Improvement in Motor and Walking Capacity during Multisegmental Transcutaneous Spinal Stimulation in Individuals with Incomplete Spinal Cord Injury
Abstract
:1. Introduction
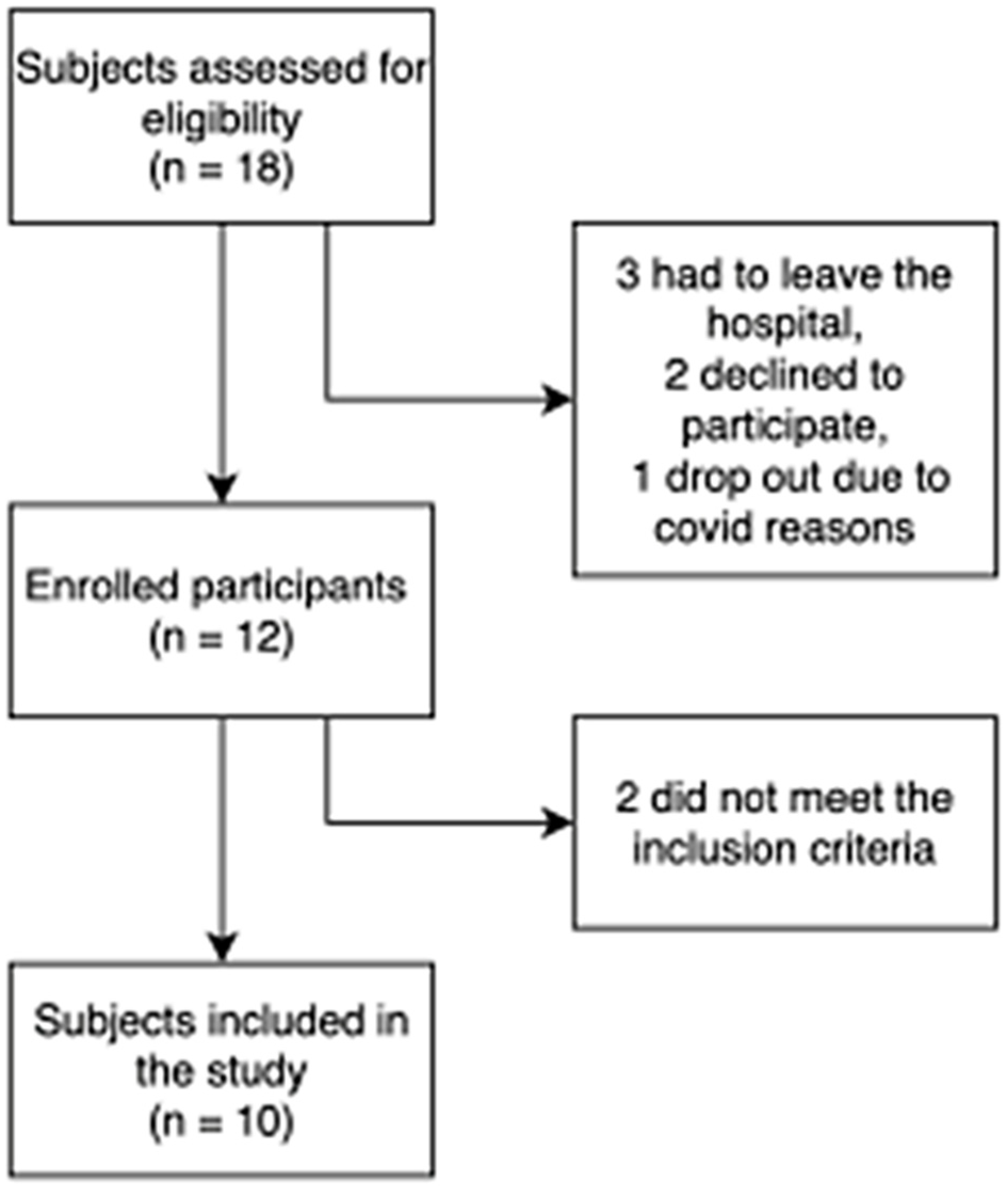
2. Results
2.1. 6 Meter Walking Test (6MWT)
2.2. Maximum Voluntary Contraction of the Quadriceps Muscle (MVC-QM)
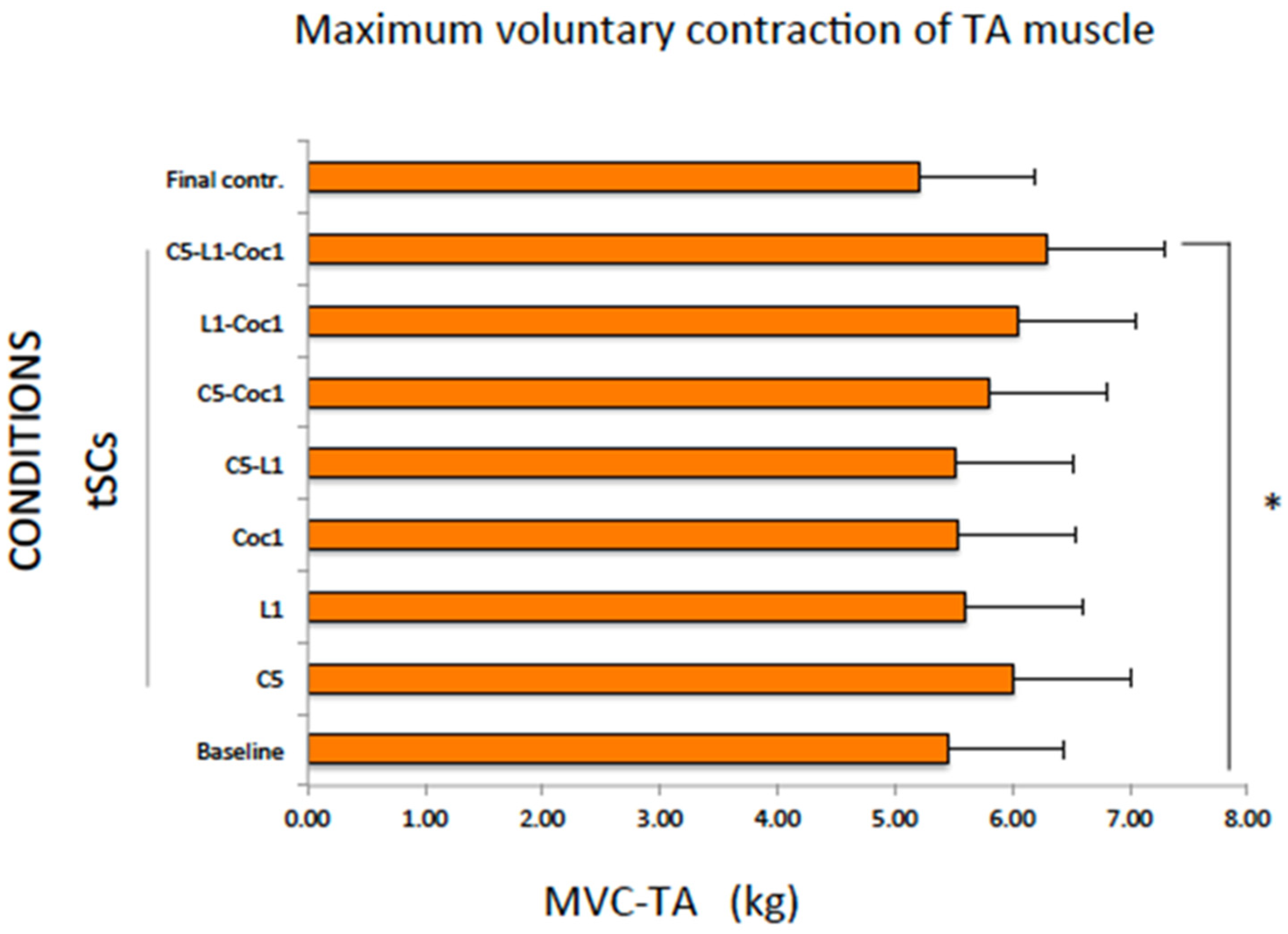
2.3. Maximum Voluntary Contraction of the TA Muscle (MVC-TA)
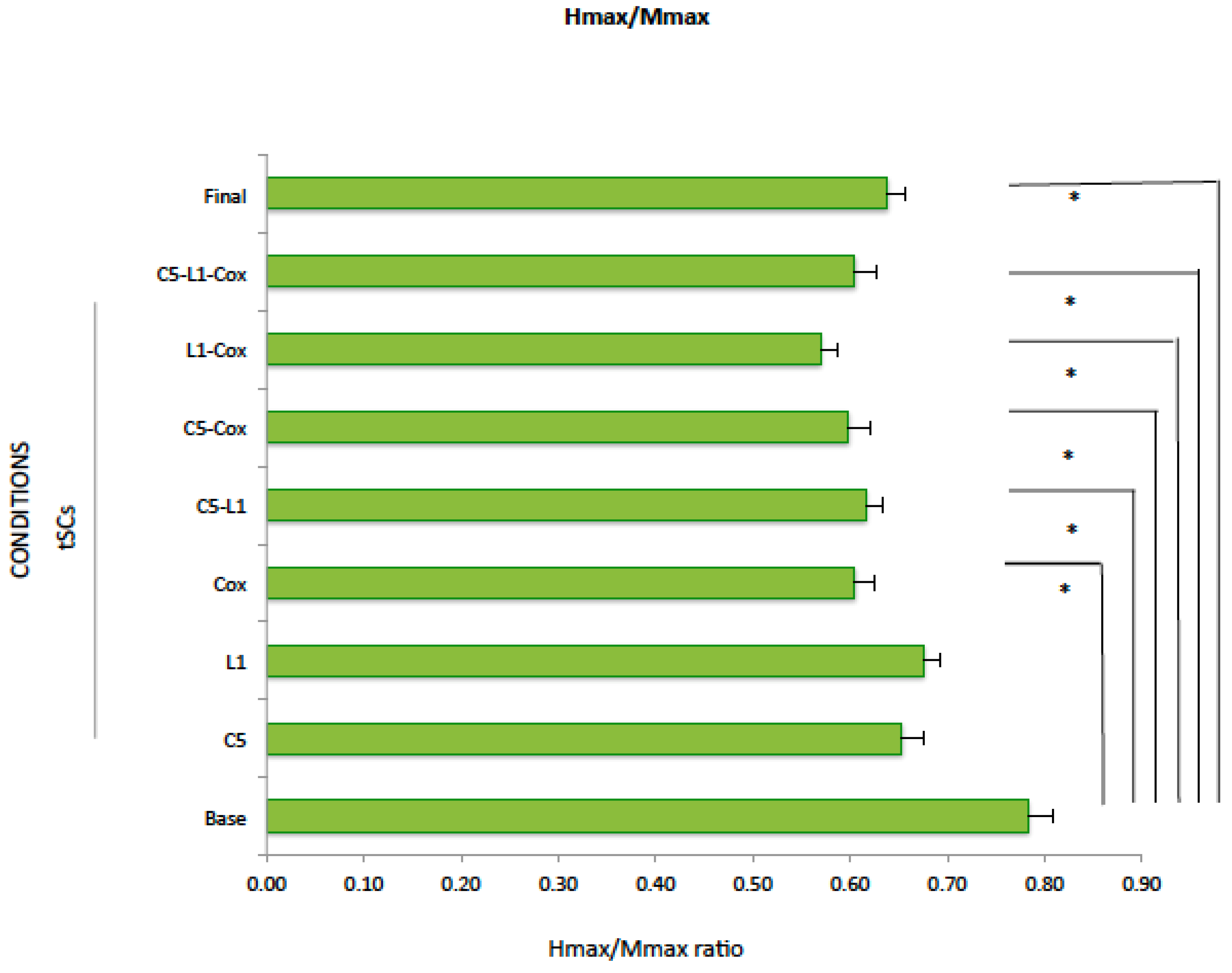
2.4. Hmax/Mmax
3. Discussion
4. Conclusion
5. Methods
5.1. Clinical Assessments
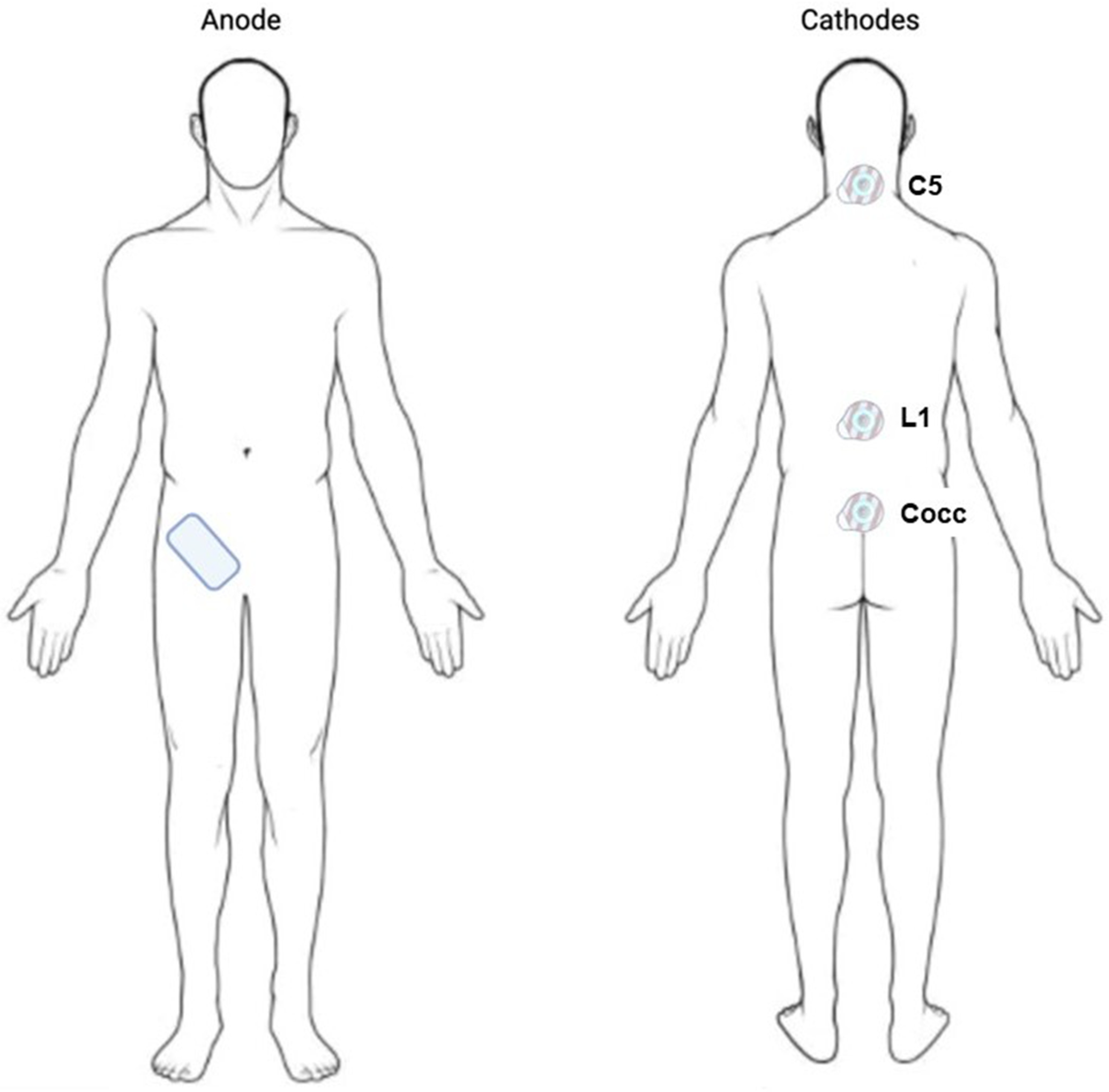
5.2. Experimental Setup
5.3. Study Conditions
5.4. Stimulation
6. Data Analysis and Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ditunno, P.L.; Patrick, M.; Stineman, M.; Ditunno, J.F. Who wants to walk? Preferences for recovery after SCI: A longitudinal and cross-sectional study. Spinal Cord 2008, 46, 500–506. [Google Scholar] [CrossRef]
- Pagliacci, M.C.; Celani, M.G.; Spizzichino, L.; Zampolini, M.; Aito, S.; Citterio, A.; Finali, G.; Loria, D.; Ricci, S.; Taricco, M.; et al. Gruppo Italiano Studio Epidemiologico Mielolesioni (GISEM) Group. spinal cord lesion management in Italy: A 2-year survey. Spinal Cord 2003, 41, 620–628. [Google Scholar] [CrossRef]
- Fouad, K.; Pearson, K. Restoring walking after spinal cord injury. Prog. Neurobiol. 2004, 73, 107–126. [Google Scholar] [CrossRef]
- Awai, L.; Curt, A. Comprehensive assessment of walking function after human spinal cord injury. Prog. Brain Res. 2015, 218, 1–14. [Google Scholar] [CrossRef]
- Domingo, A.; Al-Yahya, A.A.; Asiri, Y.; Eng, J.J.; Tania Lam, T.; Spinal Cord Injury Rehabilitation Evidence Research Team. A systematic review of the effects of pharmacological agents on walking function in people with spinal cord injury. J. Neurotrauma 2012, 29, 865–879. [Google Scholar] [CrossRef]
- Hasegawa, T.; Uchiyama, Y.; Uemura, K.; Harada, Y.; Sugiyama, M.; Tanaka, H. Physical impairment and walking function required for community ambulation in patients with cervical incomplete spinal cord injury. Spinal Cord 2014, 52, 396–399. [Google Scholar] [CrossRef]
- Benito-Penalva, J.; Edwards, D.J.; Opisso, E.; Cortes, M.; Lopez-Blazquez, R.; Murillo, N.; Costa, U.; Tormos, J.M.; Vidal-Samsó, J.; Valls-Solé, J.; et al. Gait training in human spinal cord injury using electromechanical systems: Effect of device type and patient characteristics. Arch. Phys. Med. Rehabil. 2012, 93, 404–412. [Google Scholar] [CrossRef]
- Hofer, A.-S.; Schwab, M.E. Enhancing rehabilitation and functional recovery after brain and spinal cord trauma with electrical neuromodulation. Curr. Opin. Neurol. 2019, 32, 828–835. [Google Scholar] [CrossRef]
- Rahman, M.A.; Tharu, N.S.; Gustin, S.M.; Zheng, Y.P.; Alam, M. Trans-Spinal Electrical Stimulation Therapy for Functional Rehabilitation after Spinal Cord Injury: Review. J. Clin. Med. 2022, 11, 1550. [Google Scholar] [CrossRef]
- Inanici, F.; Brighton, L.N.; Samejima, S.; Hofstetter, C.P.; Moritz, C.T. Transcutaneous Spinal Cord Stimulation Restores Hand and Arm Function after Spinal Cord Injury. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 310–319. [Google Scholar] [CrossRef]
- Gad, P.; Gerasimenko, Y.; Zdunowski, S.; Turner, A.; Sayenko, D.; Lu, D.C.; Edgerton, V.R. Weight bearing over-ground stepping in an Exoskeleton with Non-invasive spinal cord neuromodulation after complete motor complete paraplegia. Front. Neurosci. 2017, 11, 333. [Google Scholar] [CrossRef]
- Minassian, K.; Hofstoetter, U.S.; Danner, S.M.; Mayr, W.; Bruce, J.A.; McKay, W.B.; Tansey, K.E. Spinal Rhythm Generation by Step-Induced Feedback and Transcutaneous Posterior Root Stimulation in Complete Spinal Cord–Injured Individuals. Neurorehabil. Neural Repair 2016, 30, 233–243. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Freundl, B.; Danner, S.M.; Krenn, M.J.; Mayr, W.; Binder, H.; Minassian, K. Transcutaneous spinal cord stimulation induces temporary attenuation of spasticity in individuals with spinal cord injury. J. Neurotrauma 2020, 37, 481–493. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Krenn, M.; Danner, S.M.; Hofer, C.; Kern, H.; McKay, W.B.; Mayr, W.; Minassian, K. Augmentation of Voluntary Locomotor Activity by Transcutaneous Spinal Cord Stimulation in Motor-Incomplete Spinal Cord-Injured Individuals: Augmentation of Locomotion by tSCS in Incomplete SCI. Artif. Organs 2015, 39, E176–E186. [Google Scholar] [CrossRef]
- Kumru, H.; García-Alén, L.; Ros-Alsina, A.; Albu, S.; Valles, M.; Vidal, J. Transcutaneous Spinal Cord Stimulation Improves Respiratory Muscle Strength and Function in Subjects with Cervical Spinal Cord Injury: Original Research. Biomedicines 2023, 11, 2121. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Bhagat, N.A.; Ramdeo, R.; Ebrahimi, S.; Sharma, P.D.; Griffin, D.G.; Stein, A.; Harkema, S.J.; Bouton, C.E. Targeted transcutaneous spinal cord stimulation promotes persistent recovery of upper limb strength and tactile sensation in spinal cord injury: A pilot study. Front. Neurosci. 2023, 17, 1210328. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Freundl, B.; Lackner, P.; Binder, H. Transcutaneous Spinal Cord Stimulation Enhances Walking Performance and Reduces Spasticity in Individuals with Multiple Sclerosis. Brain Sci. 2021, 11, 472. [Google Scholar] [CrossRef]
- Tator, C.H.; Minassian, K.; Mushahwar, V.K. Spinal cord stimulation: Therapeutic benefits and movement generation after spinal cord injury. Handb. Clin. Neurol. 2012, 109, 283–296. [Google Scholar] [CrossRef]
- Gerasimenko, Y.; Gorodnichev, R.; Puhov, A.; Moshonkina, T.; Savochin, A.; Selionov, V.; Roy, R.R.; Lu, D.C.; Edgerton, V.R. Initiation and modulation of locomotor circuitry output with multisite transcutaneous electrical stimulation of the spinal cord in noninjured humans. J. Neurophysiol. 2015, 113, 834–842. [Google Scholar] [CrossRef]
- Gerasimenko, Y.P.; Lu, D.C.; Modaber, M.; Zdunowski, S.; Gad, P.; Sayenko, D.G.; Morikawa, E.; Haakana, P.; Ferguson, A.R.; Roy, R.R.; et al. Noninvasive Reactivation of Motor Descending Control after Paralysis. J. Neurotrauma. 2015, 32, 1968–1980. [Google Scholar] [CrossRef]
- Kumru, H.; Flores, Á.; Rodríguez-Cañón, M.; Edgerton, V.R.; García, L.; Benito-Penalva, J.; Navarro, X.; Gerasimenko, Y.; García-Alías, G.; Vidal, J. Cervical Electrical Neuromodulation Effectively Enhances Hand Motor Output in Healthy Subjects by Engaging a Use-Dependent Intervention. J. Clin. Med. 2021, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Parhizi, B.; Barss, T.S.; Mushahwar, V.K. Cervical and Lumbar Spinal Cord Stimulation Induces Facilitation of Both Spinal and Corticospinal Circuitry in Humans. Front. Neurosci. 2021, 15, 615103. [Google Scholar] [CrossRef] [PubMed]
- Barss, T.S.; Parhizi, B.; Mushahwar, V.K. Transcutaneous spinal cord stimulation of the cervical cord modulates lumbar networks. J. Neurophysiol. 2020, 123, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Angeli, C.A.; Gerasimenko, Y. Combined cervical transcutaneous with lumbosacral epidural stimulation improves voluntary control of stepping movements in spinal cord injured individuals. Front. Bioeng. Biotechnol. 2023, 11, 1073716. [Google Scholar] [CrossRef]
- Murray, L.M.; Knikou, M. Transspinal stimulation increases motoneuron output of multiple segments in human spinal cord injury. PLoS ONE 2019, 14, e0213696. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, M.; Masugi, Y.; Sasaki, A.; Sayenko, D.G.; Nakazawa, K. On the reflex mechanisms of cervical transcutaneous spinal cord stimulation in human subjects. J. Neurophysiol. 2019, 121, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- McCrea, D.A.; Rybak, I.A. Organization of mammalian locomotor rhythm and pattern generation. Brain Res. Rev. 2008, 57, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Whelan, P.; Bonnot, A.; O’Donovan, J.M. Properties of rhythmic activity generated by the isolated spinal cord of the neonatal mouse. J. Neurophysiol. 2000, 84, 2821–2833. [Google Scholar] [CrossRef]
- Etlin, A.; Blivis, D.; Ben-Zwi, M.; Lev-Tov, A. Long and short multifunicular projections of sacral neurons are activated by sensory input to produce locomotor activity in the absence of supraspinal control. J. Neurosci. 2010, 30, 10324–10336. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, M.M.R.; Dimitrijevic, M.M.R.; Illis, L.S.; Nakajima, K.; Sharkey, P.C.; Sherwood, A.M. Spinal cord stimulation for the control of spasticity in patients with chronic spinal cord injury: II. Neurophysiologic observations. Cent. Nerv. Syst. Trauma. 1986, 3, 145–152. [Google Scholar] [CrossRef]
- Jung, Y.; Breitbart, S.; Malvea, A.; Bhatia, A.; Ibrahim, G.M.; Gorodetsky, C. Epidural Spinal Cord Stimulation for Spasticity: A Systematic Review of the Literature. World Neurosurg. 2024, 183, 227–238.e5. [Google Scholar] [CrossRef]
- Simpson, R.K., Jr.; Robertson, C.S.; Goodman, J.C. Segmental recovery of amino acid neurotransmitters during posterior epidural stimulation after spinal cord injury. J. Am. Paraplegia Soc. 1993, 16, 34–41. [Google Scholar] [CrossRef]
- Islam, M.A.; Pulverenti, T.S.; Knikou, M. Neuronal Actions of Transspinal Stimulation on Locomotor Networks and Reflex Excitability During Walking in Humans With and Without Spinal Cord Injury. Front. Hum. Neurosci. 2021, 15, 620414. [Google Scholar] [CrossRef]
- Knikou, M.; Murray, L.M. Repeated transspinal stimulation decreases soleus H-reflex excitability and restores spinal inhibition in human spinal cord injury. PLoS ONE 2019, 14, e0223135. [Google Scholar] [CrossRef]
- Kirshblum, S.C.; Burns, S.P.; Biering-Sorensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.J.; et al. International standards for neurological classification of spinal cord injury (Revised 2011). J. Spinal Cord Med. 2011, 34, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Grosprêtre, S.; Martin, A. H reflex and spinal excitability: Methodological considerations. J. Neurophysiol. 2012, 107, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Emch, G.S.; Johnson, C.S.; Wrathall, J.R. Effect of spinal cord injury severity on alterations of the H-reflex. Exp. Neurol. 2005, 196, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Misiaszek, J.E. The H-reflex as a tool in neurophysiology: Its limitations and uses in understanding nervous system function. Muscle Nerve 2003, 28, 144–160. [Google Scholar] [CrossRef]

| Demographic Data | Intensity of tSCS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Age | Gender | AIS | NLI | Etiology | Time since SCI (Months) | Total Motor Score | LEMS | C5 (mA) | L1 (mA) | Coc1 (mA) |
| tSCS 1 | 32 | M | D | C4 | T | 6 | 90 | 45 | 25 | 42 | 56 |
| tSCS 2 | 28 | M | D | C8 | T | 6 | 89 | 39 | 28 | 58 | 60 |
| tSCS 3 | 48 | M | D | C4 | NT | 6 | 96 | 49 | 25 | 55 | 53 |
| tSCS 4 | 55 | M | D | C3 | T | 6 | 88 | 46 | 40 | 50 | 40 |
| tSCS 5 | 61 | F | D | C4 | T | 72 | 81 | 38 | 44 | 50 | 62 |
| tSCS 6 | 42 | M | D | C5 | T | 6 | 81 | 43 | 50 | 44 | 62 |
| tSCS 7 | 47 | M | D | C6 | T | 11 | 57 | 26 | 40 | 49 | 60 |
| tSCS 8 | 26 | M | D | C7 | T | 6 | 90 | 45 | 46 | 30 | 46 |
| tSCS 9 | 50 | M | D | C6 | T | 8 | 70 | 30 | 47 | 41 | 50 |
| tSCS 10 | 43 | M | D | C3 | T | 8 | 83 | 47 | 60 | 60 | 75 |
| Subject | Baseline | C5 | L1 | Coc1 | C5-L1 | C5-Coc1 | L1-Coc1 | C5-L1-Coc1 | Final Control |
|---|---|---|---|---|---|---|---|---|---|
| tSCS 1 | 6.09 | 6.88 | 6.67 | 6.75 | 6.51 | 6.19 | 6.58 | 5.94 | 5.99 |
| tSCS 2 | 7.80 | 8.80 | 10.43 | 8.09 | 8.59 | 8.03 | 8.57 | 7.78 | 8.19 |
| tSCS 3 | 13.36 | 11.22 | 11.13 | 11.29 | 11.06 | 10.81 | 10.75 | 11.11 | 11.16 |
| tSCS 4 | 7.07 | 5.97 | 6.58 | 6.16 | 6.39 | 6.08 | 6.05 | 5.99 | 5.81 |
| tSCS 5 | 23.81 | 23.82 | 24.40 | 21.38 | 23.85 | 23.34 | 23.02 | 24.14 | 23.20 |
| tSCS 6 | 13.48 | 11.48 | 9.76 | 9.45 | 13.83 | 12.27 | 12.11 | 11.52 | 9.86 |
| tSCS 7 | 39.70 | 37.77 | 36.41 | 37.58 | 30.99 | 30.67 | 34.60 | 29.64 | 32.50 |
| tSCS 8 | 9.43 | 8.89 | 8.73 | 8.71 | 8.54 | 8.62 | 8.47 | 8.29 | 8.44 |
| tSCS 9 | 36.02 | 29.19 | 30.58 | 36.07 | 31.13 | 31.59 | 29.24 | 35.89 | 34.76 |
| tSCS 10 | 26.51 | 26.15 | 25.66 | 26.24 | 26.58 | 22.63 | 23.92 | 23.40 | 28.36 |
| Mean | 18.33 | 17.02 | 17.03 | 17.17 * | 16.74 | 16.02 * | 16.33 * | 16.37* | 16.82 |
| SE | 3.92 | 3.55 | 3.50 | 3.88 | 3.24 | 3.18 | 3.29 | 3.45 | 3.66 |
| Subjects | Baseline | C5 | L1 | Coc1 | C5-L1 | C5-Coc1 | L1-Coc1 | C5-L1-Coc1 | Final Control |
|---|---|---|---|---|---|---|---|---|---|
| tSCS 1 | 15.59 | 13.56 | 12.93 | 13.18 | 12.49 | 12.15 | 14.78 | 12.79 | 12.39 |
| tSCS 2 | 7.30 | 6.81 | 8.49 | 9.59 | 8.84 | 10.06 | 11.48 | 10.26 | 8.74 |
| tSCS 3 | 11.80 | 26.58 | 23.49 | 21.80 | 19.24 | 20.81 | 14.44 | 24.43 | 22.20 |
| tSCS 4 | 26.47 | 30.00 | 28.45 | 27.25 | 29.34 | 29.72 | 27.20 | 28.67 | 28.85 |
| tSCS 5 | 6.80 | 8.58 | 6.27 | 6.20 | 5.80 | 6.91 | 5.37 | 6.65 | 8.05 |
| tSCS 6 | 17.07 | 16.41 | 15.94 | 16.90 | 15.88 | 16.63 | 16.00 | 16.60 | 17.27 |
| tSCS 7 | 18.41 | 14.69 | 16.23 | 15.08 | 16.65 | 16.56 | 15.47 | 16.50 | 17.25 |
| tSCS 8 | 25.92 | 29.07 | 30.00 | 26.89 | 28.14 | 30.00 | 26.67 | 29.50 | 27.42 |
| tSCS 9 | 14.30 | 10.73 | 13.80 | 12.06 | 14.90 | 11.89 | 12.66 | 11.25 | 11.18 |
| tSCS 10 | 19.41 | 18.47 | 19.69 | 19.27 | 19.21 | 19.30 | 17.74 | 19.59 | 20.15 |
| Mean | 16.30 | 17.49 | 17.53 | 16.82 | 17.05 | 17.40 | 16.18 | 17.62 | 17.35 |
| SE | 2.13 | 2.66 | 2.50 | 2.22 | 2.37 | 2.47 | 2.08 | 2.48 | 2.33 |
| Subject | Baseline | C5 | L1 | Coc1 | C5-L1 | C5-Coc1 | L1-Coc1 | C5-L1-Coc1 | Final Control |
|---|---|---|---|---|---|---|---|---|---|
| tSCS 1 | 7.62 | 7.46 | 8.75 | 8.85 | 7.54 | 8.03 | 11.25 | 11.00 | 8.04 |
| tSCS 2 | 0.64 | 0.79 | 0.68 | 0.80 | 0.07 | 1.03 | 0.54 | 0.56 | 0.74 |
| tSCS 3 | 3.12 | 3.02 | 3.27 | 2.35 | 1.55 | 4.66 | 5.16 | 2.40 | 2.49 |
| tSCS 4 | 13.02 | 13.33 | 13.64 | 13.94 | 13.56 | 13.46 | 11.91 | 15.11 | 13.61 |
| tSCS 5 | 0.35 | 1.03 | 0.90 | 1.51 | 1.09 | 1.03 | 0.30 | 0.64 | 0.41 |
| tSCS 6 | 8.44 | 7.90 | 7.43 | 7.29 | 9.96 | 7.23 | 10.22 | 9.41 | 7.39 |
| tSCS 7 | 2.84 | 4.14 | 3.17 | 2.10 | 3.25 | 3.73 | 3.02 | 3.10 | 3.33 |
| tSCS 8 | 7.34 | 11.01 | 7.78 | 8.30 | 8.48 | 8.93 | 8.57 | 9.20 | 7.54 |
| tSCS 9 | 4.90 | 5.27 | 4.97 | 3.70 | 4.84 | 4.80 | 4.56 | 5.05 | 3.45 |
| tSCS 10 | 6.13 | 6.09 | 5.35 | 6.46 | 4.81 | 5.09 | 4.94 | 6.40 | 4.96 |
| Mean | 5.44 | 6.00 | 5.59 | 5.53 | 5.51 | 5.80 | 6.05 | 6.29 * | 5.20 |
| SE | 0.54 | 0.60 | 0.56 | 0.55 | 0.55 | 0.58 | 0.60 | 0.63 | 0.52 |
| A | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hmax (microV) | Mmax (microV) | |||||||||||||||||
| Base | C5 | L1 | Coc1 | C5-L1 | C5-Coc1 | L1-Coc1 | C5-L1-Coc1 | Final | Base | C5 | L1 | Coc1 | C5-L1 | C5-Coc1 | L1-Coc1 | C5-L1-Coc1 | Final | |
| tSCS 1 | 5122 | 2756 | 3659 | 3476 | 2561 | 2634 | 4561 | 2659 | 3000 | 8171 | 8354 | 9085 | 9024 | 7866 | 8842 | 8964 | 9024 | 9146 |
| tSCS 2 | 5000 | 4390 | 4902 | 4293 | 3122 | 4390 | 4951 | 4268 | 4073 | 7500 | 5317 | 7098 | 6634 | 6317 | 6878 | 6365 | 5713 | 6146 |
| tSCS 3 | 4298 | 4634 | 4573 | 4573 | 3659 | 4146 | 3792 | 3963 | 3969 | 5603 | 5634 | 6281 | 5122 | 5366 | 5854 | 7273 | 6159 | 6780 |
| tSCS 4 | 7505 | 7285 | 6688 | 7096 | 6633 | 6407 | 8358 | 7322 | 7286 | 11,926 | 11,030 | 11,913 | 13,048 | 13,700 | 10,993 | 13,946 | 13,060 | 12,317 |
| tSCS 5 | 2524 | 1152 | 2835 | 1829 | 1402 | 1225 | ND | 1823 | 2414 | 3396 | 2707 | 3493 | 2878 | 2884 | 2804 | ND | 3231 | 3213 |
| tSCS 6 | 5341 | 4341 | 3914 | 3384 | 4778 | 4701 | 4341 | 2914 | 4646 | 6103 | 8042 | 8407 | 8656 | 8887 | 8548 | 7743 | 8048 | 8657 |
| tSCS 7 | 1342 | 1781 | 2049 | 1512 | 2146 | 1853 | 1537 | 1781 | 1488 | 3732 | 3659 | 2976 | 4610 | 2902 | 4488 | 4756 | 5439 | 2756 |
| tSCS 8 | 4146 | 3122 | 3659 | 3512 | 3598 | 3073 | 2683 | 4171 | 3537 | 4561 | 6073 | 5915 | 6293 | 5671 | 6707 | 6342 | 6171 | 6281 |
| tSCS 9 | 4634 | 4000 | 4195 | 3854 | 4073 | 4024 | 3805 | 3781 | 3902 | 3537 | 4000 | 4439 | 4390 | 4659 | 3951 | 4366 | 4048 | 3756 |
| tSCS 10 | 5463 | 5854 | 5390 | 4781 | 5024 | 6037 | 5659 | 5407 | 5549 | 5829 | 6342 | 6415 | 6098 | 5585 | 6890 | 6171 | 5732 | 7073 |
| Mean | 4537.5 | 3931.5 | 4186.4 | 3831.0 * | 3699.6 * | 3849.0 * | 4409.7 * | 3808.9 | 3986.4 * | 6035.8 | 6115.8 | 6602.2 | 6675.3 | 6383.7 | 6595.5 | 7325.1 * | 6662.5 | 6612.5 |
| SE | 167.8 | 183.2 | 130.8 | 156.8 | 152.7 | 168.0 | 213.2 | 168.1 | 162.2 | 263.2 | 249.3 | 270.8 | 292.2 | 318.9 | 246.6 | 317.4 | 280.5 | 295.0 |
| B | ||||||||||||||||||
| Hmax/Mmax | ||||||||||||||||||
| Subject | Baseline | C5 | L1 | Coc1 | C5-L1 | C5-Coc1 | L1-Coc1 | C5-L1-Coc1 | Final Control | |||||||||
| tSCS 1 | 0.63 | 0.33 | 0.40 | 0.39 | 0.33 | 0.30 | 0.51 | 0.29 | 0.33 | |||||||||
| tSCS 2 | 0.67 | 0.83 | 0.69 | 0.65 | 0.49 | 0.64 | 0.78 | 0.74 | 0.66 | |||||||||
| tSCS 3 | 0.77 | 0.82 | 0.73 | 0.89 | 0.68 | 0.71 | 0.52 | 0.64 | 0.59 | |||||||||
| tSCS 4 | 0.63 | 0.66 | 0.56 | 0.54 | 0.48 | 0.58 | 0.56 | 0.56 | 0.59 | |||||||||
| tSCS 5 | 0.74 | 0.43 | 0.81 | 0.64 | 0.49 | 0.44 | ND | 0.56 | 0.75 | |||||||||
| tSCS 6 | 0.88 | 0.54 | 0.47 | 0.39 | 0.54 | 0.55 | 0.56 | 0.36 | 0.54 | |||||||||
| tSCS 7 | 0.36 | 0.49 | 0.69 | 0.33 | 0.74 | 0.41 | 0.32 | 0.33 | 0.54 | |||||||||
| tSCS 8 | 0.91 | 0.51 | 0.62 | 0.56 | 0.63 | 0.46 | 0.42 | 0.68 | 0.56 | |||||||||
| tSCS 9 | 1.31 | 1.00 | 0.95 | 0.88 | 0.87 | 1.02 | 0.87 | 0.94 | 1.04 | |||||||||
| tSCS 10 | 0.94 | 0.92 | 0.84 | 0.78 | 0.90 | 0.88 | 0.54 | 1.00 | 0.78 | |||||||||
| Mean | 0.78 | 0.65 | 0.68 | 0.60 * | 0.62 * | 0.60 * | 0.56 * | 0.60 * | 0.64 * | |||||||||
| SE | 0.08 | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumru, H.; Ros-Alsina, A.; García Alén, L.; Vidal, J.; Gerasimenko, Y.; Hernandez, A.; Wrigth, M. Improvement in Motor and Walking Capacity during Multisegmental Transcutaneous Spinal Stimulation in Individuals with Incomplete Spinal Cord Injury. Int. J. Mol. Sci. 2024, 25, 4480. https://doi.org/10.3390/ijms25084480
Kumru H, Ros-Alsina A, García Alén L, Vidal J, Gerasimenko Y, Hernandez A, Wrigth M. Improvement in Motor and Walking Capacity during Multisegmental Transcutaneous Spinal Stimulation in Individuals with Incomplete Spinal Cord Injury. International Journal of Molecular Sciences. 2024; 25(8):4480. https://doi.org/10.3390/ijms25084480
Chicago/Turabian StyleKumru, Hatice, Aina Ros-Alsina, Loreto García Alén, Joan Vidal, Yury Gerasimenko, Agusti Hernandez, and Mark Wrigth. 2024. "Improvement in Motor and Walking Capacity during Multisegmental Transcutaneous Spinal Stimulation in Individuals with Incomplete Spinal Cord Injury" International Journal of Molecular Sciences 25, no. 8: 4480. https://doi.org/10.3390/ijms25084480





