Dysregulated VEGF/VEGFR-2 Signaling and Plexogenic Lesions in the Embryonic Lungs of Chickens Predisposed to Pulmonary Arterial Hypertension
Abstract
:1. Introduction
2. Results
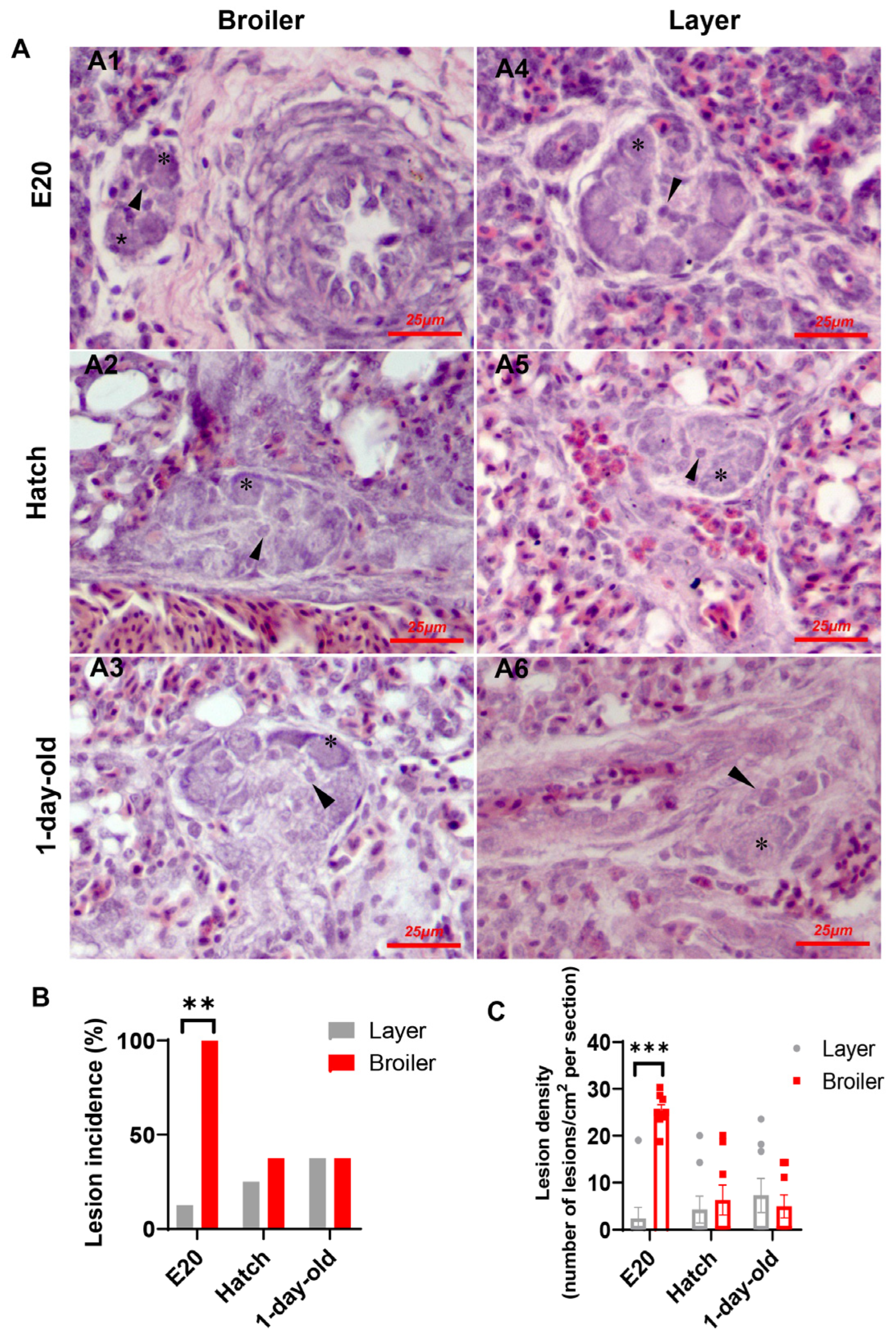
2.1. Plexiform Lesion Incidence and Density in Broilers and Layers
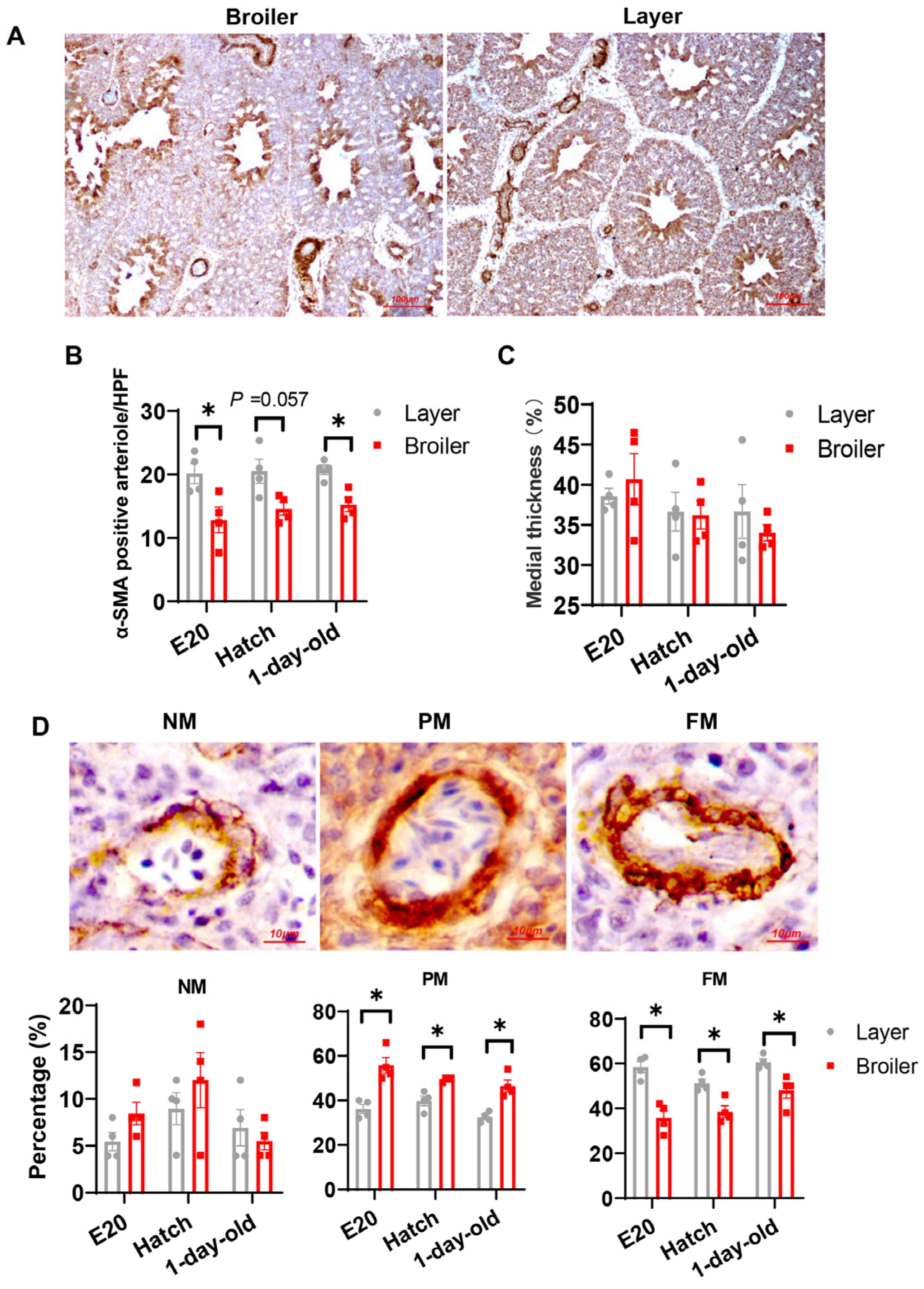
2.2. Phenotypic Characteristics of Cells in Plexiform Lesions
2.3. Broilers Have Decreased Arteriogenesis in Their Lung Compared to Layers
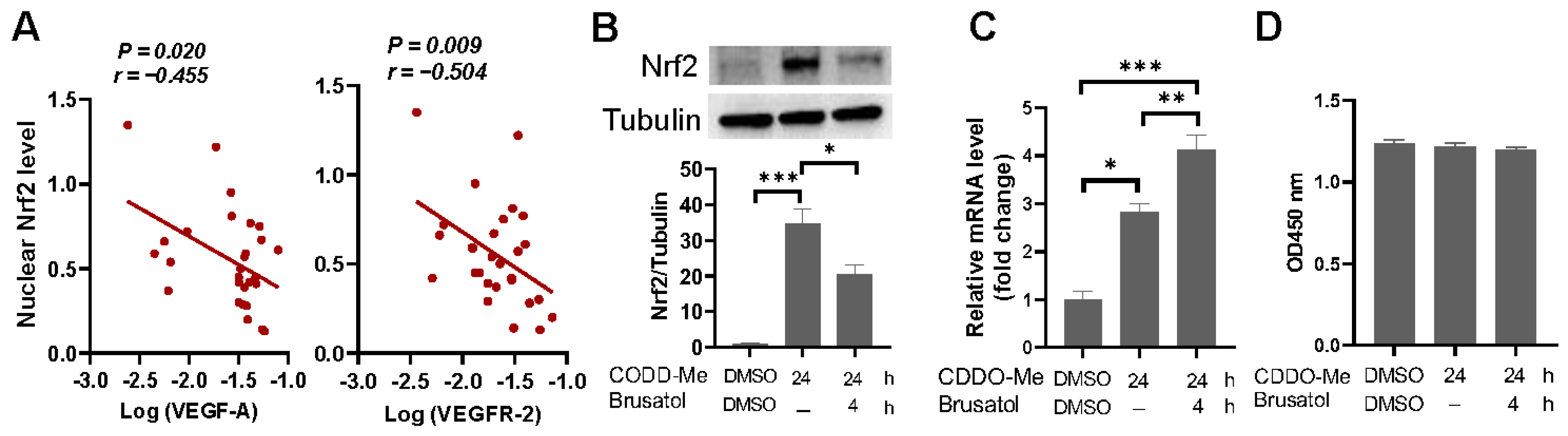
2.4. Correlations between Lung Angiogenic Factors and Morphometric Parameters Measured
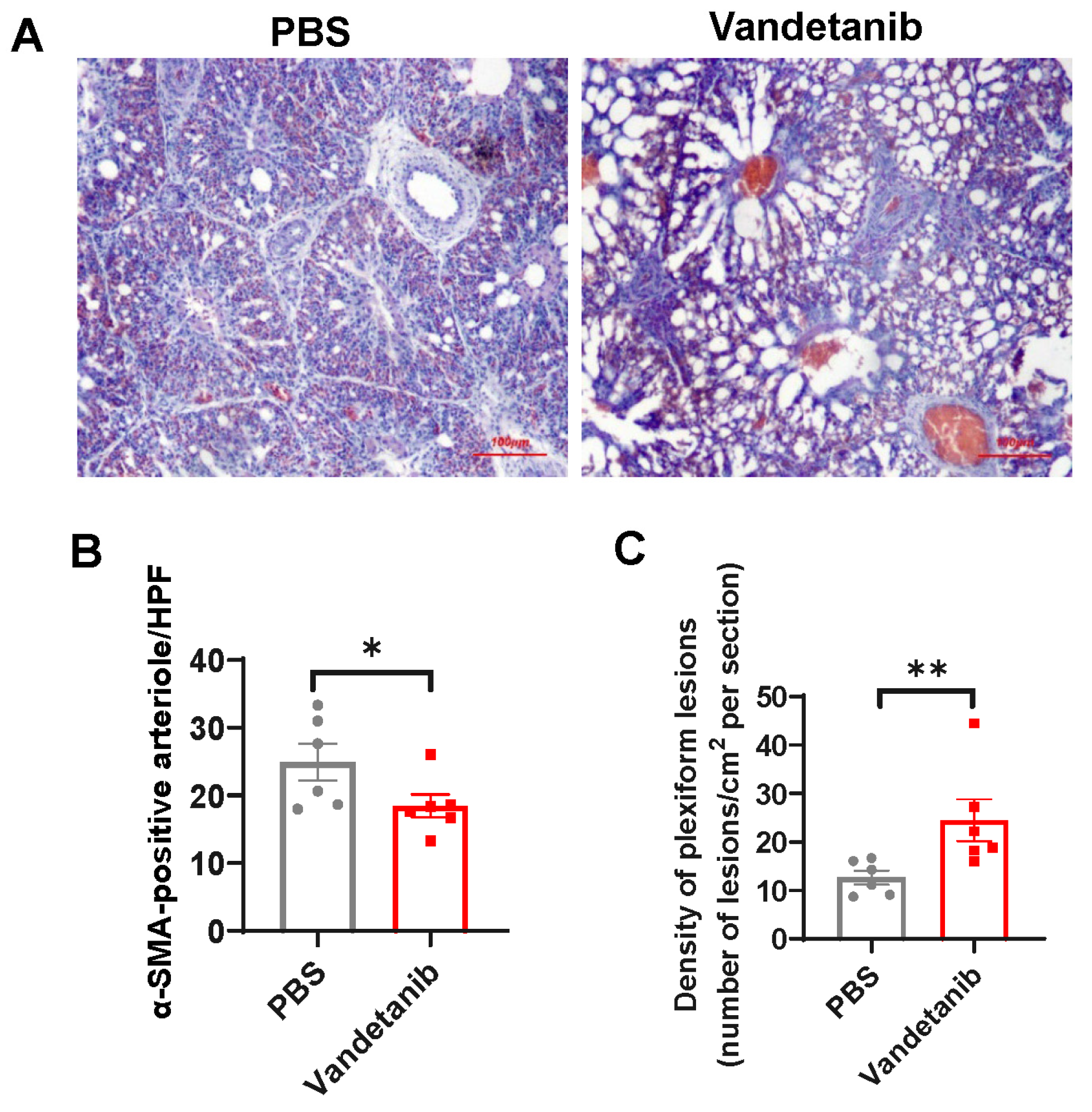
2.5. Pharmaceutical Inhibition of VEGFR-2 Promotes the Development of Plexiform Lesions in the Embryonic Lung of Layers
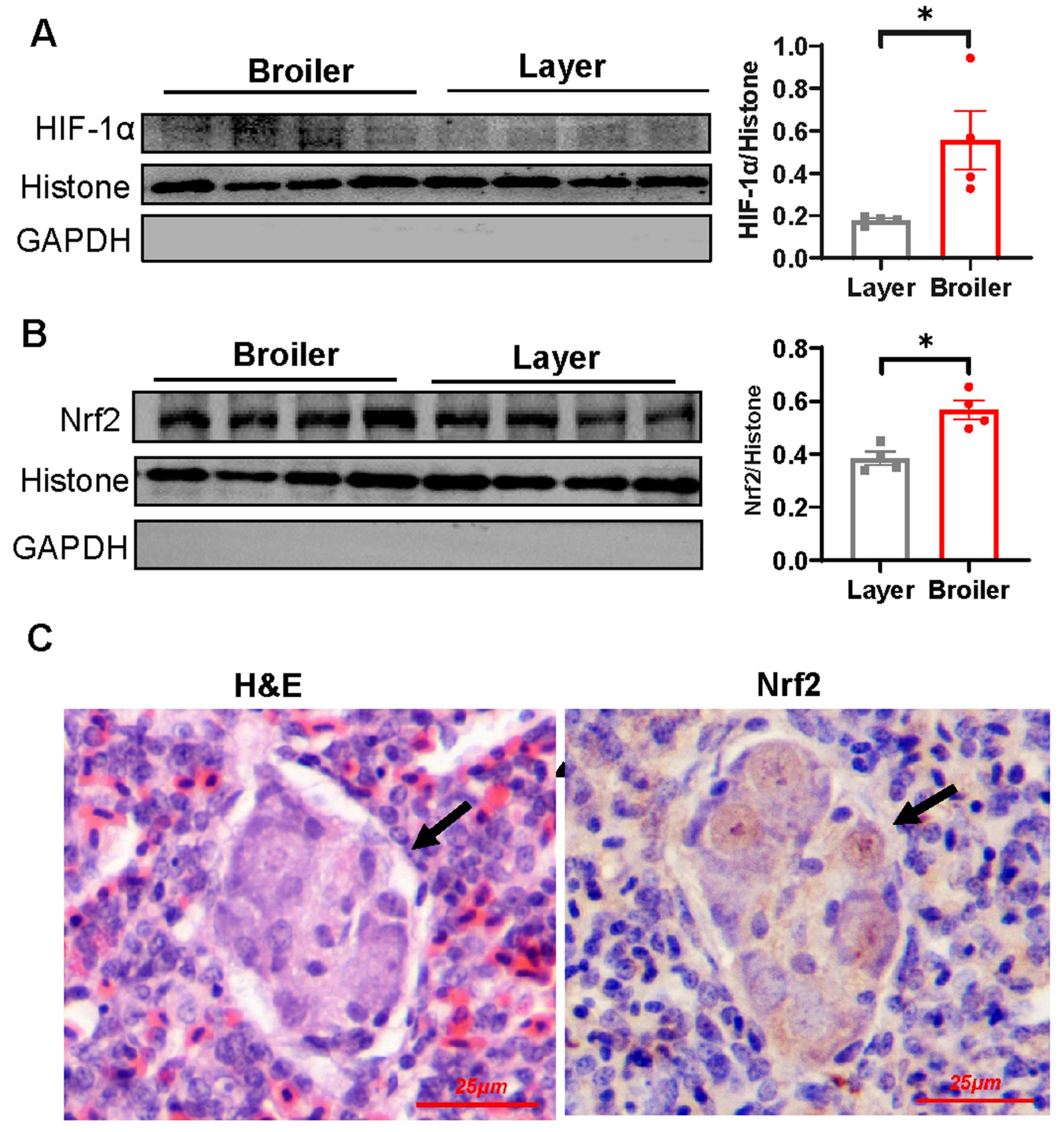
2.6. Nrf2 Is Hyperactivated in Broiler Embryonic Lung and Overexpression of Nrf2 Suppresses VEGF and VEGFR-2 Transcription
3. Discussion
4. Materials and Methods
4.1. Animal Ethics
4.2. Embryo and Chick Preparation
4.3. Vandetanib Inoculation
4.4. Lung Sampling
4.5. Histological and Immunohistochemical Staining
4.6. TUNEL Assay
4.7. Quantitative Real-Time RT–PCR (qPCR)
4.8. Western Blot Analysis
4.9. Luciferase Assay
4.10. Ex Vivo Expansion of Endothelial Progenitor Cells and Treatments
4.11. Cell Viability Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rafikova, O.; Al, G.I.; Rafikov, R. Focus on Early Events: Pathogenesis of Pulmonary Arterial Hypertension Development. Antioxid. Redox Sign. 2019, 31, 933–953. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, S.; Yu, Y.A. The Pathobiology of Pulmonary Arterial Hypertension. Cardiol. Clin. 2022, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cool, C.D.; Stewart, J.S.; Werahera, P.; Miller, G.J.; Williams, R.L.; Voelkel, N.F.; Tuder, R.M. Three-Dimensional Reconstruction of Pulmonary Arteries in Plexiform Pulmonary Hypertension Using Cell-Specific Markers. Evidence for a Dynamic and Heterogeneous Process of Pulmonary Endothelial Cell Growth. Am. J. Pathol. 1999, 155, 411–419. [Google Scholar] [CrossRef]
- Voelkel, N.F.; Bogaard, H.J. Adding Complexity to Plexogenic Arteriopathy. Eur. Respir. J. 2016, 48, 1553–1555. [Google Scholar] [CrossRef] [PubMed]
- Yamaki, S.; Wagenvoort, C.A. Comparison of Primary Plexogenic Arteriopathy in Adults and Children. A morphometric study in 40 patients. Br. Heart J. 1985, 54, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Oyer, C.E.; Feit, L.R.; Rogers, B.B.; Kuhn, C. In Utero Development of Hypertensive Necrotizing Pulmonary Arterial Lesions: Report of a Case Associated with Premature Closure of the Ductus Arteriosus and Pulmonary Hypoplasia. Cardiovasc. Pathol. 2000, 9, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Palevsky, H.I.; Schloo, B.L.; Pietra, G.G.; Weber, K.T.; Janicki, J.S.; Rubin, E.; Fishmasn, A.P. Primary Pulmonary Hypertension. Vascular Structure, Morphometry, and Responsiveness to Vasodilator Agents. Circulation 1989, 80, 1207–1221. [Google Scholar] [CrossRef] [PubMed]
- Hirose, S.; Hosoda, Y.; Furuya, S.; Otsuki, T.; Ikeda, E. Expression of Vascular Endothelial Growth Factor and Its Receptors Correlates Closely with Formation of the Plexiform Lesion in Human Pulmonary Hypertension. Pathol. Int. 2000, 50, 472–479. [Google Scholar] [CrossRef]
- Tuder, R.M.; Chacon, M.; Alger, L.; Wang, J.; Taraseviciene-Stewart, L.; Kasahara, Y.; Cool, C.D.; Bishop, A.E.; Geraci, M.; Semenza, G.L.; et al. Expression of Angiogenesis-Related Molecules in Plexiform Lesions in Severe Pulmonary Hypertension: Evidence for a Process of Disordered Angiogenesis. J. Pathol. 2001, 195, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Masri, F.A.; Xu, W.; Comhair, S.A.; Asosingh, K.; Ko, M.; Vasanji, A.; Drazba, J.; Anand-Apte, B.; Erzurum, S.C. Hyperproliferative Apoptosis-Resistant Endothelial Cells in Idiopathic Pulmonary Arterial Hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, L548–L554. [Google Scholar] [CrossRef] [PubMed]
- Majka, S.M.; Skokan, M.; Wheeler, L.; Harral, J.; Gladson, S.; Burnham, E.; Loyd, J.E.; Stenmark, K.R.; Varella-Garcia, M.; West, J. Evidence for Cell Fusion Is Absent in Vascular Lesions Associated with Pulmonary Arterial Hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L1028–L1039. [Google Scholar] [CrossRef] [PubMed]
- Montani, D.; Perros, F.; Gambaryan, N.; Girerd, B.; Dorfmuller, P.; Price, L.C.; Huertas, A.; Hammad, H.; Lambrecht, B.; Simonneau, G.; et al. C-Kit-Positive Cells Accumulate in Remodeled Vessels of Idiopathic Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care 2011, 184, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Toshner, M.; Voswinckel, R.; Southwood, M.; Al-Lamki, R.; Howard, L.S.; Marchesan, D.; Yang, J.; Suntharalingam, J.; Soon, E.; Exley, A.; et al. Evidence of Dysfunction of Endothelial Progenitors in Pulmonary Arterial Hypertension. Am. J. Resp. Crit. Care 2009, 180, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Asosingh, K.; Aldred, M.A.; Vasanji, A.; Drazba, J.; Sharp, J.; Farver, C.; Comhair, S.A.; Xu, W.; Licina, L.; Huang, L.; et al. Circulating Angiogenic Precursors in Idiopathic Pulmonary Arterial Hypertension. Am. J. Pathol. 2008, 172, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.T.; Comhair, S.A.; Aldred, M.A.; Mavrakis, L.; Savasky, B.M.; Erzurum, S.C.; Asosingh, K. Pulmonary Artery Endothelium Resident Endothelial Colony-Forming Cells in Pulmonary Arterial Hypertension. Pulm. Circ. 2011, 1, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Carman, B.L.; Predescu, D.N.; Machado, R.; Predescu, S.A. Plexiform Arteriopathy in Rodent Models of Pulmonary Arterial Hypertension. Am. J. Pathol. 2019, 189, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Meoli, D.F.; Swarthout, R.F.; Kallop, D.Y.; Galaria, I.I.; Harvey, J.L.; Miller, C.M.; Blaxall, B.C.; Hall, C.M.; Pierce, R.A.; et al. Plexiform-Like Lesions and Increased Tissue Factor Expression in a Rat Model of Severe Pulmonary Arterial Hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, L583–L590. [Google Scholar] [CrossRef]
- Coste, F.; Guibert, C.; Magat, J.; Abell, E.; Vaillant, F.; Dubois, M.; Courtois, A.; Diolez, P.; Quesson, B.; Marthan, R.; et al. Chronic Hypoxia Aggravates Monocrotaline-Induced Pulmonary Arterial Hypertension: A Rodent Relevant Model to the Human Severe Form of the Disease. Respir. Res. 2017, 18, 47. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Toba, M.; Alzoubi, A.; Ito, M.; Fagan, K.A.; Cool, C.D.; Voelkel, N.F.; McMurtry, I.F.; Oka, M. Formation of Plexiform Lesions in Experimental Severe Pulmonary Arterial Hypertension. Circulation 2010, 121, 2747–2754. [Google Scholar] [CrossRef] [PubMed]
- Happe, C.M.; de Raaf, M.A.; Rol, N.; Schalij, I.; Vonk-Noordegraaf, A.; Westerhof, N.; Voelkel, N.F.; de Man, F.S.; Bogaard, H.J. Pneumonectomy Combined with SU5416 Induces Severe Pulmonary Hypertension in Rats. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L1088–L1097. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Ortega, J.; Ruiz-Feria, C.A. Pulmonary Vascular Remodeling in Broiler and Leghorn Chickens after Unilateral Pulmonary Artery Occlusion. Poult. Sci. 2012, 91, 2904–2911. [Google Scholar] [CrossRef] [PubMed]
- Kluess, H.A.; Stafford, J.; Evanson, K.W.; Stone, A.J.; Worley, J.; Wideman, R.F. Intrapulmonary Arteries Respond to Serotonin and Adenosine Triphosphate in Broiler Chickens Susceptible to Idiopathic Pulmonary Arterial Hypertension. Poult. Sci. 2012, 91, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Chai, J.; Bi, S.C.; Li, J.J.; Li, W.W.; Zhou, J.Y. Involvement of Matrix Metalloproteinase-2 in Medial Hypertrophy of Pulmonary Arterioles in Broiler Chickens with Pulmonary Arterial Hypertension. Vet. J. 2012, 193, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Pan, J.Q.; Li, J.C.; Liu, Y.J.; Sun, W.D.; Wang, X.L. L-Arginine Inhibiting Pulmonary Vascular Remodelling Is Associated with Promotion of Apoptosis in Pulmonary Arterioles Smooth Muscle Cells in Broilers. Res. Vet. Sci. 2005, 79, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Wideman, R.J.; Hamal, K.R. Idiopathic Pulmonary Arterial Hypertension: An Avian Model for Plexogenic Arteriopathy and Serotonergic Vasoconstriction. J. Pharmacol. Toxicol. Methods 2011, 63, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Hamal, K.R.; Erf, G.F.; Anthony, N.B.; Wideman, R.F. Immunohistochemical Examination of Plexiform-Like Complex Vascular Lesions in the Lungs of Broiler Chickens Selected for Susceptibility to Idiopathic Pulmonary Arterial Hypertension. Avian Pathol. 2012, 41, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Liu, R.; Tan, X.; Zhang, Q.; Ye, L.; Yan, B.; Zhuang, Y.; Xu, J. MSC Transplantation Attenuates Inflammation, Prevents Endothelial Damage and Enhances the Angiogenic Potency of Endogenous MSCs in a Model of Pulmonary Arterial Hypertension. J. Inflamm. Res. 2022, 15, 2087–2101. [Google Scholar] [CrossRef] [PubMed]
- Wideman, R.F.; Hamal, K.R.; Bayona, M.T.; Lorenzoni, A.G.; Cross, D.; Khajali, F.; Rhoads, D.D.; Erf, G.F.; Anthony, N.B. Plexiform Lesions in the Lungs of Domestic Fowl Selected for Susceptibility to Pulmonary Arterial Hypertension: Incidence and Histology. Anat. Rec. 2011, 294, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Wideman, R.J.; Mason, J.G.; Anthony, N.B.; Cross, D. Plexogenic Arteriopathy in Broiler Lungs: Evaluation of Line, Age, and Sex Influences. Poult. Sci. 2015, 94, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.J.; Guo, X.L.; Xu, J.X.; Liu, R.; Li, D.Y.; Li, Q.H.; Zhou, T.; Fang, C.; Tan, X. Potential Contribution of Early Endothelial Progenitor Cell (eEPC)-to-Macrophage Switching in the Development of Pulmonary Plexogenic Lesion. Respir. Res. 2022, 23, 290. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Juan, F.G.; Shah, A.Q. Involvement of Endothelial Progenitor Cells in the Formation of Plexiform Lesions in Broiler Chickens: Possible Role of Local Immune/Inflammatory Response. J. Zhejiang Univ. Sci. B 2017, 18, 59–69. [Google Scholar] [CrossRef]
- Tan, X.; Shao, F.J.; Fan, G.J.; Ying, Y.T. Expression of Angiogenic Factors and Plexiform Lesions in the Lungs of Broiler and Layer Chickens: A Comparison. Poult. Sci. 2018, 97, 1526–1535. [Google Scholar] [CrossRef]
- Patan, S. Vasculogenesis and Angiogenesis as Mechanisms of Vascular Network Formation, Growth and Remodeling. J. Neuro-Oncol. 2000, 50, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Schlenger, K.; Hockel, M.; Yuan, F. Quantitative Angiogenesis Assays: Progress and Problems. Nat. Med. 1997, 3, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Role of Vascular Endothelial Growth Factor in Regulation of Physiological Angiogenesis. Am. J. Physiol. Cell Physiol. 2001, 280, C1358–C1366. [Google Scholar] [CrossRef] [PubMed]
- Pugh, C.W.; Ratcliffe, P.J. Regulation of Angiogenesis by Hypoxia: Role of the HIF System. Nat. Med. 2003, 9, 677–684. [Google Scholar] [CrossRef]
- Eberhard, A.; Kahlert, S.; Goede, V.; Hemmerlein, B.; Plate, K.H.; Augustin, H.G. Heterogeneity of Angiogenesis and Blood Vessel Maturation in Human Tumors: Implications for Antiangiogenic Tumor Therapies. Cancer Res. 2000, 60, 1388–1393. [Google Scholar] [PubMed]
- Olszewska-Pazdrak, B.; Hein, T.W.; Olszewska, P.; Carney, D.H. Chronic Hypoxia Attenuates VEGF Signaling and Angiogenic Responses by Downregulation of KDR in Human Endothelial Cells. Am. J. Physiol. Cell Physiol. 2009, 296, C1162–C1170. [Google Scholar] [CrossRef]
- Marsboom, G.; Pokreisz, P.; Gheysens, O.; Vermeersch, P.; Gillijns, H.; Pellens, M.; Liu, X.; Collen, D.; Janssens, S. Sustained endothelial progenitor cell dysfunction after chronic hypoxia-induced pulmonary hypertension. Stem Cells 2008, 26, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Druyan, S. The Effects of Genetic Line (Broilers vs. Layers) on Embryo Development. Poult. Sci. 2010, 89, 1457–1467. [Google Scholar] [CrossRef]
- Chen, J.; Lippo, L.; Labella, R.; Tan, S.L.; Marsden, B.D.; Dustin, M.L.; Ramasamy, S.K.; Kusumbe, A.P. Decreased Blood Vessel Density and Endothelial Cell Subset Dynamics during Ageing of the Endocrine System. EMBO J. 2021, 40, e105242. [Google Scholar] [CrossRef] [PubMed]
- Makanya, A.N.; Hlushchuk, R.; Baum, O.; Velinov, N.; Ochs, M.; Djonov, V. Microvascular Endowment in the Developing Chicken Embryo Lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L1136–L1146. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Oshima, K.; Crockett, E.S.; Joshi, S.R.; McLendon, J.M.; Matsumoto, Y.; McMurtry, I.F.; Abe, K.; Oka, M. Aneurysm-Type Plexiform Lesions Form in Supernumerary Arteries in Pulmonary Arterial Hypertension: Potential Therapeutic Implications. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 317, L805–L815. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Firth, A.L.; Sacks, R.S.; Ogawa, A.; Auger, W.R.; Fedullo, P.F.; Madani, M.M.; Lin, G.Y.; Sakakibara, N.; Thistlethwaite, P.A.; et al. Identification of Putative Endothelial Progenitor Cells (CD34+CD133+Flk-1+) in Endarterectomized Tissue of Patients with Chronic Thromboembolic Pulmonary Hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 296, L870–L878. [Google Scholar] [CrossRef] [PubMed]
- Jonigk, D.; Golpon, H.; Bockmeyer, C.L.; Maegel, L.; Hoeper, M.M.; Gottlieb, J.; Nickel, N.; Hussein, K.; Maus, U.; Lehmann, U.; et al. Plexiform Lesions in Pulmonary Arterial Hypertension Composition, Architecture, and Microenvironment. Am. J. Pathol. 2011, 179, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Jakkula, M.; Le Cras, T.D.; Gebb, S.; Hirth, K.P.; Tuder, R.M.; Voelkel, N.F.; Abman, S.H. Inhibition of Angiogenesis Decreases Alveolarization in the Developing Rat Lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L600–L607. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.C.; Keith, B. The Role of Oxygen Availability in Embryonic Development and Stem Cell Function. Nat. Rev. Mol. Cell Biol. 2008, 9, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Manuelli, V.; Pecorari, C.; Filomeni, G.; Zito, E. Regulation of Redox Signaling in HIF-1-Dependent Tumor Angiogenesis. FEBS J. 2022, 289, 5413–5425. [Google Scholar] [CrossRef]
- Fijalkowska, I.; Xu, W.; Comhair, S.A.; Janocha, A.J.; Mavrakis, L.A.; Krishnamachary, B.; Zhen, L.; Mao, T.; Richter, A.; Erzurum, S.C.; et al. Hypoxia Inducible-Factor1alpha Regulates the Metabolic Shift of Pulmonary Hypertensive Endothelial Cells. Am. J. Pathol. 2010, 176, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Teng, X.; Zhang, L.; Chen, J.; Liu, Z.; Chen, X.; Zhao, S.; Yang, S.; Feng, J.; Yan, X. CD146-HIF-1alpha Hypoxic Reprogramming Drives Vascular Remodeling and Pulmonary Arterial Hypertension. Nat. Commun. 2019, 10, 3551. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.Y.; Shimoda, L.A.; Iyer, N.V.; Huso, D.L.; Sun, X.; McWilliams, R.; Beaty, T.; Sham, J.S.; Wiener, C.M.; Sylvester, J.T.; et al. Impaired Physiological Responses to Chronic Hypoxia in Mice Partially Deficient for Hypoxia-Inducible Factor 1alpha. J. Clin. Investig. 1999, 103, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Florczyk, U.; Jazwa, A.; Maleszewska, M.; Mendel, M.; Szade, K.; Kozakowska, M.; Grochot-Przeczek, A.; Viscardi, M.; Czauderna, S.; Bukowska-Strakova, K.; et al. Nrf2 Regulates Angiogenesis: Effect on Endothelial Cells, Bone Marrow-Derived Proangiogenic Cells and Hind Limb Ischemia. Antioxid. Redox Sign. 2014, 20, 1693–1708. [Google Scholar] [CrossRef] [PubMed]
- Valcarcel-Ares, M.N.; Gautam, T.; Warrington, J.P.; Bailey-Downs, L.; Sosnowska, D.; de Cabo, R.; Losonczy, G.; Sonntag, W.E.; Ungvari, Z.; Csiszar, A. Disruption of Nrf2 Signaling Impairs Angiogenic Capacity of Endothelial Cells: Implications for Microvascular Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Nezu, M.; Souma, T.; Yu, L.; Sekine, H.; Takahashi, N.; Wei, A.Z.; Ito, S.; Fukamizu, A.; Zsengeller, Z.K.; Nakamura, T.; et al. Nrf2 Inactivation Enhances Placental Angiogenesis in a Preeclampsia Mouse Model and Improves Maternal and Fetal Outcomes. Sci. Signal. 2017, 10, eaam5711. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, S.; Yamada, Y.; Liu, F.; Murohara, T.; Itoh, K.; Yamamoto, M.; Ichihara, G. Ablation of the Transcription Factor Nrf2 Promotes Ischemia-Induced Neovascularization by Enhancing the Inflammatory Response. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Korn, C.; Augustin, H.G. Mechanisms of Vessel Pruning and Regression. Dev. Cell 2015, 34, 5–17. [Google Scholar] [CrossRef]
- Ormea, N.M.; Millerb, D.V.; Edwards, W.D. Age-related Changes Among 25 Patients with Congenital Cardiac Left-To-Right Shunts and Irreversible Plexogenic Pulmonary Arteriopathy. Cardiovasc. Pathol. 2008, 17, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Mushaben, E.M.; Hershey, G.K.; Pauciulo, M.W.; Nichols, W.C.; Le Cras, T.D. Chronic Allergic Inflammation Causes Vascular Remodeling and Pulmonary Hypertension in BMPR2 Hypomorph and Wild-Type Mice. PLoS ONE 2012, 7, e32468. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.F.; Lv, Y.; Gu, W.Z.; Tang, L.L.; Wei, J.K.; Zhang, L.Y.; Du, L.Z. Epigenetics of Hypoxic Pulmonary Arterial Hypertension Following Intrauterine Growth Retardation Rat: Epigenetics in PAH Following IUGR. Respir. Res. 2013, 14, 20. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, L.; Liu, R.; Li, Q.; Zhou, C.; Tan, X. Dysregulated VEGF/VEGFR-2 Signaling and Plexogenic Lesions in the Embryonic Lungs of Chickens Predisposed to Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2024, 25, 4489. https://doi.org/10.3390/ijms25084489
Ye L, Liu R, Li Q, Zhou C, Tan X. Dysregulated VEGF/VEGFR-2 Signaling and Plexogenic Lesions in the Embryonic Lungs of Chickens Predisposed to Pulmonary Arterial Hypertension. International Journal of Molecular Sciences. 2024; 25(8):4489. https://doi.org/10.3390/ijms25084489
Chicago/Turabian StyleYe, Lujie, Rui Liu, Qinghao Li, Chunzhen Zhou, and Xun Tan. 2024. "Dysregulated VEGF/VEGFR-2 Signaling and Plexogenic Lesions in the Embryonic Lungs of Chickens Predisposed to Pulmonary Arterial Hypertension" International Journal of Molecular Sciences 25, no. 8: 4489. https://doi.org/10.3390/ijms25084489




