The Effects of Chronic Immunosuppressive Treatment on Morphological Changes in Cardiac Tissue and the Balance between Matrix Metalloproteinases (MMP-2 and MMP-9) and Their Inhibitors in the Rat Heart
Abstract
:1. Introduction
Metalloproteinases and Their Inhibitors in Heart Diseases
2. Results
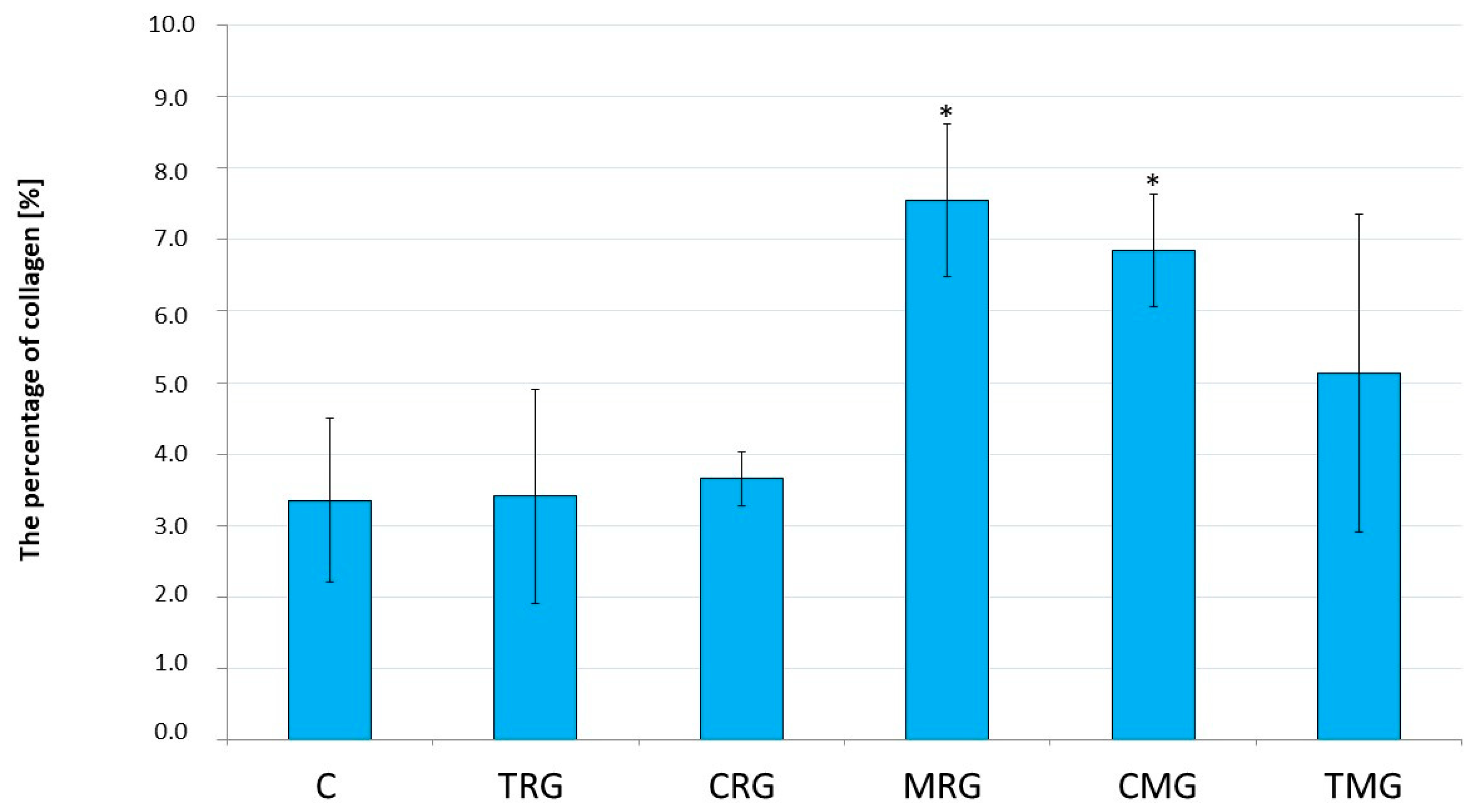
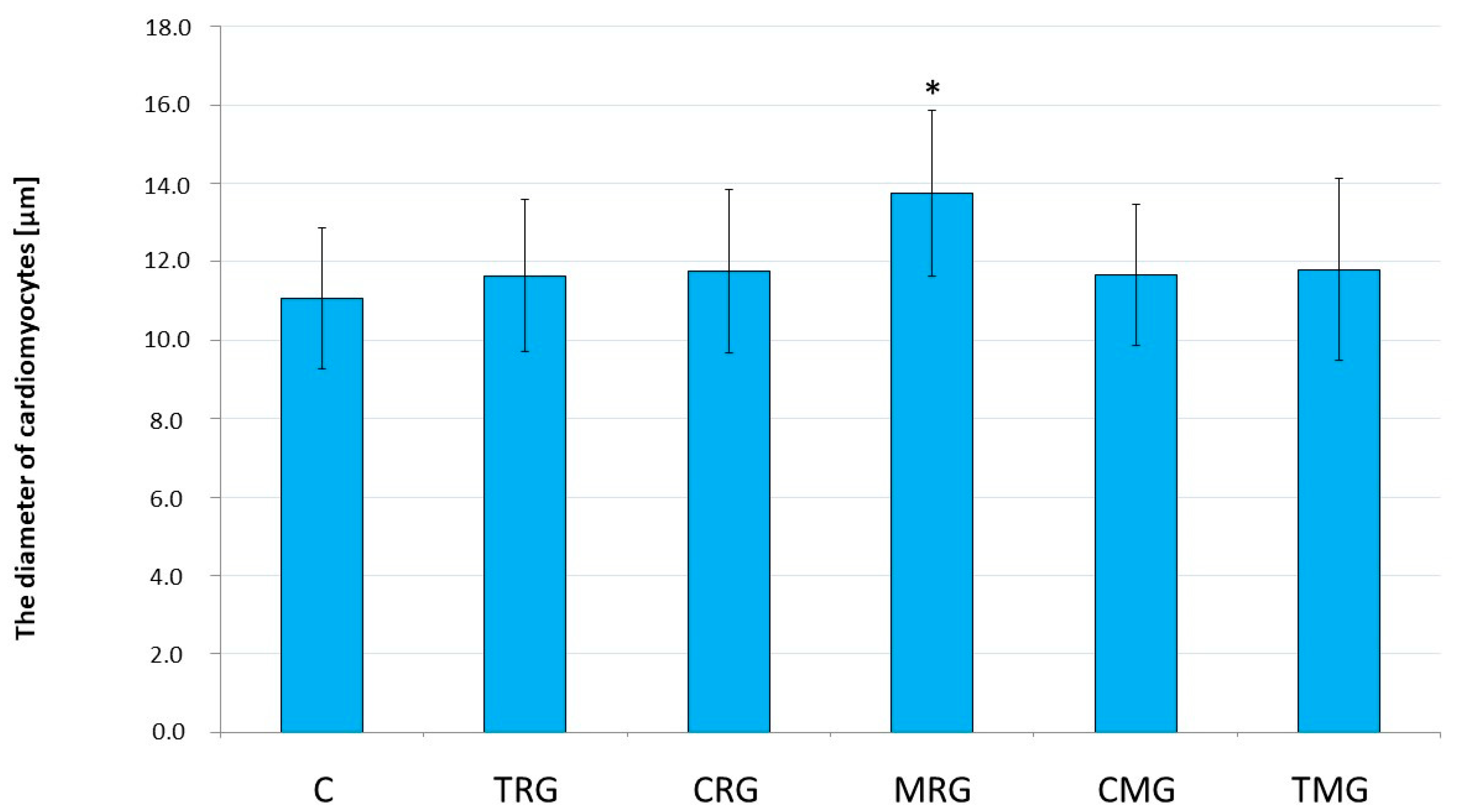
2.1. Histological Analysis
2.2. Activities of MMP-2 and MMP-9 and Expressions of TIMP-1 and TIMP-2
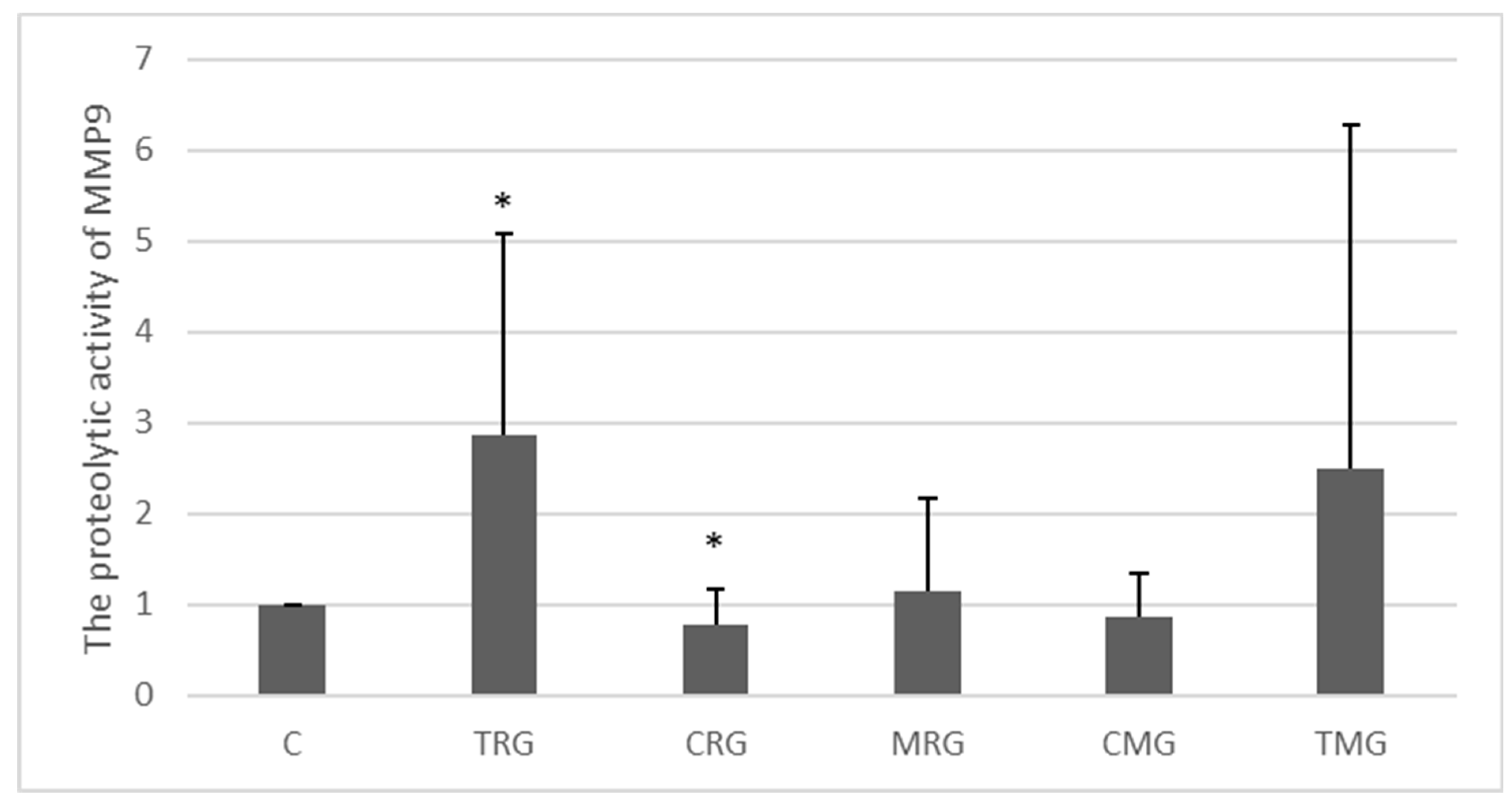
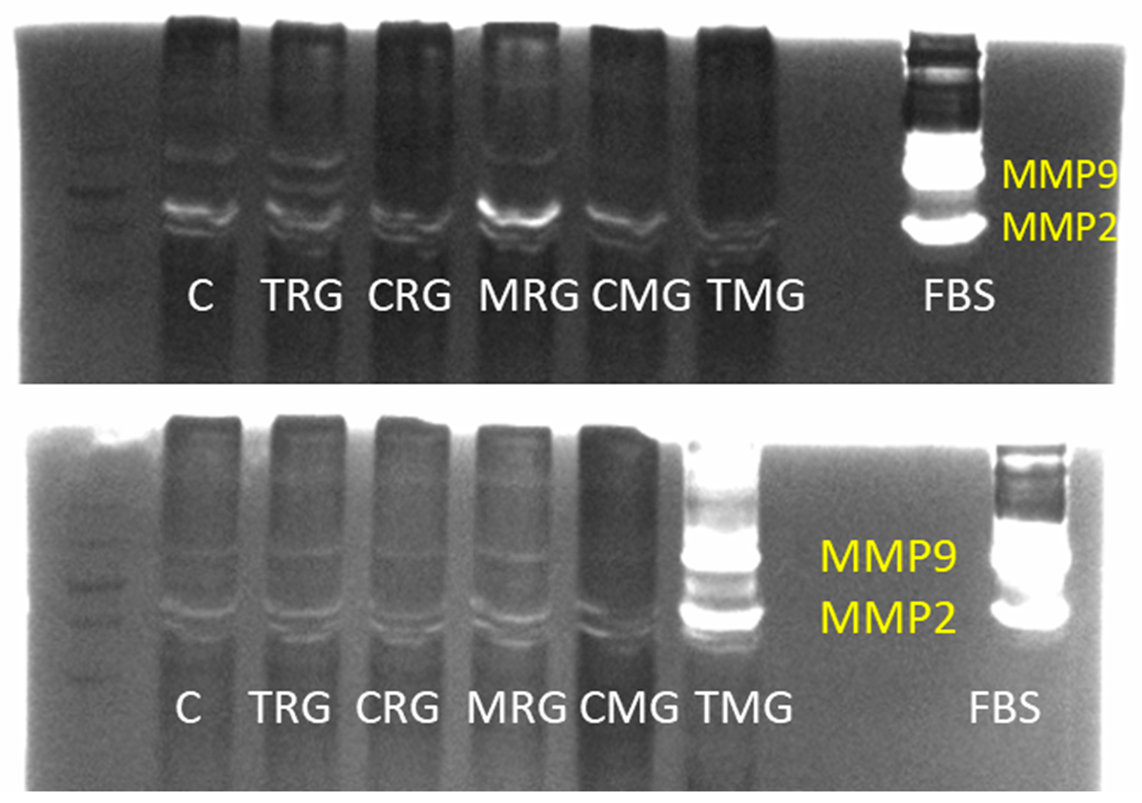
2.2.1. MMP-9 Activity (Zymography)
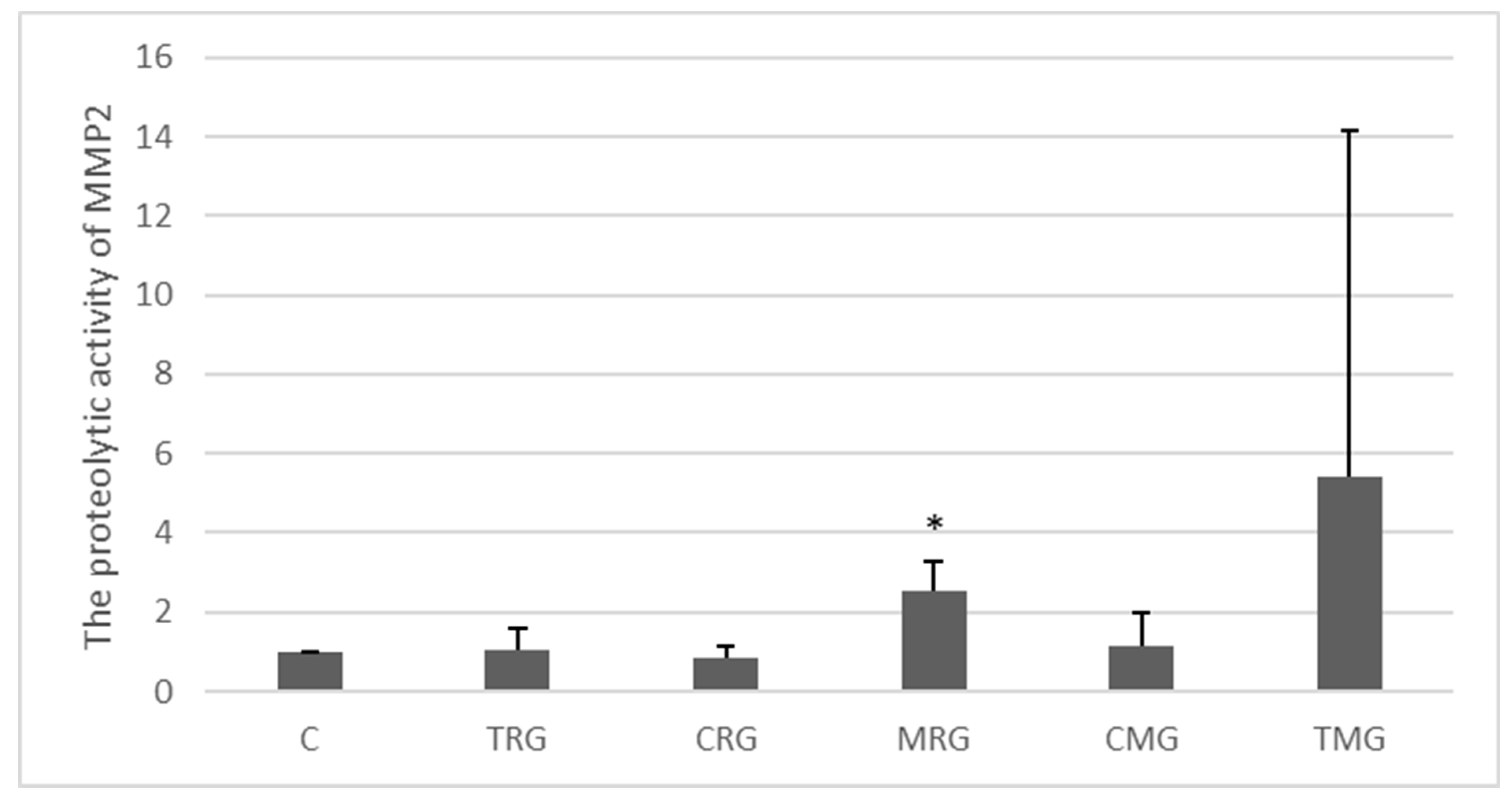
2.2.2. MMP-2 Activity (Zymography)
2.2.3. TIMP-1 Expression (Western Blot)
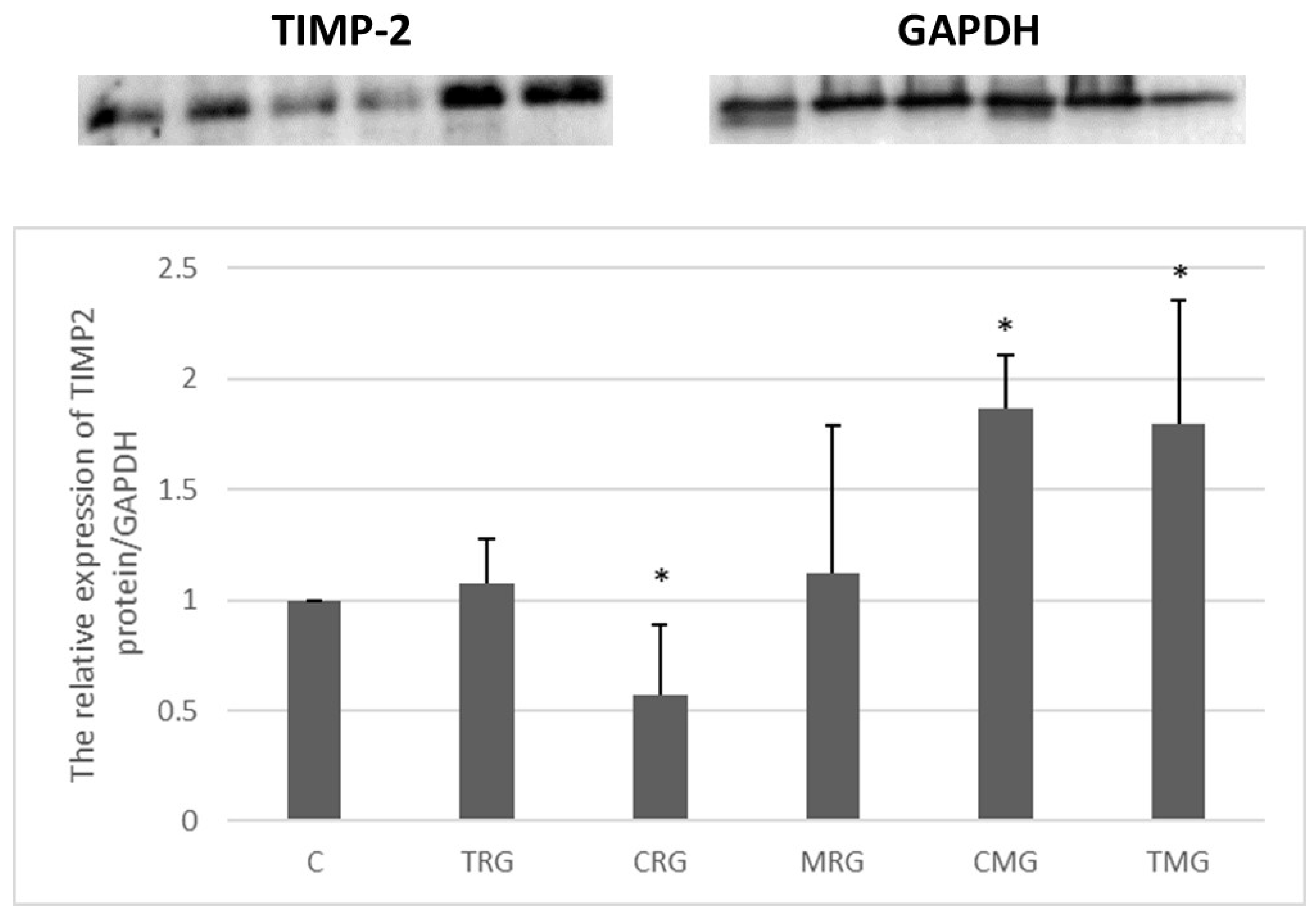
2.2.4. TIMP-2 Expression (Western Blot)
3. Discussion
4. Material and Methods
4.1. Animals and Protocol
4.2. Histological Analysis
4.3. Quantitative Analysis of Cardiomyocyte Diameter and Mallory Trichrome Staining
4.4. Homogenization of Samples and Determination of Protein Concentration
4.5. Activities of MMP-2 and MMP-9
4.6. Expressions of TIMP-1 and TIMP-2
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartono, C.; Muthukumar, T.; Suthanthiran, M. Immunosuppressive Drug Therapy. Cold Spring Harb. Perspect Med. 2013, 3, a015487. [Google Scholar] [CrossRef] [PubMed]
- Meneghini, M.; Bestard, O.; Grinyo, J.M. Immunosuppressive drugs modes of action. Best Pract. Res. Clin. Gastroenterol. 2021, 54–55, 101757. [Google Scholar] [CrossRef] [PubMed]
- Newland, D.M.; Nemeth, T.L. Induction and Standard Immunosuppression. In Solid Organ Transplantation in Infants and Children [Internet]; Organ and Tissue Transplantation; Dunn, S.P., Horslen, S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 149–182. [Google Scholar] [CrossRef]
- Chang, D.H.; Youn, J.-C.; Dilibero, D.; Patel, J.K.; Kobashigawa, J.A. Heart Transplant Immunosuppression Strategies at Cedars-Sinai Medical Center. Int. J. Heart Fail. 2020, 3, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Arnol, M.; Naumovic, R.; Dimitrov, E.P.; Racki, S.; Bucsa, C.A.; Covic, A.; Mitic, I.; Vavic, N.; Radovanovic, R.M.V.; Zibar, L.; et al. Immunosuppressive regimens following kidney transplantation in five European countries: The observational RECORD study. Transplant. Rep. 2020, 5, 100061. [Google Scholar] [CrossRef]
- Neuwirt, H.; Rudnicki, M.; Schratzberger, P.; Pirklbauer, M.; Kronbichler, A.; Mayer, G. Immunosuppression after renal transplantation. Memo—Mag. Eur. Med. Oncol. 2019, 12, 216–221. [Google Scholar] [CrossRef]
- Surówka, A.; Prowans, P.; Żołnierczuk, M.; Miśkiewicz, M.; Wawrowski, T.; Skodda, M.; Markowska, M.; Kędzierska-Kapuza, K. The Effect of Calcineurin Inhibitors on MMPs Activity in Heart and Their Side Effects—A Review of Literature. Int. J. Mol. Sci. 2023, 24, 10291. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Y.; Khan, H. Immunosuppressive Drugs. Encycl. Infect. Immun. 2022, 726–740. [Google Scholar] [CrossRef]
- Wijdicks, E.F. Neurotoxicity of immunosuppressive drugs. Liver Transpl. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transpl. Soc. 2001, 7, 937–942. [Google Scholar] [CrossRef]
- Tran-Minh, M.-L.; Sousa, P.; Maillet, M.; Allez, M.; Gornet, J.-M. Hepatic complications induced by immunosuppressants and biologics in inflammatory bowel disease. World J. Hepatol. 2017, 9, 613–626. [Google Scholar] [CrossRef]
- De Mattos, A.M.; Olyaei, A.J.; Bennett, W.M. Nephrotoxicity of immunosuppressive drugs: Long-term consequences and challenges for the future. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2000, 35, 333–346. [Google Scholar] [CrossRef]
- Vial, T.; Descotes, J. Immunosuppressive drugs and cancer. Toxicology 2003, 185, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.A. Opportunistic Infections—Coming to the Limits of Immunosuppression? Cold Spring Harb. Perspect Med. 2013, 3, a015669. [Google Scholar] [CrossRef]
- Sertić, Z.; Letilović, T.; Kanižaj, T.F.; Knotek, M.; Hadžibegović, I.; Starovečki, I.; Jerkić, H. Cardiovascular mortality in liver and kidney transplant recipients. Medicine 2021, 100, e26019. [Google Scholar] [CrossRef]
- Subbiah, A.K.; Chhabra, Y.K.; Mahajan, S. Cardiovascular disease in patients with chronic kidney disease: A neglected subgroup. Heart Asia 2016, 8, 56–61. [Google Scholar] [CrossRef]
- Miller, L.W. Cardiovascular toxicities of immunosuppressive agents. Am. J. Transpl. Off. J. Am. Soc. Transpl. Am. Soc. Transpl. Surg. 2002, 2, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Opałka, B.; Żołnierczuk, M.; Grabowska, M. Immunosuppressive Agents—Effects on the Cardiovascular System and Selected Metabolic Aspects: A Review. J. Clin. Med. 2023, 12, 6935. [Google Scholar] [CrossRef]
- Euler, G.; Locquet, F.; Kociszewska, J.; Osygus, Y.; Heger, J.; Schreckenberg, R.; Schlüter, K.-D.; Kenyeres, É.; Szabados, T.; Bencsik, P.; et al. Matrix Metalloproteinases Repress Hypertrophic Growth in Cardiac Myocytes. Cardiovasc. Drugs Ther. 2021, 35, 353–365. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; la Rosa, C.C.-D.; Ramirez-Acuña, J.M.; Perez-Romero, A.B.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Nikolov, A.; Popovski, N. Role of Gelatinases MMP-2 and MMP-9 in Healthy and Complicated Pregnancy and Their Future Potential as Preeclampsia Biomarkers. Diagnostics 2021, 11, 480. [Google Scholar] [CrossRef]
- Gonçalves, P.R.; Nascimento, L.D.; Gerlach, R.F.; Rodrigues, K.E.; Prado, A.F. Matrix Metalloproteinase 2 as a Pharmacological Target in Heart Failure. Pharmaceuticals 2022, 15, 920. [Google Scholar] [CrossRef]
- Yabluchanskiy, A.; Ma, Y.; Iyer, R.P.; Hall, M.E.; Lindsey, M.L. Matrix Metalloproteinase-9: Many Shades of Function in Cardiovascular Disease. Physiology 2013, 28, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.F.; Agha, A.M.; Dorbala, P.; Claggett, B.L.; Yu, B.; Hussain, A.; Nambi, V.; Chen, L.Y.; Matsushita, K.; Hoogeveen, R.C.; et al. MMP-2 Associates with Incident Heart Failure and Atrial Fibrillation: The ARIC Study. Circ. Heart Fail. 2023, 16, e010849. [Google Scholar] [CrossRef] [PubMed]
- Ezhov, M.; Safarova, M.; Afanasieva, O.; Mitroshkin, M.; Matchin, Y.; Pokrovsky, S. Matrix Metalloproteinase 9 as a Predictor of Coronary Atherosclerotic Plaque Instability in Stable Coronary Heart Disease Patients with Elevated Lipoprotein(a) Levels. Biomolecules 2019, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Hirohata, S.; Kusachi, S.; Murakami, M.; Murakami, T.; Sano, I.; Watanabe, T.; Komatsubara, I.; Kondo, J.; Tsuji, T. Time dependent alterations of serum matrix metalloproteinase-1 and metalloproteinase-1 tissue inhibitor after successful reperfusion of acute myocardial infarction. Heart 1997, 78, 278–284. [Google Scholar] [CrossRef]
- Blankenberg, S.; Rupprecht, H.J.; Poirier, O.; Bickel, C.; Smieja, M.; Hafner, G.; Meyer, J.; Cambien, F.; Tiret, L.; AtheroGene Investigators. Plasma Concentrations and Genetic Variation of Matrix Metalloproteinase 9 and Prognosis of Patients with Cardiovascular Disease. Circulation 2003, 107, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Kai, H.; Ikeda, H.; Yasukawa, H.; Kai, M.; Seki, Y.; Kuwahara, F.; Ueno, T.; Sugi, K.; Imaizumi, T. Peripheral blood levels of matrix metalloproteases-2 and -9 are elevated in patients with acute coronary syndromes. J. Am. Coll. Cardiol. 1998, 32, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.-Y.; Sawicki, G.; Wozniak, M.; Wang, W.; Radomski, M.W.; Schulz, R. Matrix Metalloproteinase-2 Contributes to Ischemia-Reperfusion Injury in the Heart. Circulation 2000, 101, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Niida, S.; Dote, K.; Takenaka, S.; Hirao, H.; Miura, F.; Ishida, M.; Shingu, T.; Sueda, T.; Yoshizumi, M.; et al. Matrix metalloproteinase-9 contributes to human atrial remodeling during atrial fibrillation. J. Am. Coll. Cardiol. 2004, 43, 818–825. [Google Scholar] [CrossRef]
- Huxley, R.R.; Lopez, F.L.; MacLehose, R.F.; Eckfeldt, J.H.; Couper, D.; Leiendecker-Foster, C.; Hoogeveen, R.C.; Chen, L.Y.; Soliman, E.Z.; Agarwal, S.K.; et al. Novel Association between Plasma Matrix Metalloproteinase-9 and Risk of Incident Atrial Fibrillation in a Case-Cohort Study: The Atherosclerosis Risk in Communities Study. PLoS ONE 2013, 8, e59052. [Google Scholar] [CrossRef]
- Thuny, F.; Habib, G.; Le Dolley, Y.; Canault, M.; Casalta, J.-P.; Verdier, M.; Avierinos, J.-F.; Raoult, D.; Mege, J.-L.; Morange, P.-E.; et al. Circulating Matrix Metalloproteinases in Infective Endocarditis: A Possible Marker of the Embolic Risk. PLoS ONE 2011, 6, e18830. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Park, I.-K.; Kohyama, K. Matrix Metalloproteinase (MMP)-9, but Not MMP-2, Is Involved in the Development and Progression of C Protein-Induced Myocarditis and Subsequent Dilated Cardiomyopathy. J. Immunol. 2009, 183, 4773–4781. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Patal, S.; Wexler, D.; Roth, A.; Sheps, D.; Keren, G. Circulating matrix metalloproteinase-2 but not matrix metalloproteinase-3, matrix metalloproteinase-9, or tissue inhibitor of metalloproteinase-1 predicts outcome in patients with congestive heart failure. Am. Heart J. 2005, 150, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Jordán, A.; Roldán, V.; García, M.; Monmeneu, J.; De Burgos, F.G.; Lip, G.Y.H.; Marín, F. Matrix metalloproteinase-1 and its inhibitor, TIMP-1, in systolic heart failure: Relation to functional data and prognosis. J. Intern. Med. 2007, 262, 385–392. [Google Scholar] [CrossRef]
- Cheung, C.; Luo, H.; Yanagawa, B.; Leong, H.S.; Samarasekera, D.; Lai, J.C.; Suarez, A.; Zhang, J.; McManus, B.M. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in coxsackievirus-induced myocarditis. Cardiovasc. Pathol. 2006, 15, 63–74. [Google Scholar] [CrossRef]
- Kobusiak-Prokopowicz, M.; Kaaz, K.; Marciniak, D.; Karolko, B.; Mysiak, A. Relationships between Circulating Matrix Metalloproteinases, Tissue Inhibitor TIMP-2, and Renal Function in Patients with Myocarditis. Kidney Blood Press. Res. 2021, 46, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Patrichi, G.; Patrichi, A.; Satala, C.-B.; Sin, A.I. Matrix Metalloproteinases and Heart Transplantation—A Pathophysiological and Clinical View. Medicina 2023, 59, 1295. [Google Scholar] [CrossRef] [PubMed]
- Najafi, F.; Jamrozik, K.; Dobson, A.J. Understanding the ‘epidemic of heart failure’: A systematic review of trends in determinants of heart failure. Eur. J. Heart Fail. 2009, 11, 472–479. [Google Scholar] [CrossRef]
- Poulter, N. Global risk of cardiovascular disease. Heart 2003, 89, ii2–ii5. [Google Scholar] [CrossRef]
- Wakatsuki, T.; Schlessinger, J.; Elson, E.L. The biochemical response of the heart to hypertension and exercise. Trends Biochem. Sci. 2004, 29, 609–617. [Google Scholar] [CrossRef]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Elezaby, A.; Dexheimer, R.; Sallam, K. Cardiovascular effects of immunosuppression agents. Front. Cardiovasc. Med. 2022, 9, 981838. [Google Scholar] [CrossRef] [PubMed]
- Schäfers, M.; Schober, O.; Hermann, S. Matrix-Metalloproteinases as Imaging Targets for Inflammatory Activity in Atherosclerotic Plaques. J. Nucl. Med.Off. Publ. Soc. Nucl. Med. 2010, 51, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Woessner, J.F. Matrix Metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef] [PubMed]
- Järveläinen, H.; Sainio, A.; Koulu, M.; Wight, T.N.; Penttinen, R. Extracellular Matrix Molecules: Potential Targets in Pharmacotherapy. Pharmacol. Rev. 2009, 61, 198–223. [Google Scholar] [CrossRef]
- Al-Roub, A.; Akhter, N.; Al-Rashed, F.; Wilson, A.; Alzaid, F.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. TNFα induces matrix metalloproteinase-9 expression in monocytic cells through ACSL1/JNK/ERK/NF-kB signaling pathways. Sci Rep. 2023, 13, 14351. [Google Scholar] [CrossRef]
- DeCoux, A.; Lindsey, M.L.; Villarreal, F.; Garcia, R.A.; Schulz, R. Myocardial matrix metalloproteinase-2: Inside out and upside down. J. Mol. Cell. Cardiol. 2014, 77, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.-M.; Chen, Y.-S.; Harn, H.-J. The Versatile Role of Matrix Metalloproteinase for the Diverse Results of Fibrosis Treatment. Molecules 2019, 24, 4188. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Cook, L.G.; Hamilton, S.L.; Wu, G.-Y.; Mitchell, B.M. FK506 Binding Protein 12/12.6 Depletion Increases Endothelial Nitric Oxide Synthase Threonine 495 Phosphorylation and Blood Pressure. Hypertension 2007, 49, 569–576. [Google Scholar] [CrossRef]
- Bianchi, R.; Rodella, L.; Rezzani, R. Cyclosporine A up-regulates expression of matrix metalloproteinase 2 and vascular endothelial growth factor in rat heart. Int. Immunopharmacol. 2003, 3, 427–433. [Google Scholar] [CrossRef]
- Janicki, J.S.; Brower, G.L.; Gardner, J.D.; Chancey, A.L.; Stewart, J.J.A. The Dynamic Interaction Between Matrix Metalloproteinase Activity and Adverse Myocardial Remodeling. Heart Fail. Rev. 2004, 9, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Berthier, C.; Pally, C.; Weckbecker, G.; Raulf, F.; Rehrauer, H.; Wagner, U.; Le Hir, M.; Marti, H.P. Experimental heart transplantation: Effect of cyclosporine on expression and activity of metzincins. Swiss Med. Wkly. 2009, 139, 233. [Google Scholar] [CrossRef] [PubMed]
- Kobashigawa, J.A.; Miller, L.W.; Russell, S.D.; Ewald, G.A.; Zucker, M.J.; Goldberg, L.R.; Eisen, H.J.; Salm, K.; Tolzman, D.; Gao, J.; et al. Tacrolimus with Mycophenolate Mofetil (MMF) or Sirolimus vs. Cyclosporine with MMF in Cardiac Transplant Patients: 1-Year Report. Am. J. Transplant. 2006, 6, 1377–1386. [Google Scholar] [CrossRef]
- Brook, N.R.; Waller, J.R.; Bicknell, G.R.; Nicholson, M.L. Cyclosporine and rapamycin act in a synergistic and dose-dependent manner in a model of immunosuppressant-induced kidney damage. Transplant. Proc. 2005, 37, 837–838. [Google Scholar] [CrossRef] [PubMed]



| Disease Entity | MMP | Effect | Reference |
|---|---|---|---|
| Myocardial infarction | MMP-1 TIMP-1 | Increased serum MMP1 and TIMP-1 levels in post-MI patients undergoing coronary artery reperfusion | [26] |
| MMP-9 | Increased serum MMP-9 levels as a predictive factor in patients with coronary artery disease | [27] | |
| MMP-2 MMP-9 | Increased serum levels of MMP-2 and MMP-9 in patients with myocardial ischemia | [28] | |
| MMP-2 | Increased tissue expression of MMP-2 in rat hearts after reperfusion following myocardial ischemia | [29] | |
| Atrial fibrillation | MMP-9 | Increased tissue expression of the active form of MMP-9 in patients with atrial fibrillation compared with controls without a history of AF | [30] |
| MMP-9 | Increased serum levels of the active form of MMP-9 in patients with atrial fibrillation compared with controls without a history of AF | [31] | |
| Endocarditis | MMP-9 | Increased serum MMP-9 levels in patients with an embolic episode in infective endocarditis | [32] |
| Cardiomyopathy | MMP-2 MMP-9 | Increased tissue expressions of MMP-2 and MMP-9 in hearts of rats with inflammation and fibrosis compared to non-diseased hearts of healthy rats | [33] |
| Heart failure | MMP-2 MMP-9 TIMP-1 | Increased serum levels of MMP-2 and MMP-9 and TIMP-1 in patients with heart failure compared to controls | [34] |
| MMP-1 TIMP-1 | Higher serum TIMP-1 and lower serum MMP-1 levels in patients with heart failure; TIMP-1 levels and the TIMP-1/MMP-1 ratio correlate negatively with peak VO2 | [35] | |
| Myocarditis | MMP-2 MMP-9 | Increased tissue expressions of MMP-2 and MMP-9 in Coxsackie B3 virus-induced myocarditis in mice | [36] |
| MMP-2 | Negative correlation of cardiac ejection fraction and serum MMP-2 levels in patients with myocarditis | [37] |
| Drug Name | Dose | Trade Name |
|---|---|---|
| Cyclosporin A | 5 mg/kg/day | Sandimmun-Neoral |
| Tacrolimus | 4 mg/kg/day | Prograf |
| Mycophenolate mofetil | 20 mg/kg/day | Cellcept |
| Rapamycin | 0.5 mg/kg/day | Rapamune |
| Prednisone | 4 mg/kg/day | Encorton |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surówka, A.; Żołnierczuk, M.; Prowans, P.; Grabowska, M.; Kupnicka, P.; Markowska, M.; Olejnik-Wojciechowska, J.; Szlosser, Z.; Wilk, A.; Szumilas, K.; et al. The Effects of Chronic Immunosuppressive Treatment on Morphological Changes in Cardiac Tissue and the Balance between Matrix Metalloproteinases (MMP-2 and MMP-9) and Their Inhibitors in the Rat Heart. Int. J. Mol. Sci. 2024, 25, 4468. https://doi.org/10.3390/ijms25084468
Surówka A, Żołnierczuk M, Prowans P, Grabowska M, Kupnicka P, Markowska M, Olejnik-Wojciechowska J, Szlosser Z, Wilk A, Szumilas K, et al. The Effects of Chronic Immunosuppressive Treatment on Morphological Changes in Cardiac Tissue and the Balance between Matrix Metalloproteinases (MMP-2 and MMP-9) and Their Inhibitors in the Rat Heart. International Journal of Molecular Sciences. 2024; 25(8):4468. https://doi.org/10.3390/ijms25084468
Chicago/Turabian StyleSurówka, Anna, Michał Żołnierczuk, Piotr Prowans, Marta Grabowska, Patrycja Kupnicka, Marta Markowska, Joanna Olejnik-Wojciechowska, Zbigniew Szlosser, Aleksandra Wilk, Kamila Szumilas, and et al. 2024. "The Effects of Chronic Immunosuppressive Treatment on Morphological Changes in Cardiac Tissue and the Balance between Matrix Metalloproteinases (MMP-2 and MMP-9) and Their Inhibitors in the Rat Heart" International Journal of Molecular Sciences 25, no. 8: 4468. https://doi.org/10.3390/ijms25084468






