An Antimicrobial Copper–Plastic Composite Coating: Characterization and In Situ Study in a Hospital Environment
Abstract
:1. Introduction
2. Results and Discussion
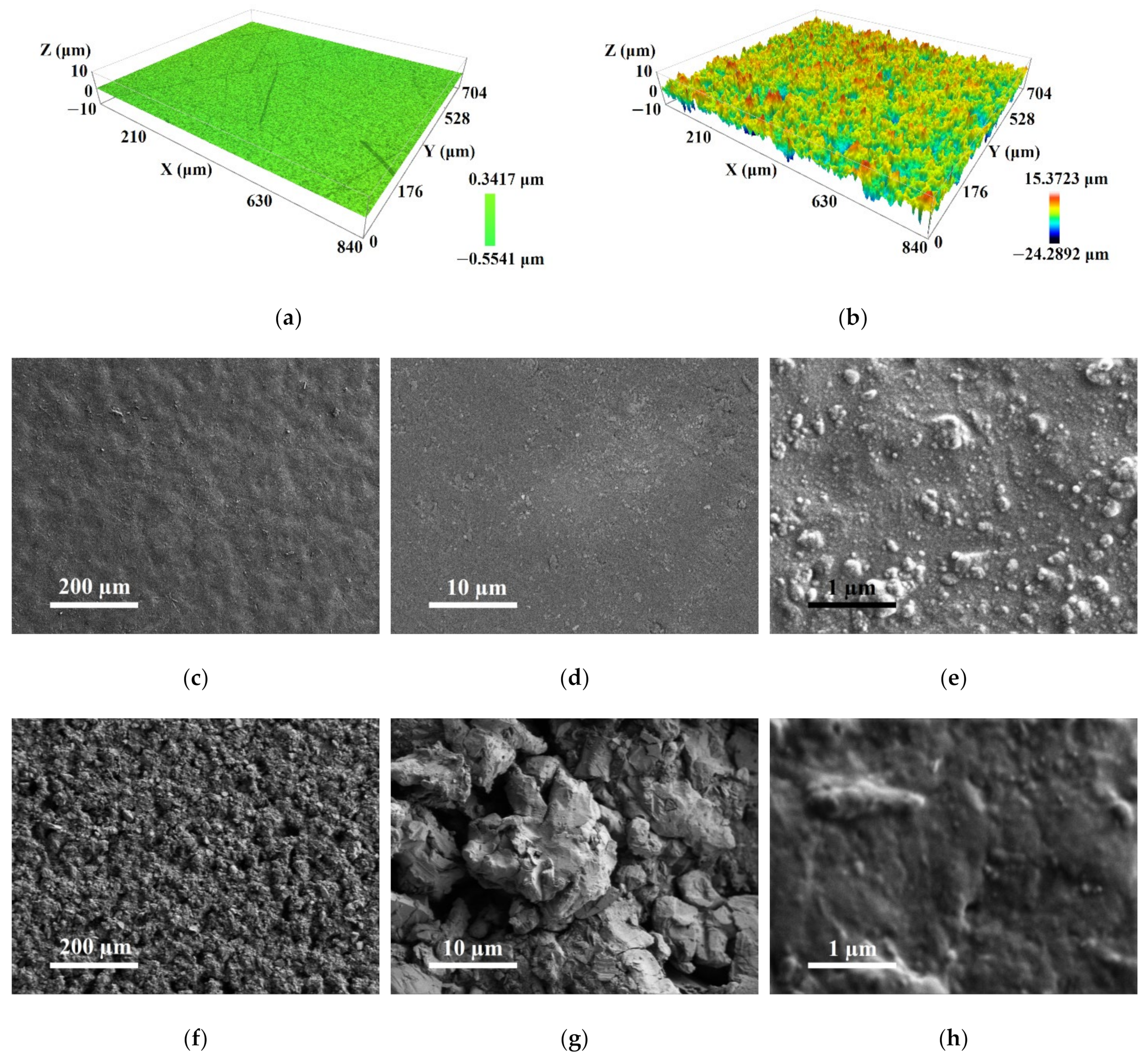
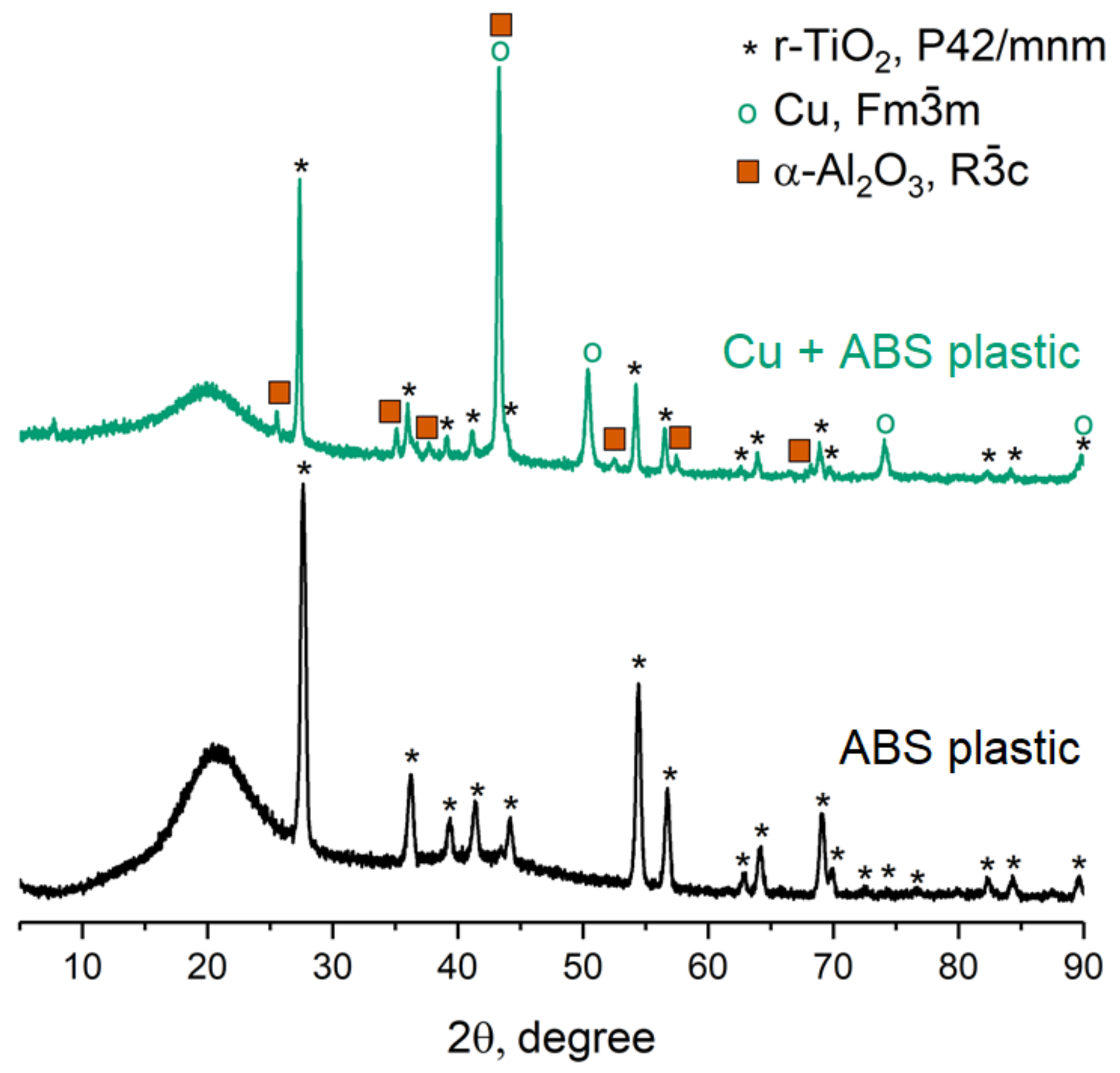
2.1. Physico-Chemical Properties of the Materials under Study
2.2. Study of Bactericidal Properties
3. Materials and Methods
3.1. Objectives and General Design of the Study
3.2. Materials
3.3. Preparation of the Copper Coatings
3.4. Characterization of the Coatings
3.5. Collection of Swabs
3.6. Assessment of the Test Surface Contamination and Identification of the Contaminants
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenthal, V.D.; Yin, R.; Lu, Y.; Rodrigues, C.; Myatra, S.N.; Kharbanda, M.; Valderrama-Beltran, S.L.; Mehta, Y.; Daboor, M.A.; Todi, S.K.; et al. The Impact of Healthcare-Associated Infections on Mortality in ICU: A Prospective Study in Asia, Africa, Eastern Europe, Latin America, and the Middle East. Am. J. Infect. Control 2023, 51, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Gidey, K.; Gidey, M.T.; Hailu, B.Y.; Gebreamlak, Z.B.; Niriayo, Y.L. Clinical and Economic Burden of Healthcare-Associated Infections: A Prospective Cohort Study. PLoS ONE 2023, 18, e0282141. [Google Scholar] [CrossRef]
- Shulakova, N.I.; Tutelyan, A.V.; Maleev, V.V.; Akimkin, V.G. Risks of HAIs: Problems and Pitfalls. Health Risk Anal. 2023, 2023, 104–114. [Google Scholar] [CrossRef]
- Zimlichman, E.; Henderson, D.; Tamir, O.; Franz, C.; Song, P.; Yamin, C.K.; Keohane, C.; Denham, C.R.; Bates, D.W. Health Care–Associated Infections: A Meta-Analysis of Costs and Financial Impact on the US Health Care System. JAMA Intern. Med. 2013, 173, 2039–2046. [Google Scholar] [CrossRef]
- Skachkova, T.S.; Zamyatin, M.N.; Orlova, O.A.; Yumtsunova, N.A.; Lashenkova, N.N.; Fomina, V.S.; Gusarov, V.G.; Mikhaylova, Y.V.; Shelenkov, A.A.; Goloveshkina, E.N.; et al. Monitoring Methicillin-Resistant Staphylococcus Strains in the Moscow Medical and Surgical Center Using Molecular-Biological Methods. Epidemiol. Vaccinal Prev. 2021, 20, 44–50. [Google Scholar] [CrossRef]
- Pelfrene, E.; Botgros, R.; Cavaleri, M. Antimicrobial Multidrug Resistance in the Era of COVID-19: A Forgotten Plight? Antimicrob. Resist. Infect. Control 2021, 10, 21. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug Resistance (MDR): A Widespread Phenomenon in Pharmacological Therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef]
- Saha, M.; Sarkar, A. Review on Multiple Facets of Drug Resistance: A Rising Challenge in the 21st Century. J. Xenobiot. 2021, 11, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Cruz-López, F.; Martínez-Meléndez, A.; Garza-González, E. How Does Hospital Microbiota Contribute to Healthcare-Associated Infections? Microorganisms 2023, 11, 192. [Google Scholar] [CrossRef]
- Khan, I.D.; Basu, A.; Kiran, S.; Trivedi, S.; Pandit, P.; Chattoraj, A. Device-Associated Healthcare-Associated Infections (DA-HAI) and the Caveat of Multiresistance in a Multidisciplinary Intensive Care Unit. Med. J. Armed Forces India 2017, 73, 222–231. [Google Scholar] [CrossRef]
- Mateescu, M.C.; Grigorescu, S.; Socea, B.; Bloanca, V.; Grigorescu, O.D. Contribution to the Personalized Management of the Nosocomial Infections: A New Paradigm Regarding the Influence of the Community Microbial Environment on the Incidence of the Healthcare-Associated Infections (HAI) in Emergency Hospital Surgical Departments. J. Pers. Med. 2023, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Pereira da Fonseca, T.A.; Pessôa, R.; Felix, A.C.; Sanabani, S.S. Diversity of Bacterial Communities on Four Frequently Used Surfaces in a Large Brazilian Teaching Hospital. Int. J. Environ. Res. Public Health 2016, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.S.; Pachpute, S.R. Surveillance of Bacterial Carriage in the Nose and Hands of Healthcare Workers and Patients Attending Maternity and Children’s Hospital. J. Fam. Med. Prim. Care 2023, 12, 3262–3265. [Google Scholar] [CrossRef]
- Zhen, X.; Lundborg, C.S.; Sun, X.; Hu, X.; Dong, H. Economic Burden of Antibiotic Resistance in ESKAPE Organisms: A Systematic Review. Antimicrob. Resist. Infect. Control 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Yong, L.X.; Calautit, J.K. A Comprehensive Review on the Integration of Antimicrobial Technologies onto Various Surfaces of the Built Environment. Sustainability 2023, 15, 3394. [Google Scholar] [CrossRef]
- Siegel, J.; Vyhnálková, B.; Savenkova, T.; Pryjmaková, J.; Slepička, P.; Šlouf, M.; Hubáček, T. Surface Engineering of AgNPs-Decorated Polyetheretherketone. Int. J. Mol. Sci. 2023, 24, 1432. [Google Scholar] [CrossRef] [PubMed]
- Boinovich, L.; Kaminsky, V.; Domantovsky, A.; Emelyanenko, K.; Aleshkin, A.; Zulkarneev, E.; Kiseleva, I.; Emelyanenko, A. Bactericidal Activity of Superhydrophobic and Superhydrophilic Copper in Bacterial Dispersions. Langmuir 2019, 35, 2832–2841. [Google Scholar] [CrossRef] [PubMed]
- Emelyanenko, A.M.; Pytskii, I.S.; Kaminsky, V.V.; Chulkova, E.V.; Domantovsky, A.G.; Emelyanenko, K.A.; Sobolev, V.D.; Aleshkin, A.V.; Boinovich, L.B. Superhydrophobic Copper in Biological Liquids: Antibacterial Activity and Microbiologically Induced or Inhibited Corrosion. Colloids Surf. B 2020, 185, 110622. [Google Scholar] [CrossRef]
- Baranwal, A.; Srivastava, A.; Kumar, P.; Bajpai, V.K.; Maurya, P.K.; Chandra, P. Prospects of Nanostructure Materials and their Composites as Antimicrobial Agents. Front. Microbiol. 2018, 9, 422. [Google Scholar] [CrossRef]
- Makvandi, P.; Gu, J.T.; Zare, E.N.; Ashtari, B.; Moeini, A.; Tay, F.R.; Niu, L. Polymeric and Inorganic Nanoscopical Antimicrobial Fillers in Dentistry. Acta Biomater. 2020, 101, 69–101. [Google Scholar] [CrossRef]
- Nazarov, D.; Kozlova, L.; Rogacheva, E.; Kraeva, L.; Maximov, M. Atomic Layer Deposition of Antibacterial Nanocoatings: A Review. Antibiotics 2023, 12, 1656. [Google Scholar] [CrossRef] [PubMed]
- Bondareva, N.E.; Sheremet, A.B.; Morgunova, E.Y.; Khisaeva, I.R.; Parfenova, A.S.; Chernukha, M.Y.; Omran, F.S.; Emelyanenko, A.M.; Boinovich, L.B. Study of the Antibacterial Activity of Superhydrophilic and Superhydrophobic Copper Substrates against Multi-Drug Resistant Hospital-Acquired Pseudomonas aeruginosa Isolates. Int. J. Mol. Sci. 2024, 25, 779. [Google Scholar] [CrossRef] [PubMed]
- Linklater, D.P.; Mah, S.W.L.; Tzanov, V.; Baulin, V.; Borg, N.A.; Moad, G.; Simons, R.; O’Connor, A.J.; Ivanova, E.P. Current Perspectives on the Development of Virucidal Nano Surfaces. Curr. Opin. Colloid Interface Sci. 2023, 67, 101720. [Google Scholar] [CrossRef]
- Muller, M.P.; Macdougall, C.; Lim, M.; Armstrong, I.; Bialachowski, A.; Callery, S.; Ciccotelli, W.; Cividino, M.; Dennis, J.; Hota, S.; et al. Antimicrobial Surfaces to Prevent Healthcare-Associated Infections: A Systematic Review. J. Hosp. Infect. 2016, 92, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Karpanen, T.J.; Casey, A.L.; Lambert, P.A.; Cookson, B.D.; Nightingale, P.; Miruszenko, L.; Elliott, T.S.J. The Antimicrobial Efficacy of Copper Alloy Furnishing in the Clinical Environment: A Crossover Study. Infect. Control Hosp. Epidemiol. 2012, 33, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Palza, H.; Nuñez, M.; Bastías, R.; Delgado, K. In situ Antimicrobial Behavior of Materials with Copper-Based Additives in a Hospital Environment. Int. J. Antimicrob. Agents 2018, 51, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Sifri, C.D.; Burke, G.H.; Enfield, K.B. Reduced Health Care-Associated Infections in an Acute Care Community Hospital Using a Combination of Self-Disinfecting Copper-Impregnated Composite Hard Surfaces and Linens. Am. J. Infect. Control 2016, 44, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Kalb, L.; Bäßler, P.; Schneider-Brachert, W.; Eckl, D.B. Antimicrobial Photodynamic Coatings Reduce the Microbial Burden on Environmental Surfaces in Public Transportation—A Field Study in Buses. Int. J. Environ. Res. Public Health 2022, 19, 2325. [Google Scholar] [CrossRef]
- Mbhele, Z.N.; Shobo, C.O.; Amoako, D.G.; Zishiri, O.T.; Bester, L.A. Occurrence, Antibiotic Resistance, Virulence Factors, and Genetic Diversity of Bacillus spp. from Public Hospital Environments in South Africa. Microb. Drug Resist. 2021, 27, 1692–1704. [Google Scholar] [CrossRef]
- Dollwet, H.H.A.; Sorenson, J.R.J. Historic uses of copper compounds in medicine. Trace Elem. Med. 1985, 2, 80–87. [Google Scholar]
- Bataglioli, R.A.; Rocha Neto, J.B.M.; Calais, G.B.; Lopes, L.M.; Tsukamoto, J.; de Moraes, A.P.; Arns, C.W.; Beppu, M.M. Hybrid Alginate–Copper Sulfate Textile Coating for Coronavirus Inactivation. J. Am. Ceram. Soc. 2021, 105, 1748–1752. [Google Scholar] [CrossRef]
- Mu, L.; Rutkowski, S.; Gai, M.; Tverdokhlebov, S.I.; Frueh, J. Copper Alginate Surface for Perpetual Self-Polishing and Anti-Biofouling Compound Release. Appl. Surf. Sci. 2021, 569, 151087. [Google Scholar] [CrossRef]
- Ramos-Zúñiga, J.; Bruna, N.; Pérez-Donoso, J.M. Toxicity Mechanisms of Copper Nanoparticles and Copper Surfaces on Bacterial Cells and Viruses. Int. J. Mol. Sci. 2023, 24, 10503. [Google Scholar] [CrossRef] [PubMed]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds: Tables of Spectral Data; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Alassali, A.; Picuno, C.; Bébien, T.; Fiore, S.; Kuchta, K. Validation of Near Infrared Spectroscopy as an Age-Prediction Method for Plastics. Resour. Conserv. Recycl. 2020, 154, 104555. [Google Scholar] [CrossRef]
- Shimada, J.; Kabuki, K. The Mechanism of Oxidative Degradation of ABS Resin. Part I. The Mechanism of Thermooxidative Degradation. J. Appl. Polym. Sci. 1968, 12, 655–669. [Google Scholar] [CrossRef]
- Ceretti, D.V.A.; Edeleva, M.; Cardon, L.; D’hooge, D.R. Molecular Pathways for Polymer Degradation During Conventional Processing, Additive Manufacturing, and Mechanical Recycling. Molecules 2023, 28, 2344. [Google Scholar] [CrossRef] [PubMed]
- Kent, T. Water Content of Latent Fingerprints—Dispelling the Myth. Forensic Sci. Int. 2016, 266, 134–138. [Google Scholar] [CrossRef]
- ISO 25178-2:2021; Geometrical Product Specifications (GPS)—Surface Texture: Areal—Part 2: Terms, Definitions and Surface Texture Parameters. ISO (International Organization for Standardization): Geneva, Switzerland, 2021.
- Georgieva, M.; Chakarova, V.; Petrova, M.; Lazarova, D.; Dobrev, D. Pre-Treatment of Dielectrics and Technological Process for Deposition of Chemical Copper Layers from Copper Solution with Improved Ecological Impact. Trans. IMF 2020, 98, 81–87. [Google Scholar] [CrossRef]
- Badaraev, A.D.; Lerner, M.I.; Bakina, O.V.; Sidelev, D.V.; Tran, T.H.; Krinitcyn, M.G.; Tverdokhlebov, S.I. Antibacterial Activity and Cytocompatibility of Electrospun PLGA Scaffolds Surface-Modified by Pulsed DC Magnetron Co-Sputtering of Copper and Titanium. Pharmaceutics 2023, 15, 939. [Google Scholar] [CrossRef]
- Abdidizaji, S.; Yalabadi, A.K.; Yazdani-Jahromi, M.; Garibay, O.O.; Garibay, I. Agent-Based Modeling of C. difficile Spread in Hospitals: Assessing Contribution of High-Touch vs. Low-Touch Surfaces and Inoculations’ Containment Impact. arXiv 2024, arXiv:2401.11656. [Google Scholar]
- Nygren, E.; Gonzales Strömberg, L.; Logenius, J.; Husmark, U.; Löfström, C.; Bergström, B. Potential Sources of Contamination on Textiles and Hard Surfaces Identified as High-Touch Sites Near the Patient Environment. PLoS ONE 2023, 18, e0287855. [Google Scholar] [CrossRef]
- Bromley, K.M.; Morris, R.J.; Hobley, L.; Brandani, G.; Gillespie, R.M.; McCluskey, M.; MacPhee, C.E. Interfacial Self-Assembly of a Bacterial Hydrophobin. Proc. Natl. Acad. Sci. USA 2015, 112, 5419–5424. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Schwebke, I.; Kampf, G. How Long Do Nosocomial Pathogens Persist on Inanimate Surfaces? A Systematic Review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef] [PubMed]
- Maguire, S.M.; McClimon, J.B.; Zhang, A.C.; Keller, A.W.; Bilchak, C.R.; Ohno, K.; Carpick, R.W.; Composto, R.J. Nanoscale Structure–Property Relations in Self-Regulated Polymer-Grafted Nanoparticle Composite Structures. ACS Appl. Mater. Interfaces 2023, 15, 10974–10985. [Google Scholar] [CrossRef] [PubMed]
- Albarqouni, L.; Byambasuren, O.; Clark, J.; Scott, A.M.; Looke, D.; Glasziou, P. Does Copper Treatment of Commonly Touched Surfaces Reduce Healthcare-Acquired Infections? A Systematic Review and Meta-Analysis. J. Hosp. Infect. 2020, 106, 765–773. [Google Scholar] [CrossRef]
- Sanitary Regulations and Norms (SanPiN) 2.1.3.2630-10 “Sanitary and Epidemiological Requirements for Organizations Engaged in Medical Activities” Tsentrmag, Moscow. 2021. Available online: https://www.centrmag.ru/catalog/product/sanpin_2_1_3_2630_10_sanitarno_epidemiologicheskie_trebovaniya_k_organizatsiyam_osushchestvlyayushch/ (accessed on 18 March 2024). (In Russian).
- Irissou, E.; Legoux, J.-G.; Ryabinin, A.N.; Jodoin, B.; Moreau, C. Review on Cold Spray Process and Technology: Part I—Intellectual Property. J. Therm. Spray Technol. 2008, 17, 495–516. [Google Scholar] [CrossRef]
- Emelyanenko, A.M.; Boinovich, L.B. Application of Dynamic Thresholding of Video Images for Measuring the Interfacial Tension of Liquids and Contact Angles. Instrum. Exp. Technol. 2002, 45, 44–49. [Google Scholar] [CrossRef]
- Chamani, S.; Mobasheri, L.; Rostami, Z.; Zare, I.; Naghizadeh, A.; Mostafavi, E. Heavy Metals in Contact Dermatitis: A Review. J. Trace Elem. Med. Biol. 2023, 79, 127240. [Google Scholar] [CrossRef]



| Sample | Contact Angle, ° | Surface Tension, mN/m | Droplet Image |
|---|---|---|---|
| Initial ABS plastic switch button | 85.6 ± 1.7 | 72.1 ± 0.3 |  |
| Plastic switch button with freshly sputtered copper coating | 149.8 ± 2.6 | 72.2 ± 1.4 |  |
| Plastic switch button with copper coating after exposure in hospital environment | 104.3 ± 4.0 | 66.5 ± 2.6 |  |
| Sample | Total Swabs | Growth Cases | Maximum Total Microbial Number (CFU/mL) | Average Total Microbial Number (CFU/mL) | |
|---|---|---|---|---|---|
| Absolute | Fraction (%) | ||||
| ABS plastic switch button | 89 | 56 | 63 | 149.8 ± 2.6 | 72.2 ± 1.4 |
| Copper-coated switch button | 89 | 21 | 24 | 104.3 ± 4.0 | 66.5 ± 2.6 |
| Microorganisms | Plastic Switch Button | Copper Coated Switch Button | ||
|---|---|---|---|---|
| Number of Cases | Fraction, % | Number of Cases | Fraction, % | |
| Staphylococcus epidermidis | 38 | 59.4 | 14 | 63.6 |
| Staphylococcus aureus | 8 | 12.5 | 3 | 13.6 |
| Staphylococcus hominis | 1 | 1.6 | 0.0 | |
| Corynebacterium spp. | 3 | 4.7 | 0.0 | |
| Micrococcus luteus | 6 | 9.4 | 1 | 4.5 |
| Bacillus spp. | 4 | 6.3 | 2 | 9.1 |
| Filamentous fungi | 1 | 1.6 | 0.0 | |
| Actinomyces oris | 0.0 | 1 | 4.5 | |
| Psychrobacillus psychrotolerans | 0.0 | 1 | 4.5 | |
| Aspergillus spp. | 2 | 3.1 | 0.0 | |
| Candida Krusei | 1 | 1.6 | 0.0 | |
| Total | 64 | 22 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emelyanenko, A.M.; Omran, F.S.; Teplonogova, M.A.; Chernukha, M.Y.; Avetisyan, L.R.; Tselikina, E.G.; Putsman, G.A.; Zyryanov, S.K.; Butranova, O.I.; Emelyanenko, K.A.; et al. An Antimicrobial Copper–Plastic Composite Coating: Characterization and In Situ Study in a Hospital Environment. Int. J. Mol. Sci. 2024, 25, 4471. https://doi.org/10.3390/ijms25084471
Emelyanenko AM, Omran FS, Teplonogova MA, Chernukha MY, Avetisyan LR, Tselikina EG, Putsman GA, Zyryanov SK, Butranova OI, Emelyanenko KA, et al. An Antimicrobial Copper–Plastic Composite Coating: Characterization and In Situ Study in a Hospital Environment. International Journal of Molecular Sciences. 2024; 25(8):4471. https://doi.org/10.3390/ijms25084471
Chicago/Turabian StyleEmelyanenko, Alexandre M., Fadi S. Omran, Maria A. Teplonogova, Marina Y. Chernukha, Lusine R. Avetisyan, Eugenia G. Tselikina, Gleb A. Putsman, Sergey K. Zyryanov, Olga I. Butranova, Kirill A. Emelyanenko, and et al. 2024. "An Antimicrobial Copper–Plastic Composite Coating: Characterization and In Situ Study in a Hospital Environment" International Journal of Molecular Sciences 25, no. 8: 4471. https://doi.org/10.3390/ijms25084471






