAPKs. Glia 2018, 66, 708–724. [Google Scholar] [CrossRef]
- Apweiler, M.; Streyczek, J.; Saliba, S.W.; Ditrich, J.; Muñoz, E.; Fiebich, B.L. Anti-Inflammatory and Anti-Oxidative Effects of AM404 in IL-1β-Stimulated SK-N-SH Neuroblastoma Cells. Front. Pharmacol. 2021, 12, 789074. [Google Scholar] [CrossRef]
- Apweiler, M.; Saliba, S.W.; Streyczek, J.; Hurrle, T.; Gräßle, S.; Bräse, S.; Fiebich, B.L. Targeting Oxidative Stress: Novel Coumarin-Based Inverse Agonists of GPR55. Int. J. Mol. Sci. 2021, 22, 11665. [Google Scholar] [CrossRef]
- Akmermer, K.; Herlan, C.; Jung, N.; Bräse, S. Chemotion Repository Homepage. Available online: https://www.chemotion-repository.net/home/publications/collections/7493 (accessed on 15 February 2024). [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Wilke Saliba, S.; Apweiler, M.; Akmermer, K.; Herlan, C.; Grathwol, C.; de Oliveira, A.C.P.; Normann, C.; Jung, N.; Bräse, S.; et al. Anti-Neuroinflammatory Effects of a Macrocyclic Peptide-Peptoid Hybrid in Lipopolysaccharide-Stimulated BV2 Microglial Cells. Int. J. Mol. Sci. 2024, 25, 4462. https://doi.org/10.3390/ijms25084462
Sun L, Wilke Saliba S, Apweiler M, Akmermer K, Herlan C, Grathwol C, de Oliveira ACP, Normann C, Jung N, Bräse S, et al. Anti-Neuroinflammatory Effects of a Macrocyclic Peptide-Peptoid Hybrid in Lipopolysaccharide-Stimulated BV2 Microglial Cells. International Journal of Molecular Sciences. 2024; 25(8):4462. https://doi.org/10.3390/ijms25084462
Chicago/Turabian StyleSun, Lu, Soraya Wilke Saliba, Matthias Apweiler, Kamil Akmermer, Claudine Herlan, Christoph Grathwol, Antônio Carlos Pinheiro de Oliveira, Claus Normann, Nicole Jung, Stefan Bräse, and et al. 2024. "Anti-Neuroinflammatory Effects of a Macrocyclic Peptide-Peptoid Hybrid in Lipopolysaccharide-Stimulated BV2 Microglial Cells" International Journal of Molecular Sciences 25, no. 8: 4462. https://doi.org/10.3390/ijms25084462




