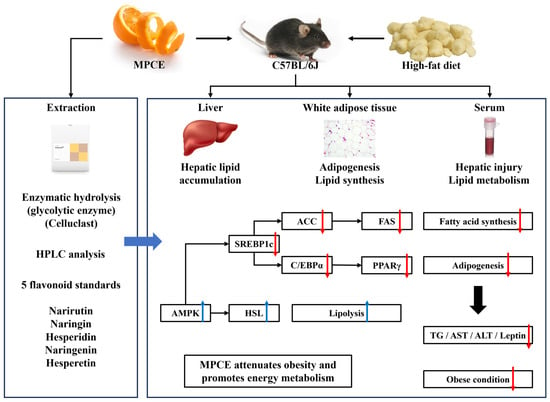
Narirutin-Rich Celluclast Extract from Mandarin (Citrus unshiu) Peel Alleviates High-Fat Diet-Induced Obesity and Promotes Energy Metabolism in C57BL/6 Mice
Abstract
:1. Introduction
2. Results
2.1. Flavonoid Composition of MPCE
2.2. Effects of MPCE on HFD-Induced Adiposity in Obese Mice
2.3. Effects of MPCE on HFD-Induced Serum Biological Parameters in Obese Mice
2.4. Effects of MPCE on Hepatic Lipid Accumulation and the Expression Levels of Lipid Metabolism-Related Proteins in HFD-Induced Obese Mice
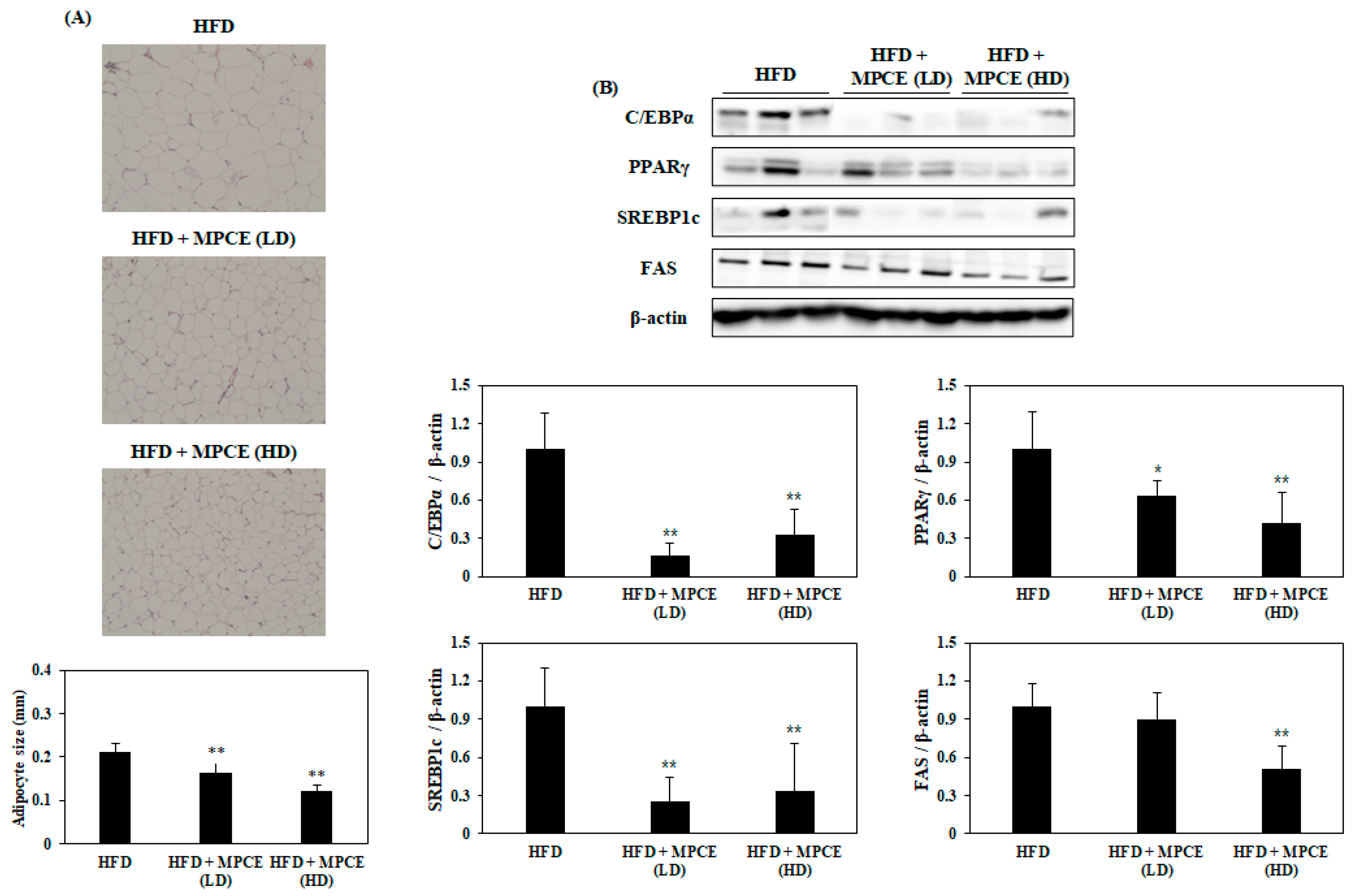
2.5. Effects of MPCE on HFD-Induced Adipogenesis and Lipogenesis in the WAT of Obese Mice
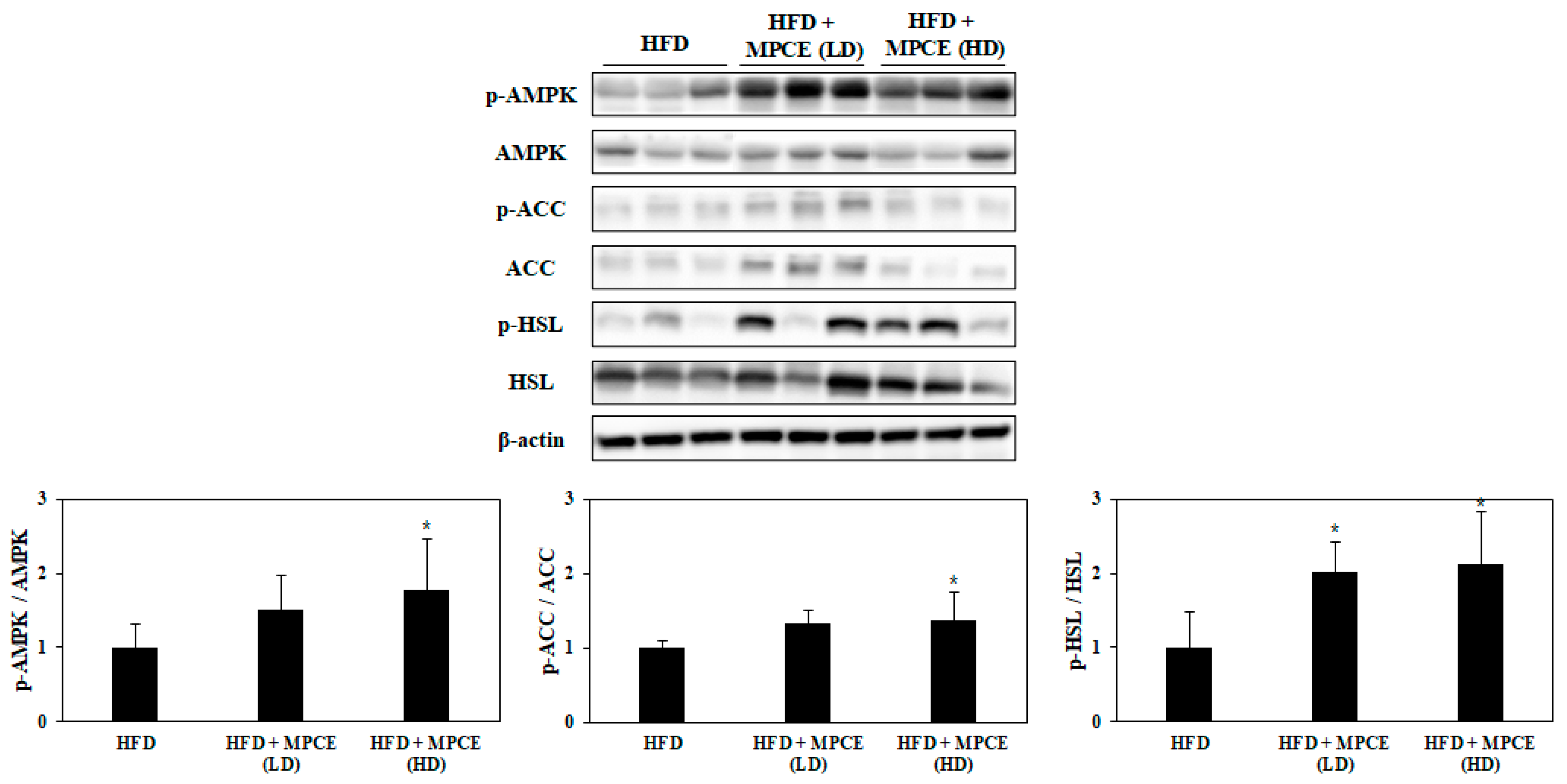
2.6. Effects of MPCE on HFD-Induced Lipolytic Metabolism in the WAT of Obese Mice
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Extraction and Chemical Composition Analysis of MPCE
4.3. Animal Experiments
4.4. Blood Parameter Analysis
4.5. Histological Analysis
4.6. Western Blot Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gadde, K.M.; Martin, C.K.; Berthoud, H.R.; Heymsfield, S.B. Obesity: Pathophysiology and management. J. Am. Coll. Cardiol. 2018, 71, 69–84. [Google Scholar] [CrossRef]
- Pi-Sunyer, F.X. The obesity epidemic: Pathophysiology and consequences of obesity. Obes. Res. 2002, 10 (Suppl. S2), 97S–104S. [Google Scholar] [CrossRef]
- Kang, M.C.; Kang, N.; Ko, S.C.; Kim, Y.B.; Jeon, Y.J. Anti-obesity effects of seaweeds of Jeju Island on the differentiation of 3T3-L1 preadipocytes and obese mice fed a high-fat diet. Food Chem. Toxicol. 2006, 90, 36–44. [Google Scholar] [CrossRef]
- Chandrasekaran, C.V.; Vijayalakshimi, M.A.; Prakash, K.; Bansal, V.S.; Meenakshi, J.; Amit, A. Review article: Herbal approach for obesity management. Am. J. Plant Sci. 2012, 3, 1003–1014. [Google Scholar] [CrossRef]
- Lee, M.R.; Kim, J.E.; Choi, J.Y.; Park, J.J.; Kim, H.R.; Song, B.R.; Choi, Y.W.; Kim, K.M.; Song, H.; Hwang, D.Y. Anti-obesity effect in high-fat-diet-induced obese C57BL/6 mice: Study of a novel extract from mulberry (Morus alba) leaves fermented with Cordyceps militaris. Exp. Ther. Med. 2019, 17, 2185–2193. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, L.; Im, S.T.; Hwang, O.; Kim, H.S.; Kang, M.C.; Lee, S.H. Anti-obesity effect of diphlorethohydroxycarmalol isolated from brown alga Ishige okamurae in high-fat diet-induced obese mice. Mar. Drugs 2019, 17, 637. [Google Scholar] [CrossRef]
- Nakajima, V.M.; Macedo, G.A.; Macedo, J.A. Citrus bioactive phenolics: Role in the obesity treatment. LWT-Food Sci. Technol. 2014, 59, 1205–1212. [Google Scholar] [CrossRef]
- Rafiq, S.; Kaul, R.; Sofi, S.A.; Bashir, N.; Nazir, F.; Nayik, G.A. Citrus peel as a source of functional ingredient: A review. J. Saudi Soc. Agric. Sci. 2018, 17, 351–358. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Landes, B.; Ramful-Baboolall, D.; Bourdon, E.; Neergheen-Bhujun, V.; Wagner, K.H.; Bahorun, T. Functional benefits of citrus fruits in the management of diabetes. Prev. Med. 2012, 54, S12–S16. [Google Scholar] [CrossRef]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant activity of citrus fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kar, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Liu, N.; Li, X.; Zhao, P.; Zhang, X.; Qiao, O.; Huang, L.; Guo, L. A review of chemical constituents and health-promoting effects of citrus peels. Food Chem. 2021, 365, 130585. [Google Scholar] [CrossRef]
- Mitra, S.; Lami, M.S.; Uddin, T.M.; Das, R.; Islam, F.; Anjum, J.; Hossain, J.; Emran, T.B. Prospective multifunctional roles and pharmacological potential of dietary flavonoid narirutin. Biomed. Pharmacother. 2022, 150, 112932. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Hassan, M.A.; Abdel-Twab, S.M.; Azeem, M.N.A. Navel orange peel hydroethanolic extract, naringin and naringenin have anti-diabetic potentials in type 2 diabetic rats. Biomed. Pharmacother. 2017, 94, 197–205. [Google Scholar] [CrossRef]
- Ha, S.K.; Park, H.Y.; Eom, H.; Kim, Y.; Choi, I. Narirutin fraction from citrus peels attenuates LPS-stimulated inflammatory response through inhibition of NF-κB and MAPKs activation. Food Chem. Toxicol. 2012, 50, 3498–3504. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, J.; Ran, Q.; Lou, G.; Peng, C.; Gan, Q.; Hu, J.; Sun, J.; Yao, R.; Huang, Q. Hesperidin: A therapeutic agent for obesity. Drug Des. Devel. Ther. 2019, 13, 3855–3866. [Google Scholar] [CrossRef]
- Qurtam, A.A.; Mechchate, H.; Es-Safi, I.; Al-Zharani, M.; Nasr, F.A.; Noman, O.M.; Aleissa, M.; Imatara, H.; Aleissa, A.M.; Bouhrim, M.; et al. Citrus flavanone narirutin, in vitro and in silico mechanistic antidiabetic potential. Pharmaceutics 2021, 13, 1818. [Google Scholar] [CrossRef]
- Inoue, T.; Tsubaki, S.; Ogawa, K.; Onishi, K.; Azuma, J. Isolation of hesperidin from peels of thinned Citrus unshiu fruits by microwave-assisted extraction. Food Chem. 2010, 123, 542–547. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, N.; Ghoul, M.; Boudhrioua, N.M. Extraction methods of citrus peel phenolic compounds. Food Rev. Int. 2014, 30, 265–290. [Google Scholar] [CrossRef]
- Li, B.B.; Smith, B.; Hossain, M.M. Extraction of phenolics from citrus peels: II. Enzyme-assisted extraction method. Sep. Purif. Technol. 2006, 48, 189–196. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.R.; Tonon, R.V.; Cabral, L.; Gottschalk, L.; Pastrana, L.; Pintado, M.E. Valorization of agricultural lignocellulosic plant byproducts through enzymatic and enzyme-assisted extraction of high-value-added compounds: A review. ACS Sustain. Chem. Eng. 2020, 8, 13112–13125. [Google Scholar] [CrossRef]
- Jang, Y.; Kang, H.; Kim, J.; Lee, S.H. Anti-obesity effects of an enzymatic extract of mandarin (Citrus unshiu) peel in 3T3-L1 adipocytes. Korean J. Food Sci. Technol. 2021, 53, 149–153. [Google Scholar]
- Hwang, H.J.; Kim, H.J.; Ko, M.J.; Chung, M.S. Recovery of hesperidin and narirutin from waste Citrus unshiu peel using subcritical water extraction aided by pulsed electric field treatment. Food Sci. Biotechnol. 2021, 30, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R. Use of high-fat diets to study rodent obesity as a model of human obesity. Int. J. Obes. 2019, 43, 1491–1492. [Google Scholar] [CrossRef]
- Koo, S.Y.; Hwang, J.H.; Yang, S.H.; Um, J.I.; Hong, K.W.; Kang, K.; Pan, C.H.; Hwang, K.T.; Kim, S.M. Anti-obesity effect of standardized extract of microalga Phaeodactylum tricornutum containing fucoxanthin. Mar. Drugs 2019, 17, 311. [Google Scholar] [CrossRef]
- Fang, C.; Pan, J.; Qu, N.; Lei, Y.; Han, J.; Zhang, J.; Han, D. The AMPK pathway in fatty liver disease. Front. Physiol. 2022, 13, 970292. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metab. Clin. Exp. 2019, 92, 6–10. [Google Scholar] [CrossRef]
- Krentz, A.J.; Fujioka, K.; Hompesch, M. Evolution of pharmacological obesity treatments: Focus on adverse side-effect profiles. Diabetes Obes. Metab. 2016, 18, 558–570. [Google Scholar] [CrossRef]
- Lu, Y.A.; Lee, H.G.; Li, X.; Hyun, J.M.; Kim, H.S.; Kim, T.H.; Kim, H.M.; Lee, J.J.; Kang, M.C.; Jeon, Y.J. Anti-obesity effects of red seaweed, Plocamium telfairiae, in C57BL/6 mice fed a high-fat diet. Food Funct. 2020, 11, 2299–2308. [Google Scholar] [CrossRef]
- Huang, Y.W.; Liu, Y.; Dushenkov, S.; Ho, C.T.; Huang, M.T. Anti-obesity effects of epigallocatechin-3-gallate, orange peel extract, black tea extract, caffeine and their combinations in a mouse model. J. Funct. Foods 2009, 1, 304–310. [Google Scholar] [CrossRef]
- Guo, J.; Cao, Y.; Ho, C.T.; Jin, S.; Huang, Q. Aged citrus peel (chenpi) extract reduces lipogenesis in differentiating 3T3-L1 adipocytes. J. Funct. Foods 2017, 34, 297–303. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, J.; Zhang, X.; Zhao, D.G.; Ma, Y.Y.; Li, D.; Ho, C.T.; Huang, Q. Aged citrus peel (chenpi) extract causes dynamic alteration of colonic microbiota in high-fat diet induced obese mice. Food Funct. 2020, 11, 2667–2678. [Google Scholar] [CrossRef] [PubMed]
- Chavan, P.; Singh, A.K.; Kaur, G. Recent progress in the utilization of industrial waste and by-products of citrus fruits: A review. J. Food Process Eng. 2018, 41, e12895. [Google Scholar] [CrossRef]
- Carota, G.; Raffaele, M.; Amenta, M.; Ballistreri, G.; Fabroni, S.; Rapisarda, P.; Sorrenti, V. In vitro effects of bioflavonoids ruch lemon extract on pre-adipocyte differentiation. Nat. Prod. Res. 2019, 35, 4774–4778. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Ramniwas, S.; Saeed, M.; Ahmad, I. A mechanistic review of the pharmacological potential of narirutin: A dietary flavonoid. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024. advance online publications. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, H.; Xiong, P. Application of Cellulase-assisted method to extract flavonoids from plants. Agric. Sci. Technol. 2014, 15, 729–732. [Google Scholar]
- Cheigh, C.I.; Chung, E.Y.; Chung, M.S. Enhanced extraction of flavanones hesperidin and narirutin from Citrus unshiu peel using subcritical water. J. Food Eng. 2012, 110, 472–477. [Google Scholar] [CrossRef]
- Lu, M.; Cao, Y.; Xiao, J.; Song, M.; Ho, C.T. Molecular mechanisms of the anti-obesity effect of bioactive ingredients in common spices: A review. Food Funct. 2018, 9, 4569–4581. [Google Scholar] [CrossRef]
- Lim, H.; Seo, J.; Chang, Y.H.; Han, B.K.; Jeong, J.K.; Park, S.B.; Choi, H.J.; Hwang, J. Anti-obesity effects of Jeju hallabong tangor (Citrus kiyomi X ponkan) peel extracts in 3T3-L1 adipocytes. J. Korean Soc. Food Sci. Nutr. 2014, 43, 1688–1694. [Google Scholar] [CrossRef]
- Karagozlu, M.Z.; Kim, M.; Lee, M. Citrus peel ethanol extract inhibits the adipogenesis cause from high fat-induced DIO model. Food Nutr. Sci. 2016, 7, 8–19. [Google Scholar]
- Kusunoki, J.; Kanatani, A.; Moller, D.E. Modulation of fatty acid metabolism as a potential approach to the treatment of obesity and the metabolic syndrome. Endocrine 2006, 29, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Ebbert, J.O.; Hensen, M.D. Fat depots, free fatty acids, and dyslipidemia. Nutrients 2013, 5, 498–508. [Google Scholar] [CrossRef]
- Kohjima, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Yada, M.; Yada, R.; Harada, N.; Enjoji, M.; et al. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int. J. Mol. Med. 2008, 21, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, M.H.; Han, J.S.; Jeong, Y.; Kim, M.; Jeon, Y.J. Bioactive compounds extracted from Gamtae (Ecklonia cava) by using enzymatic hydrolysis, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Food Sci. Biotechnol. 2012, 21, 1149–1155. [Google Scholar] [CrossRef]
- Im, S.T.; Mun, H.; Park, S.; Kang, H.; Kim, W.C.; Heo, S.J.; Lee, S.H. Ishige okamurae Celluclast extract ameliorates non-alcoholic fatty liver in high-fructose diet-fed mice by modulation of lipid metabolism and gut microbiota composition. Food Chem. Toxicol. 2023, 177, 113864. [Google Scholar] [CrossRef]



| Parameter | Group | ||
|---|---|---|---|
| HFD | HFD + MPCE (LD) | HFD + MPCE (HD) | |
| TG (mg/dL) | 343.42 ± 97.99 | 284.02 ± 19.17 | 187.01 ± 32.29 ** |
| AST (mU/mL) | 81.51 ± 22.25 | 30.07 ± 7.88 ** | 38.12 ± 9.45 ** |
| ALT (U/mL) | 81.01 ± 11.56 | 35.16 ± 4.78 ** | 43.71 ± 2.18 ** |
| Leptin (ng/mL) | 52.43 ± 8.95 | 40.67 ± 5.15 * | 37.98 ± 0.89 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, S.T.; Kang, H.; Kim, J.; Kim, S.-R.; Kim, K.-N.; Lee, S.-H. Narirutin-Rich Celluclast Extract from Mandarin (Citrus unshiu) Peel Alleviates High-Fat Diet-Induced Obesity and Promotes Energy Metabolism in C57BL/6 Mice. Int. J. Mol. Sci. 2024, 25, 4475. https://doi.org/10.3390/ijms25084475
Im ST, Kang H, Kim J, Kim S-R, Kim K-N, Lee S-H. Narirutin-Rich Celluclast Extract from Mandarin (Citrus unshiu) Peel Alleviates High-Fat Diet-Induced Obesity and Promotes Energy Metabolism in C57BL/6 Mice. International Journal of Molecular Sciences. 2024; 25(8):4475. https://doi.org/10.3390/ijms25084475
Chicago/Turabian StyleIm, Seung Tae, Heejoo Kang, Jusang Kim, Song-Rae Kim, Kil-Nam Kim, and Seung-Hong Lee. 2024. "Narirutin-Rich Celluclast Extract from Mandarin (Citrus unshiu) Peel Alleviates High-Fat Diet-Induced Obesity and Promotes Energy Metabolism in C57BL/6 Mice" International Journal of Molecular Sciences 25, no. 8: 4475. https://doi.org/10.3390/ijms25084475