PPARs as Key Transcription Regulators at the Crossroads of Metabolism and Inflammation
Funding
Conflicts of Interest
References
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Desvergne, B.; Michalik, L.; Wahli, W. Transcriptional regulation of metabolism. Physiol. Rev. 2006, 86, 465–514. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.C.; Sutter, B.M.; Ruess, H.; Barnes, S.D.; Malladi, V.S.; Tu, B.P. Glucose starvation induces a switch in the histone acetylome for activation of gluconeogenic and fat metabolism genes. Mol. Cell 2022, 82, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Ren, D.; He, Y.; Yi, H. Editorial: Epigenetic, metabolic, and transcriptional regulation of immune cell plasticity and functions in cancer and non-cancer diseases. Front. Immunol. 2023, 14, 1284124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Burch, P.E.; Cooney, A.J.; Lanz, R.B.; Pereira, F.A.; Wu, J.; Gibbs, R.A.; Weinstock, G.; Wheeler, D.A. Genomic analysis of the nuclear receptor family: New insights into structure, regulation, and evolution from the rat genome. Genome Res. 2004, 14, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Narkar, V.A. Nuclear receptors as potential therapeutic targets in peripheral arterial disease and related myopathy. FEBS J. 2023, 290, 4596–4613. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.D.; Billon, C.; Losby, M.; Bedia-Diaz, G.; Fang, Y.; Avdagic, A.; Elgendy, B.; Burris, T.P.; Griffett, K. Emerging Role of Nuclear Receptors for the Treatment of NAFLD and NASH. Metabolites 2022, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Puengel, T.; Liu, H.; Guillot, A.; Heymann, F.; Tacke, F.; Peiseler, M. Nuclear Receptors Linking Metabolism, Inflammation, and Fibrosis in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 2668. [Google Scholar] [CrossRef] [PubMed]
- Alatshan, A.; Benkő, S. Nuclear Receptors as Multiple Regulators of NLRP3 Inflammasome Function. Front. Immunol. 2021, 12, 630569. [Google Scholar] [CrossRef] [PubMed]
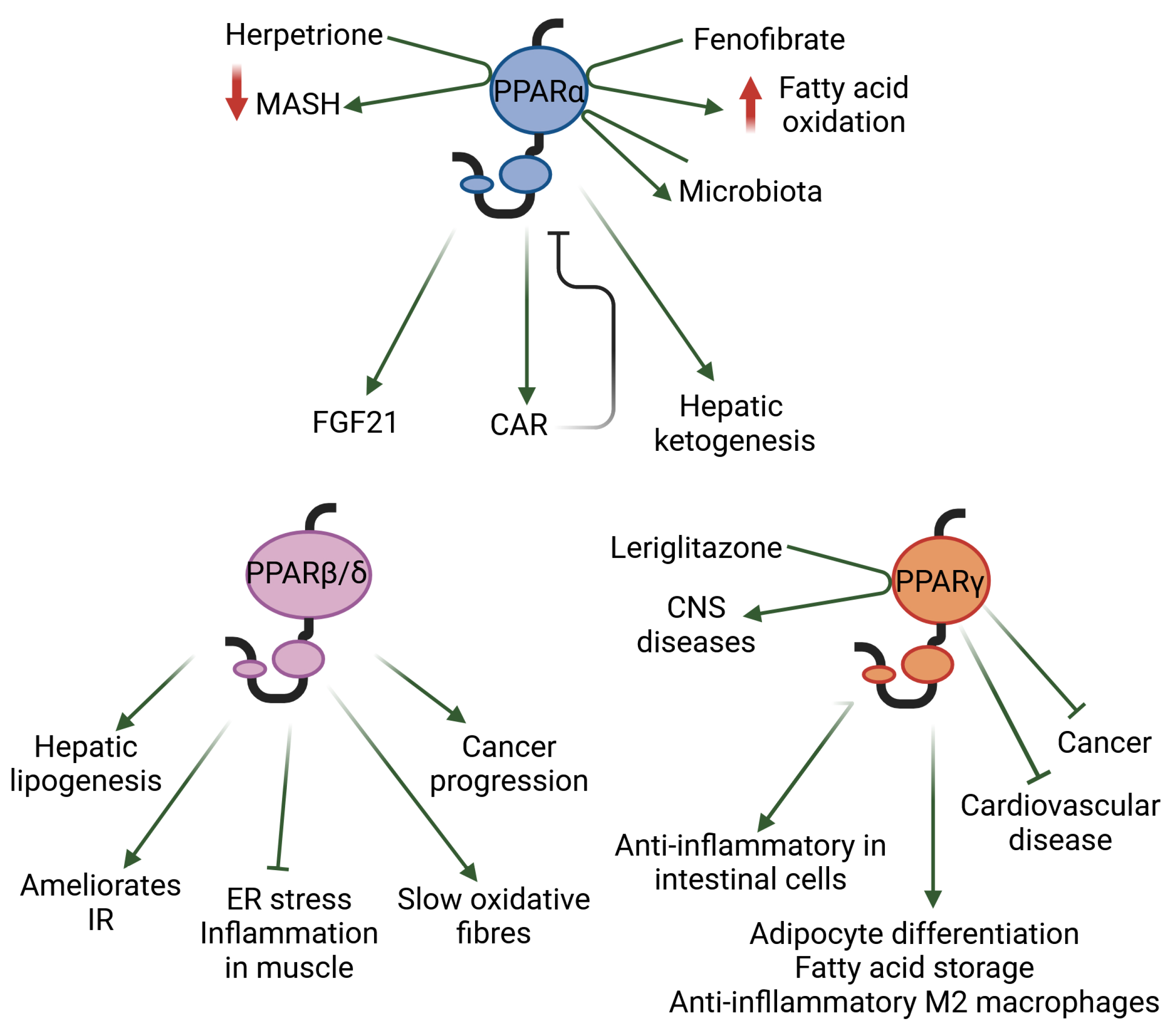
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; De Bosscher, K. Molecular Actions of PPARα in Lipid Metabolism and Inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef] [PubMed]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Wahli, W.; Michalik, L. PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol. Metab. 2012, 23, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Kamata, S.; Honda, A.; Ishii, I. Current Clinical Trial Status and Future Prospects of PPAR-Targeted Drugs for Treating Nonalcoholic Fatty Liver Disease. Biomolecules 2023, 13, 1264. [Google Scholar] [CrossRef] [PubMed]
- Montagner, A.; Polizzi, A.; Fouché, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Régnier, M.; Lukowicz, C.; Benhamed, F.; et al. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 2016, 65, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Shizu, R.; Otsuka, Y.; Ishii, C.; Ezaki, K.; Yoshinari, K. PPARα Induces the Expression of CAR That Works as a Negative Regulator of PPARα Functions in Mouse Livers. Int. J. Mol. Sci. 2023, 24, 3953. [Google Scholar] [CrossRef] [PubMed]
- Murru, E.; Muntoni, A.L.; Manca, C.; Aroni, S.; Pistis, M.; Banni, S.; Carta, G. Profound Modification of Fatty Acid Profile and Endocannabinoid-Related Mediators in PPARα Agonist Fenofibrate-Treated Mice. Int. J. Mol. Sci. 2022, 24, 709. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Li, K.; Peng, X.; Kan, Y.; Li, H.; Zhu, Y.; Wang, Z.; Li, Z.; Liu, H.Y.; Cai, D. Nuclear Receptor PPARα as a Therapeutic Target in Diseases Associated with Lipid Metabolism Disorders. Nutrients 2023, 15, 4772. [Google Scholar] [CrossRef] [PubMed]
- Linghu, L.; Zong, W.; Liao, Y.; Chen, Q.; Meng, F.; Wang, G.; Liao, Z.; Lan, X.; Chen, M. Herpetrione, a New Type of PPARα Ligand as a Therapeutic Strategy Against Nonalcoholic Steatohepatitis. Research 2023, 6, 0276. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Wang, Q.; Qin, H.; Cao, S.; Wei, Y.; Weng, J.; Yu, F.; Zeng, H. Osteoarthritis: Role of Peroxisome Proliferator-Activated Receptors. Int. J. Mol. Sci. 2023, 24, 13137. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Aguilar-Recarte, D.; Palomer, X.; Vázquez-Carrera, M. Revealing the role of peroxisome proliferator-activated receptor β/δ in nonalcoholic fatty liver disease. Metabolism 2021, 114, 154342. [Google Scholar] [CrossRef] [PubMed]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism 2021, 114, 154338. [Google Scholar] [CrossRef] [PubMed]
- Salvadó, L.; Barroso, E.; Gómez-Foix, A.M.; Palomer, X.; Michalik, L.; Wahli, W.; Vázquez-Carrera, M. PPARβ/δ prevents endoplasmic reticulum stress-associated inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia 2014, 57, 2126–2135. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.; Ali, F.; Chambon, C.; Duteil, D.; Bornert, J.M.; Tardivel, A.; Desvergne, B.; Wahli, W.; Chambon, P.; Metzger, D. PGC1alpha expression is controlled in skeletal muscles by PPARbeta, whose ablation results in fiber-type switching, obesity, and type 2 diabetes. Cell Metab. 2006, 4, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.S.; Vázquez-Carrera, M.; Montagner, A.; Sng, M.K.; Guillou, H.; Wahli, W. Transcriptional control of physiological and pathological processes by the nuclear receptor PPARβ/δ. Prog. Lipid Res. 2016, 64, 98–122. [Google Scholar] [CrossRef] [PubMed]
- Mierzejewski, K.; Kurzyńska, A.; Gerwel, Z.; Golubska, M.; Stryiński, R.; Bogacka, I. PPARβ/δ Ligands Regulate Oxidative Status and Inflammatory Response in Inflamed Corpus Luteum-An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 4993. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Wagner, N.; Wagner, K.D. The Emerging Role of PPAR Beta/Delta in Tumor Angiogenesis. PPAR Res. 2020, 2020, 3608315. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Quiles, M.; Baak, R.; Orea-Soufi, A.; Borgman, A.; den Haan, S.; Sobrevals Alcaraz, P.; Jongejan, A.; van Es, R.; Velasco, G.; Vos, H.; et al. TRIB3 Modulates PPARγ-Mediated Growth Inhibition by Interfering with the MLL Complex in Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 10535. [Google Scholar] [CrossRef] [PubMed]
- Pizcueta, P.; Vergara, C.; Emanuele, M.; Vilalta, A.; Rodríguez-Pascau, L.; Martinell, M. Development of PPARγ Agonists for the Treatment of Neuroinflammatory and Neurodegenerative Diseases: Leriglitazone as a Promising Candidate. Int. J. Mol. Sci. 2023, 24, 3201. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Shang, D.J. The role of peroxisome proliferator-activated receptor γ in lipid metabolism and inflammation in atherosclerosis. Cell Biol. Int. 2023, 47, 1469–1487. [Google Scholar] [CrossRef] [PubMed]
- Gilardi, F.; Winkler, C.; Quignodon, L.; Diserens, J.G.; Toffoli, B.; Schiffrin, M.; Sardella, C.; Preitner, F.; Desvergne, B. Systemic PPARγ deletion in mice provokes lipoatrophy, organomegaly, severe type 2 diabetes and metabolic inflexibility. Metabolism 2019, 95, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Dammone, G.; Karaz, S.; Lukjanenko, L.; Winkler, C.; Sizzano, F.; Jacot, G.; Migliavacca, E.; Palini, A.; Desvergne, B.; Gilardi, F.; et al. PPARγ Controls Ectopic Adipogenesis and Cross-Talks with Myogenesis During Skeletal Muscle Regeneration. PPARγ Controls Ectopic Adipogenesis and Cross-Talks with Myogenesis During Skeletal Muscle Regeneration. Int. J. Mol. Sci. 2018, 19, 2044. [Google Scholar] [CrossRef] [PubMed]
- Montagner, A.; Korecka, A.; Polizzi, A.; Lippi, Y.; Blum, Y.; Canlet, C.; Tremblay-Franco, M.; Gautier-Stein, A.; Burcelin, R.; Yen, Y.C.; et al. Hepatic circadian clock oscillators and nuclear receptors integrate microbiome-derived signals. Sci. Rep. 2016, 6, 20127. [Google Scholar] [CrossRef] [PubMed]
- Grabacka, M.; Płonka, P.M.; Pierzchalska, M. The PPARα Regulation of the Gut Physiology in Regard to Interaction with Microbiota, Intestinal Immunity, Metabolism, and Permeability. Int. J. Mol. Sci. 2022, 23, 14156. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Carrera, M.; Wahli, W. PPARs as Key Transcription Regulators at the Crossroads of Metabolism and Inflammation. Int. J. Mol. Sci. 2024, 25, 4467. https://doi.org/10.3390/ijms25084467
Vázquez-Carrera M, Wahli W. PPARs as Key Transcription Regulators at the Crossroads of Metabolism and Inflammation. International Journal of Molecular Sciences. 2024; 25(8):4467. https://doi.org/10.3390/ijms25084467
Chicago/Turabian StyleVázquez-Carrera, Manuel, and Walter Wahli. 2024. "PPARs as Key Transcription Regulators at the Crossroads of Metabolism and Inflammation" International Journal of Molecular Sciences 25, no. 8: 4467. https://doi.org/10.3390/ijms25084467





