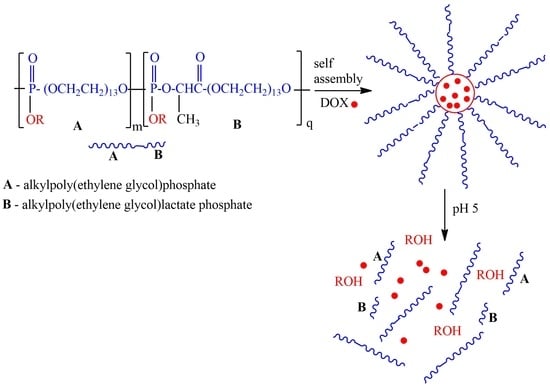
pH-Sensitive Amphiphilic Diblock Polyphosphoesters with Lactate Units: Synthesis and Application as Drug Carriers
Abstract
:1. Introduction
2. Results and Discussion
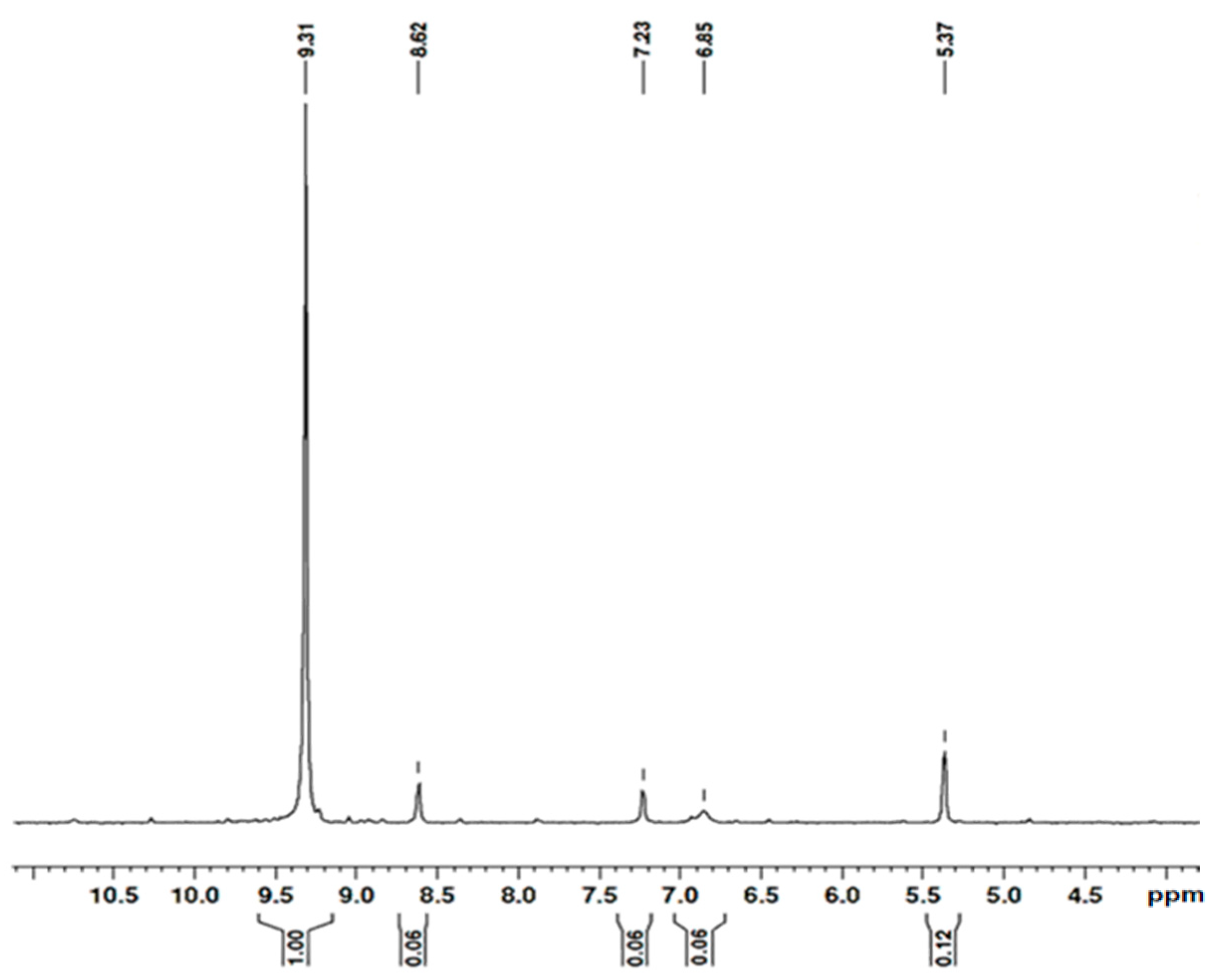
2.1. Microwave Synthesis of Poly(ethylene glycol)lactate
2.2. Synthesis of Poly[poly(ethylene glycol) H-phosphonate]-b-[poly(ethylene glycol)lactate H-phosphonate]
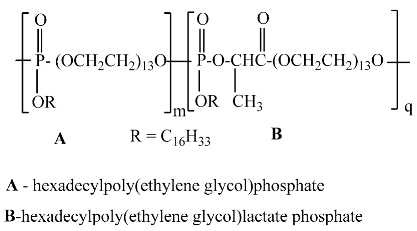
2.3. One-Pot Synthesis of Poly[alkylpoly(ethylene glycol) phosphate-b-alkylpoly(ethyleneglycol)lactate phosphate]s
One-Pot Synthesis of Poly[hexadecylpoly(ethylene glycol) phosphate)-b-hexadecyl-poly(ethylene glycol)lactate phosphate]
2.4. Self-Assembly of Poly[alkylpoly(ethylene glycol) phosphate-b-alkylpoly(ethylene glycol)lactate phosphate]s and Particle Size Distribution and Drug Loading and Encapsulation Efficiency for Doxorubicin
2.4.1. Particle Size Distribution
2.4.2. Drug Loading and Encapsulation Efficiency for Doxorubicin
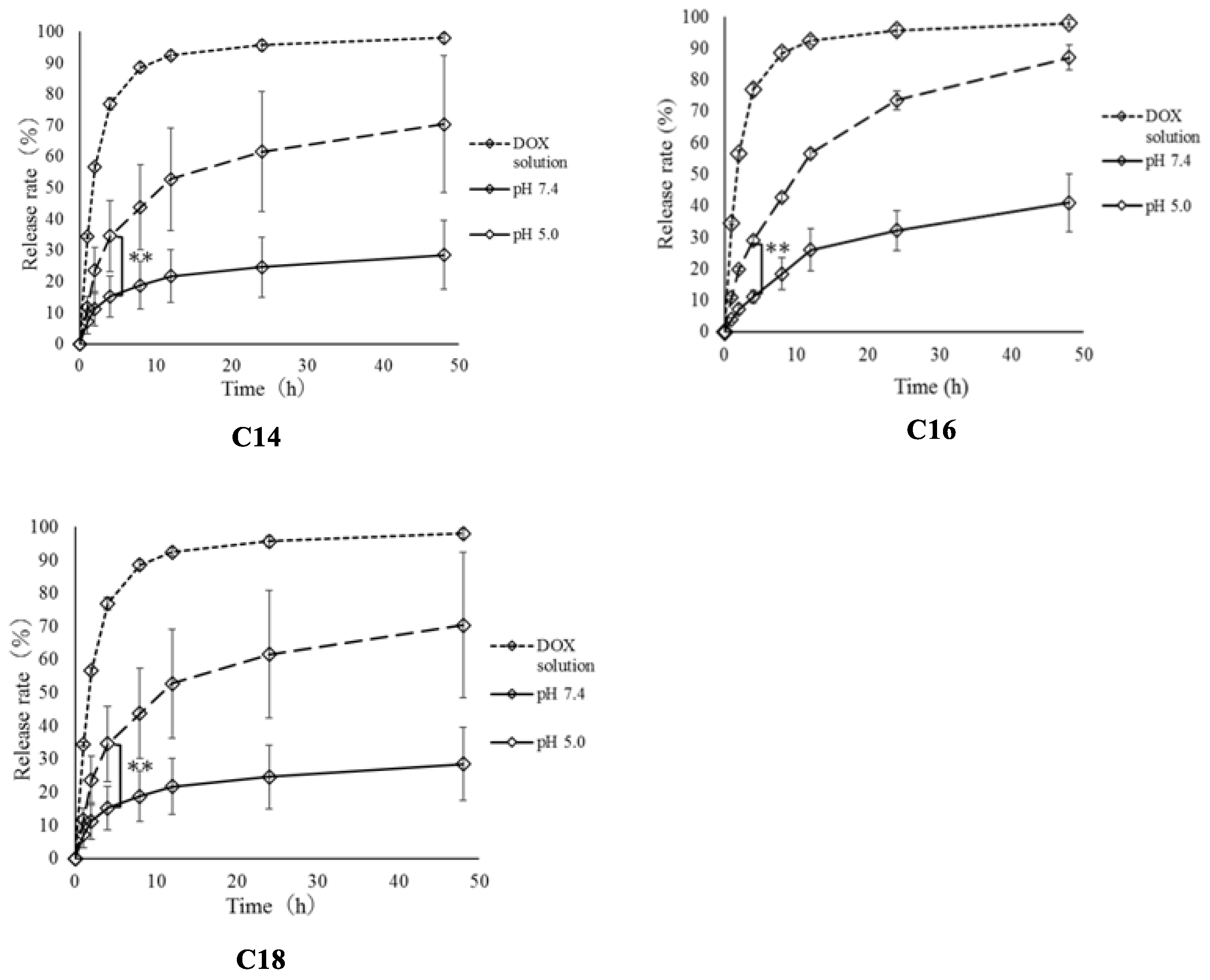
2.5. Release Rate of Loaded Doxorubicin from Micelles
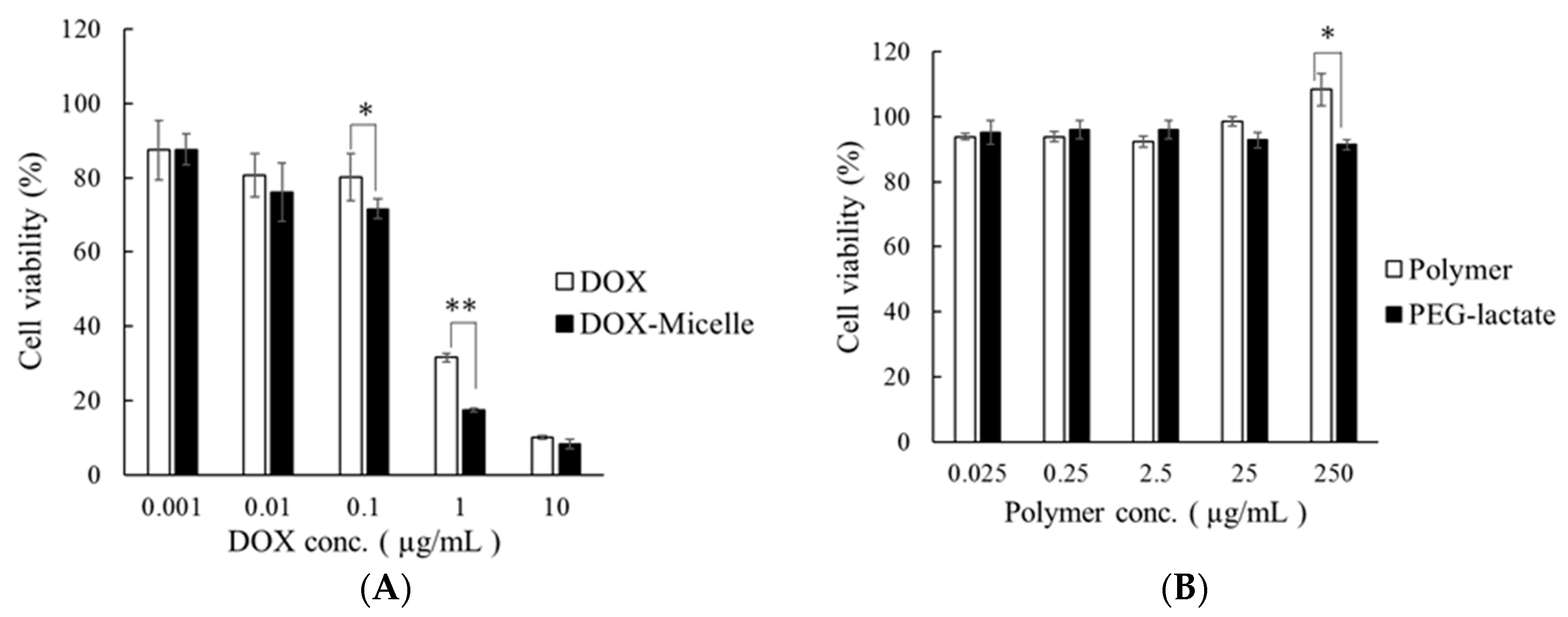
2.6. Cytotoxicity Test
2.7. Cell Uptake Test Using Confocal Laser Microscopy (CLSM)
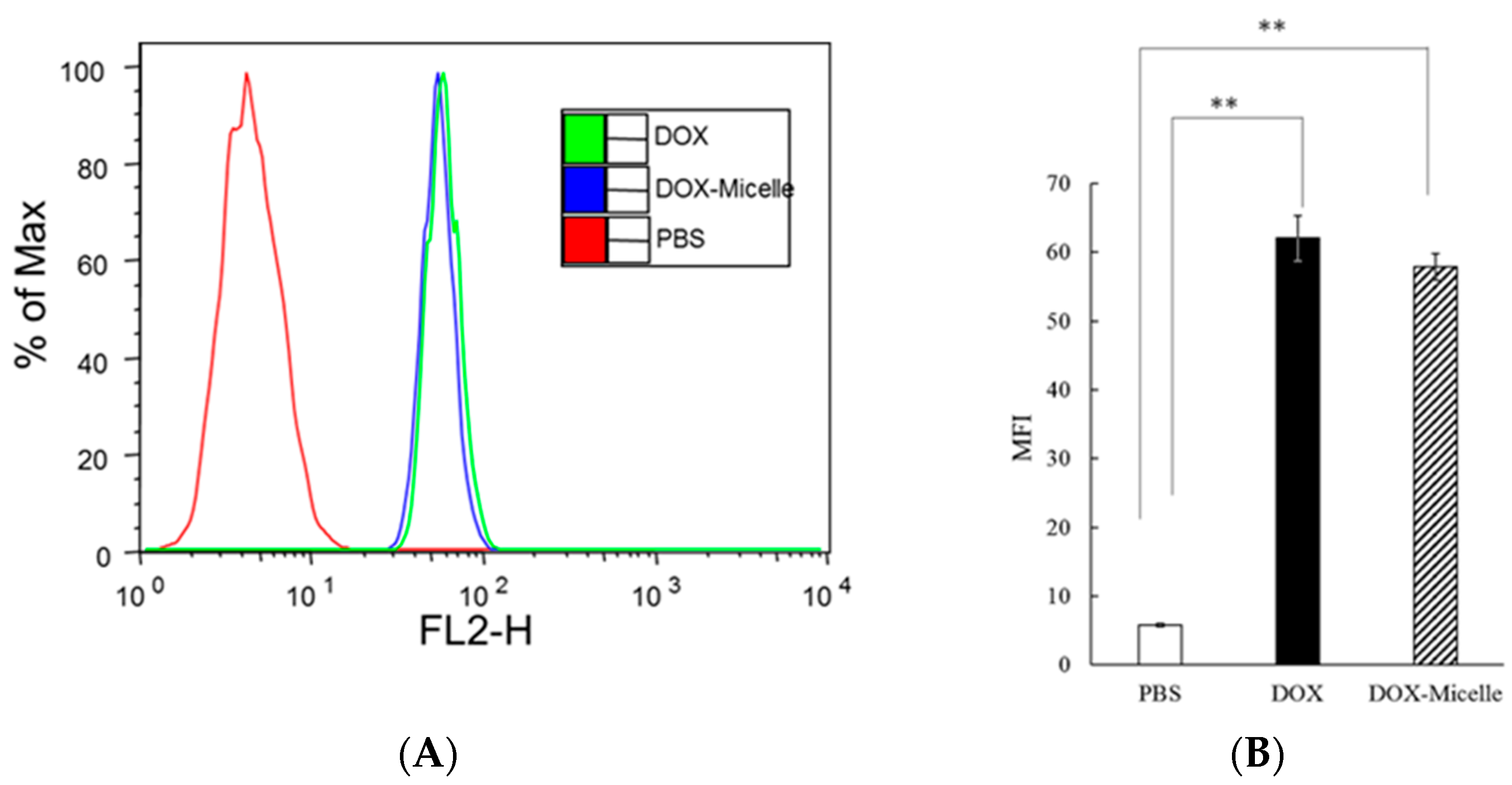
2.8. Cell Uptake Test Using Flow Cytometry (FACS)
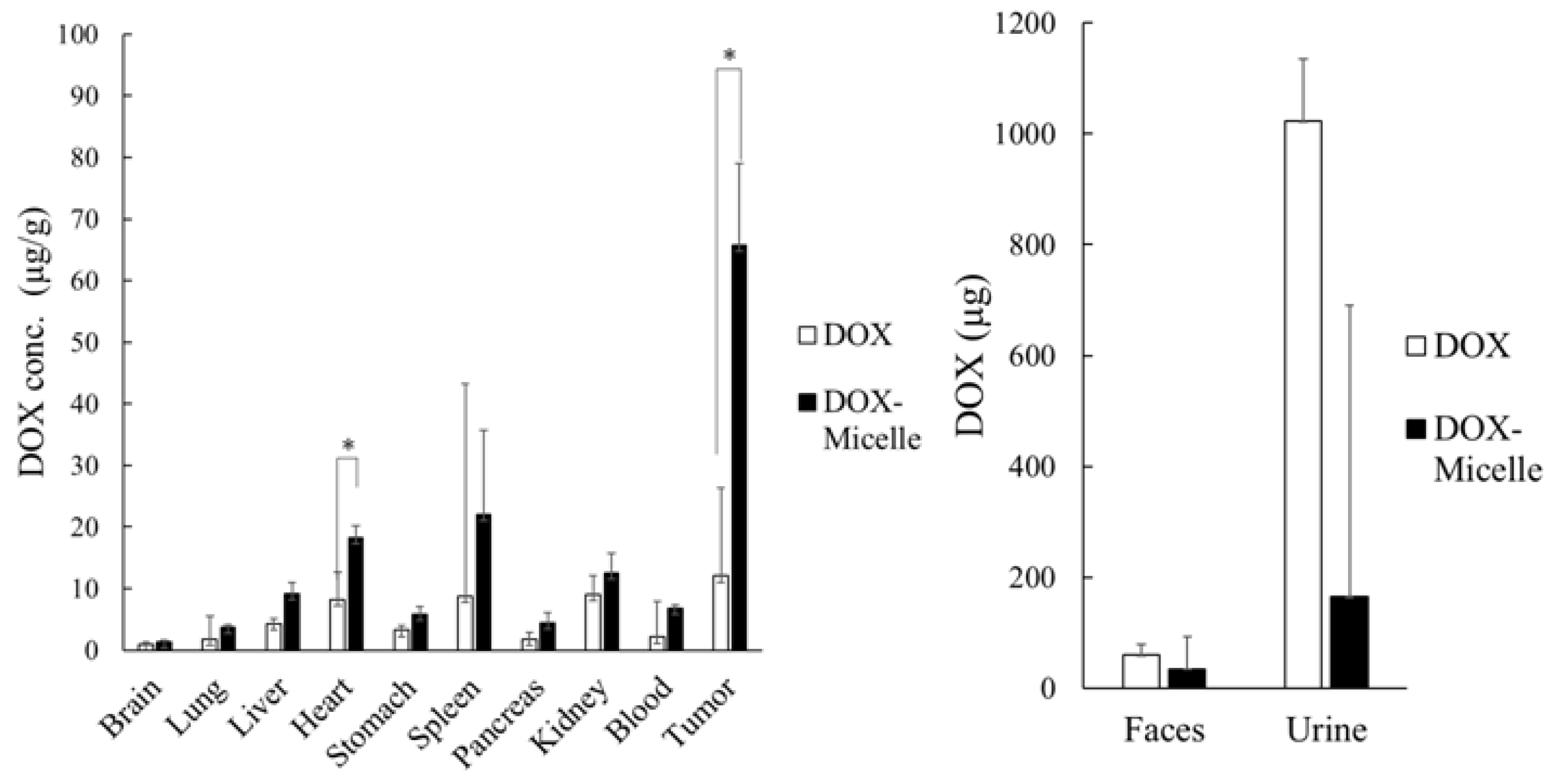
2.9. Pharmacokinetics Test Using Prepared Micelles
2.10. Comparative Analysis
3. Materials and Methods
3.1. Materials
3.2. Characterization Methods and Instruments
3.3. Microwave Synthesis of Poly(ethylene glycol)lactate
3.4. Synthesis of Poly[poly(ethylene glycol) H-phosphonate]-b-[poly(ethylene glycol)lactate H-phosphonate]
3.5. Synthesis of Poly[alkylpoly(ethylene glycol) phosphate-b-alkylpoly(ethylene glycol)lactate phosphate]s
3.5.1. Synthesis of Poly[hexadecylpoly(ethylene glycol) phosphate-b-[hexadecylpoly(ethylene glycol)lactate phosphate]
3.5.2. Synthesis of Poly[tetradecylpoly(ethylene glycol) phosphate-b-tetradecylpoly(ethylene glycol)lactate phosphate]
3.5.3. Synthesis of Poly[octadecylpoly(ethylene glycol) phosphate)-b-octadecylpoly(ethylene glycol)lactate phosphate]
3.6. Measurement of Polymeric Micelle Size
3.7. Preparation of Doxorubicin-Loaded Micelles
3.8. Measuring the Drug Loading and Encapsulation Efficiency of Micelles for Doxorubicin
3.9. Evaluation of Release Properties of Doxorubicin-Encapsulated Micelles
3.10. Cytotoxicity Test Using WST-8 Assay
3.11. Cell Uptake Test Using Confocal Laser Microscopy (CLSM)
3.12. Cell Uptake Test Using Flow Cytometry (FACS)
3.13. Pharmacokinetics Study Using Prepared Micelles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Troev, K.D. Polyphosphoesters: Chemistry and Application, 1st ed.; Elsevier: Amsterdam, The Netherlands; New York, NY, USA; Oxford, UK; Tokyo, Japan, 2012; p. 84. [Google Scholar]
- Myrex, R.D.; Farmer, B.; Gray, G.M.; Wright, Y.J.; Dees, J.; Bharara, P.C.; Byrd, H.; Branham, K.E. 31P and 1H NMR studies of the transesterification polymerization of polyphosphonate oligomers. Eur. Polym. J. 2003, 39, 1105–1115. [Google Scholar] [CrossRef]
- Oussadi, K.; Montemblault, V.; Fontaine, L. Synthesis of poly(oxyethylene phosphate)-g-poly(ethylene oxide) via the “grafting onto” approach by “click” chemistry. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 5124–5128. [Google Scholar] [CrossRef]
- Oussadi, K.; Montemblault, V.; Belbachir, M.; Fontaine, L. Ring-opening bulk polymerization of five-and six-membered cyclic phosphonates using maghnite, a nontoxic proton exchanged montmorillonite clay. J. Appl. Polym. Sci. 2011, 122, 891–897. [Google Scholar] [CrossRef]
- Branham, K.E.; Mays, J.W.; Gray, G.M.; Bharara, P.C.; Byrd, H.; Bittenger, R.; Farmer, B. Polycondensations of dimethyl phosphonate with diols: SEC and 31P and 13C NMR spectroscopic studies. Polymer 2000, 41, 3371–3379. [Google Scholar] [CrossRef]
- Pretula, J.; Penczek, S. Poly(ethylene glycol) ionomers with phosphate diester linkages. Makromol. Chem. Rapid Commun. 1988, 9, 731–737. [Google Scholar] [CrossRef]
- Pretula, J.; Penczek, S. High-molecular-weight poly (alkylene phosphonate) s by condensation of dialkylphosphonates with diols. Makromol. Chem. 1990, 191, 671–680. [Google Scholar] [CrossRef]
- Penczek, S.; Pretula, J. High-molecular-weight poly (alkylene phosphates) and preparation of amphiphilic polymers thereof. Macromolecules 1993, 26, 2228–2233. [Google Scholar] [CrossRef]
- Gitsov, I.; Johnson, F.E. Synthesis and hydrolytic stability of poly (oxyethylene-H-phosphonate) s. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 4130–4139. [Google Scholar] [CrossRef]
- Pretula, J.; Kaluzynski, K.; Szymanski, R.; Penczek, S. Preparation of poly (alkylene H-phosphonate) s and their derivatives by polycondensation of diphenyl H-phosphonate with diols and subsequent transformations. Macromolecules 1997, 30, 8172–8176. [Google Scholar] [CrossRef]
- Troev, K.; Naruoka, A.; Terada, H.; Kikuchi, A.; Makino, K. New Efficient Method of Oxidation of Poly(alkylene H-phosphonate)s—A Promising Route to Novel co-Polyphosphoesters. Macromolecules 2012, 45, 5698–5703. [Google Scholar] [CrossRef]
- Koseva, N.; Tsacheva, I.; Mitova, V.; Vodenicharova, E.; Jessica, M.; Mason, K.; Troev, K. Polymer complex of WR 2721. Synthesis and radioprotective efficiency. Eur. J. Pharm. Sci. 2014, 65, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Hristova, T.; Mitova, V.; Koda, T.; Fushimi, M.; Kuniya, M.; Makino, K.; Terada, H.; Cherkezova, R.; Yusa, S.; et al. Polyphosphoesters Based—Placlitaxel Complexes. Biological Evaluations. Anticancer Res. 2016, 36, 1613–1620. [Google Scholar] [PubMed]
- Mitova, V.; Hristova, T.; Cherkezova, R.; Koseva, N.; Yusa, S.-I.; Troev, K. Polyphosphoesters Based—Placlitaxel Complexes. J. Appl. Polym. Sci. 2015, 132, 42772. [Google Scholar] [CrossRef]
- Yamakita, Y.; Takeuchi, I.; Makino, K.; Terada, H.; Kikuchi, A.; Troev, K. Thermoresponsive Polyphosphoester via Polycondensation Reactions: Synthesis, Characterization, and Self-Assembly. Molecules 2022, 27, 6006. [Google Scholar] [CrossRef]
- Tatsuya Sakuma, T.; Makino, K.; Terada, H.; Takeuchi, I.; Mitova, M.; Kolio Troev, K. Synthesis and Characterization of Amphiphilic Diblock Polyphosphoesters Containing Lactic Acid Units for Potential Drug Delivery Applications. Molecules 2023, 28, 5243. [Google Scholar] [CrossRef]
- Schottler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohlr, K.; Landfester, K.; Mailander, V.; Wurm, F.R. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nanotechnol. 2016, 11, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.N.; Simon, J.; Mailänder, V.; Landfester, K.; Wurm, F.R. Polyphosphoester surfactants as general stealth coatings for polymeric nanocarriers. Acta Biomater. 2020, 116, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Ke, W.; Dirisala, A.; Toh, K.; Tanaka, M.; Li, J. Stealth and pseudo-stealth nanocarriers. Adv. Drug Deliv. Rev. 2023, 198, 114895. [Google Scholar] [CrossRef] [PubMed]
- Hussein, Y.H.A.; Youssry, M. Polymeric Micelles of Biodegradable Diblock Copolymers: Enhanced Encapsulation of Hydrophobic Drugs. Materials 2018, 11, 688. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Peng, Y.; Ding, J.; Zhou, W. A Smart pH-Sensitive Delivery System for Enhanced Anticancer Efficacy via Paclitaxel Endosomal Escape 10–10. Front. Pharmacol. 2019, 10, 10. [Google Scholar] [CrossRef]
- Li, F.; He, J.; Zhang, M.; Ni, P. A pH-sensitive and biodegradable supramolecular hydrogel constructed from a PEGylated polyphosphoester-doxorubicin prodrug and α-cyclodextrin. Polym. Chem. 2015, 6, 5009–5014. [Google Scholar] [CrossRef]
- Teasdale, I. Stimuli-Responsive Phosphorus-Based Polymers. Eur. J. Inorg. Chem. 2019, 2019, 1433–1706. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, A.; Sun, C. Tumor pH-Responsive Nanocarriers with Light-Activatable Drug Release for Chemo-Photodynamic Therapy of Breast Cancer. Front. Chem. 2022, 10, 905645. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Tang, L.Y.; Li, Y.; Wang, J. Thermoresponsive block copolymers of poly (ethylene glycol) and polyphosphoester: Thermo-induced self-assembly, biocompatibility, and hydrolytic degradation. Biomacromolecules 2009, 10, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Du, J.-Z.; Du, X.-J.; Mao, C.-Q.; Wang, J. Tailor-made dual pH-sensitive polymer–doxorubicin nanoparticles for efficient anticancer drug delivery. J. Am. Chem. Soc. 2011, 133, 17560–17563. [Google Scholar] [CrossRef]
- Liu, X.; Ni, P.; He, J.; Zhang, M. Synthesis and Micellization of pH/Temperature-Responsive Double-Hydrophilic Diblock Copolymers Polyphosphoester-block-poly[2-(dimethylamino)ethyl methacrylate] Prepared via ROP and ATRP. Macromolecules 2010, 43, 4771–4781. [Google Scholar] [CrossRef]
- He, J.; Ni, P.; Wang, S.; Shao, H.; Zhang, M.; Zhu, X. Synthesis and Physicochemical Characterization of Biodegradable and pH-Responsive Hydrogels Based on Polyphosphoester for Protein Delivery. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1919–1930. [Google Scholar] [CrossRef]
- Abdella, S.; Abid, F.; Youssef, S.H.; Kim, S.; Afinjuomo, F.; Malinga, C.; Song, Y.; Garg, S. pH and its applications in targeted drug delivery. Drug Discov. Today 2023, 28, 103414. [Google Scholar] [CrossRef]
- Etrych, T.; Daumová, L.; Pokorná, E.; Tušková, D.; Lidický, O.; Kolářová, V.; Pankrác, J.; Šefc, L.; Chytil, P.; Klener, P. Effective doxorubicin-based nanotherapeutics for simultaneous malignant lymphoma treatment and lymphoma growth imaging. J. Control. Release 2018, 289, 44–55. [Google Scholar] [CrossRef]
- Feng, X.; Li, D.; Han, J.; Zhuang, X.; Ding, J. Schiff base bond-linked polysaccharide–doxorubicin conjugate for upregulated cancer therapy. Mater. Sci. Eng. C 2017, 76, 1121–1128. [Google Scholar] [CrossRef]
- Huang, D.; Zhuang, Y.; Shen, H.; Yang, F.; Wang, X.; Wu, D. Acetal-linked PEGylated paclitaxel prodrugs forming free-paclitaxel-loaded pH-responsive micelles with high drug loading capacity and improved drug delivery. Mater. Sci. Eng. C 2018, 82, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, Y.; Cao, M.; Chen, P.; Yang, B.; Miao, J.; Xia, R.; Qian, J. Y-shaped copolymers of poly(ethylene glycol)-poly(e-caprolactone) with ketal bond as the branchpoint for drug delivery. Mater. Sci. Eng. C 2018, 93, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Choudhury, N.; Seal, S.; Ruidas, B.; De, P. Aromatic Nitrogen Mustard Based Autofluorescent Amphiphilic Brush Copolymer as pH-Responsive Drug Delivery Vehicle. Biomacromolecules 2019, 20, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Du, X.; Zhu, Y.; Liu, Y.; Li, Y.; Wang, J. Rational Design of Polyion Complex Nanoparticles to Overcome Cisplatin Resistance in Cancer Therapy. Adv. Mater. 2014, 26, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-X.; Gao, Y.-J.; Wang, Y.; Qiao, Z.-Y.; Fan, G.; Qiao, S.-L.; Zhang, R.-X.; Wang, L.; Wang, H. pH-Sensitive Polymeric Nanoparticles with Gold(I) Compound Payloads Synergistically Induce Cancer Cell Death through Modulation of Autophagy. Mol. Pharm. 2015, 12, 2869–2878. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Chen, T.; Teng, W.; Jin, Q.; Ji, J. Mixed-Charged Zwitterionic Polymeric Micelles for Tumor Acidic Environment Responsive Intracellular Drug Delivery. Langmuir 2019, 35, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Parrott, M.C.; Finniss, M.; Luft, J.C.; Pandya, A.; Gullapalli, A.; Napier, M.E.; DeSimone, J.M. Incorporation and Controlled Release of Silyl Ether Prodrugs from PRINT Nanoparticles. J. Am. Chem. Soc. 2012, 134, 7978–7982. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.W.; Hennink, W.E. Polymeric micelles in anticancer therapy: Targeting, imaging and triggered release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar] [CrossRef]
- Ding, H.; Tan, P.; Fu, S.; Tian, X.; Zhang, H.; Ma, X.; Gu, Z.; Luo, K. Preparation and application of pH-responsive drug delivery systems. J. Control. Release 2022, 348, 206–238. [Google Scholar] [CrossRef]
- He, H.; Chen, S.; Zhou, J.; Dou, Y.; Song, L.; Che, L.; Zhou, X.; Chen, X.; Jia, Y.; Zhang, J.; et al. Cyclodextrin-derived pH-responsive nanoparticles for delivery paclitaxel. Biomaterials 2013, 34, 5344–5358. [Google Scholar] [CrossRef]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.C. Polysaccharide based nanogels in the drug delivery system: Applicatiion as the carrier of pharmaceutical agents. Mater. Sci. Eng. C 2016, 68, 964–981. [Google Scholar] [CrossRef]
- Chaubal, M.V.; Wang, B.; Su, G.; Zhao, Z. Compositional analysis of biodegradable polyphosphoester copolymers using nmr spectroscopic methods. J. Appl. Polym. Sci. 2003, 90, 4021–4031. [Google Scholar] [CrossRef]
- Modro, A.M.; Modro, T.A. The phosphoryl and the carbonyl group as hydrogen bond acceptors. Phosphorus Sulfur Silicon Relat. Elem. 2002, 177, 2067. [Google Scholar] [CrossRef]
- Quin, L.D.; Williams, A.J. Practical Interpretation of P-31 NMR Spectra and Computer-Assisted Structure Verification; Advanced Chemistry Development: Toronto, ON, Canada, 2004; ISBN 0-9735913-0-7. [Google Scholar]
- Shin, I.L.; Kim, S.Y.; Lee, Y.M.; Cho, C.S.; Sung, Y.K. Methoxy poly(ethylene glycol)/e-caprolactone amphiphilic block copolymeric micelle containing indomethacin, I. Preparation and characterization. J. Control. Release 1998, 51, 1–11. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Huang, N.; Qiu, Z.; He, L.; Guo, J.; Xu, M.; Li, W. Effects of polymer terminal group inside micelle core on paclitaxel loading promoting and burst release suppressing. J. Gastrointest. Oncol. 2023, 14, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Deng, X.; Ding, J.; Zhou, W.; Zheng, X.; Tang, G. Mechanisms of drug release in pH-sensitive micelles for tumour targeted drug delivery system: A review. Int. J. Pharm. 2018, 535, 253–260. [Google Scholar] [CrossRef]
- Baran, J.; Penczek, S. Hydrolysis of Polyesters of Phosphoric Acid. 1. Kinetics and the pH Profile. Macromolecules 1995, 28, 5167. [Google Scholar] [CrossRef]
- Takeuchi, I.; Makino, K. Biocompatibility and effectiveness of paclitaxel-encapsulated micelle using phosphoester compounds as a carrier for cancer treatment. Colloids Surf. B 2019, 177, 356–361. [Google Scholar] [CrossRef]
- Arora, H.C.; Jensen, M.P.; Yuan, Y.; Wu, A.; Vogt, S.; Paunesku, T.; Woloschak, G.E. Nanocarriers Enhance Doxorubicin Uptake in Drug-Resistant Ovarian Cancer Cells. Cancer Res. 2012, 72, 769–778. [Google Scholar] [CrossRef]
- Rapoport, N.Y.; Kennedy, A.M.; Shea, J.E.; Scaife, C.L.; Nam, K.-H. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J. Control. Release 2009, 138, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, D.R.; Sharma, G.; Beniwal, V.; Kumar, M.N.V.R. Design of Biodegradable Nanoparticles for Oral Delivery of Doxorubicin: In vivo Pharmacokinetics and Toxicity Studies in Rats. Pharm. Res. 2009, 26, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Alhareth, K.; Vauthier, C.; Gueutin, C.; Ponchel, G.; Moussa, F. HPLC quantification of doxorubicin in plasma and tissues of rats treated with doxorubicin loaded poly(alkylcyanoacrylate) nanoparticles. J. Chromatogr. B 2012, 887–888, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Crosasso, P.; Ceruti, M.; Brusa, P.; Arpicco, S.; Dosio, F.; Cattel, L. Preparation, characterization and properties of sterically stabilized paclitaxel-containing liposomes. J. Control. Release 2000, 63, 19–30. [Google Scholar] [CrossRef]
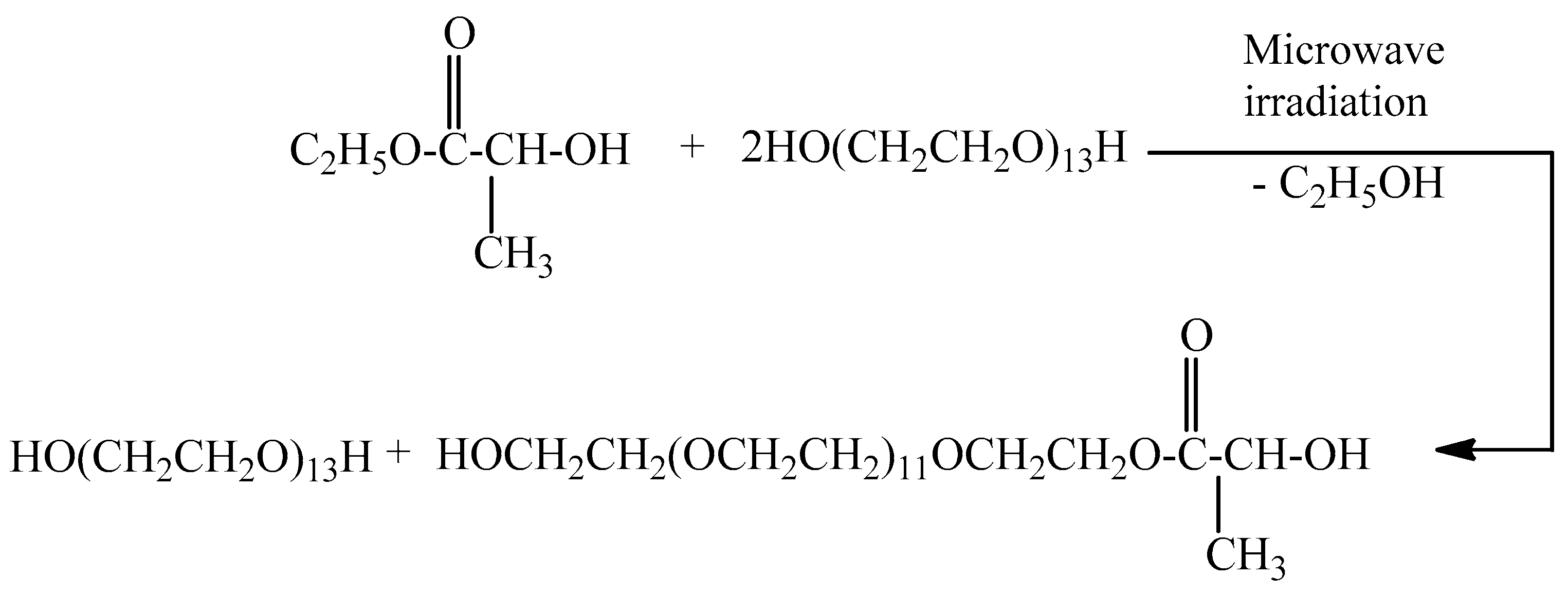
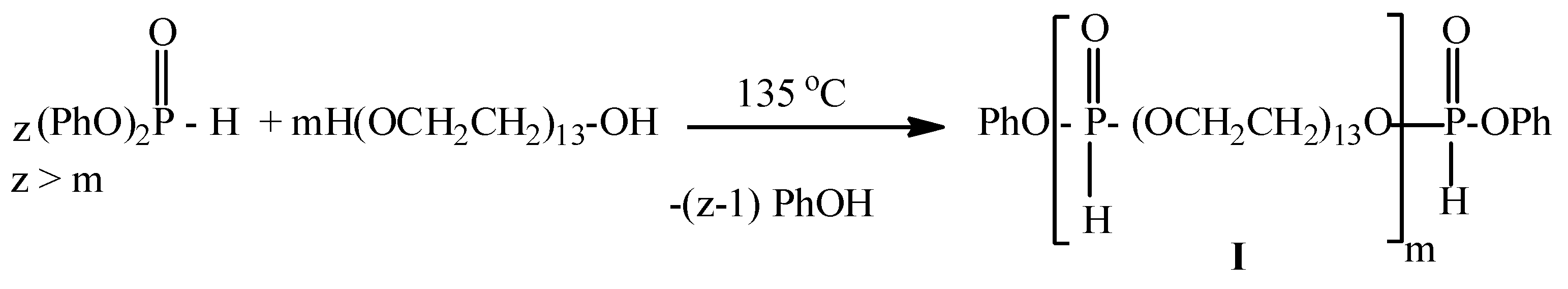
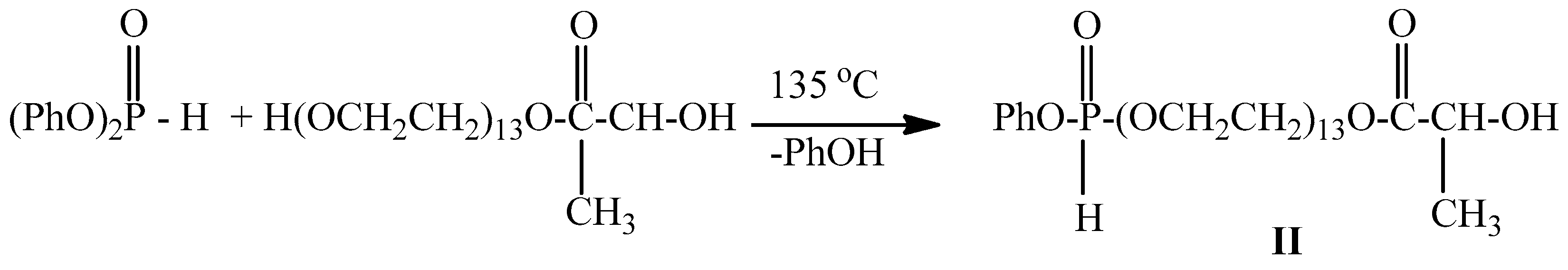
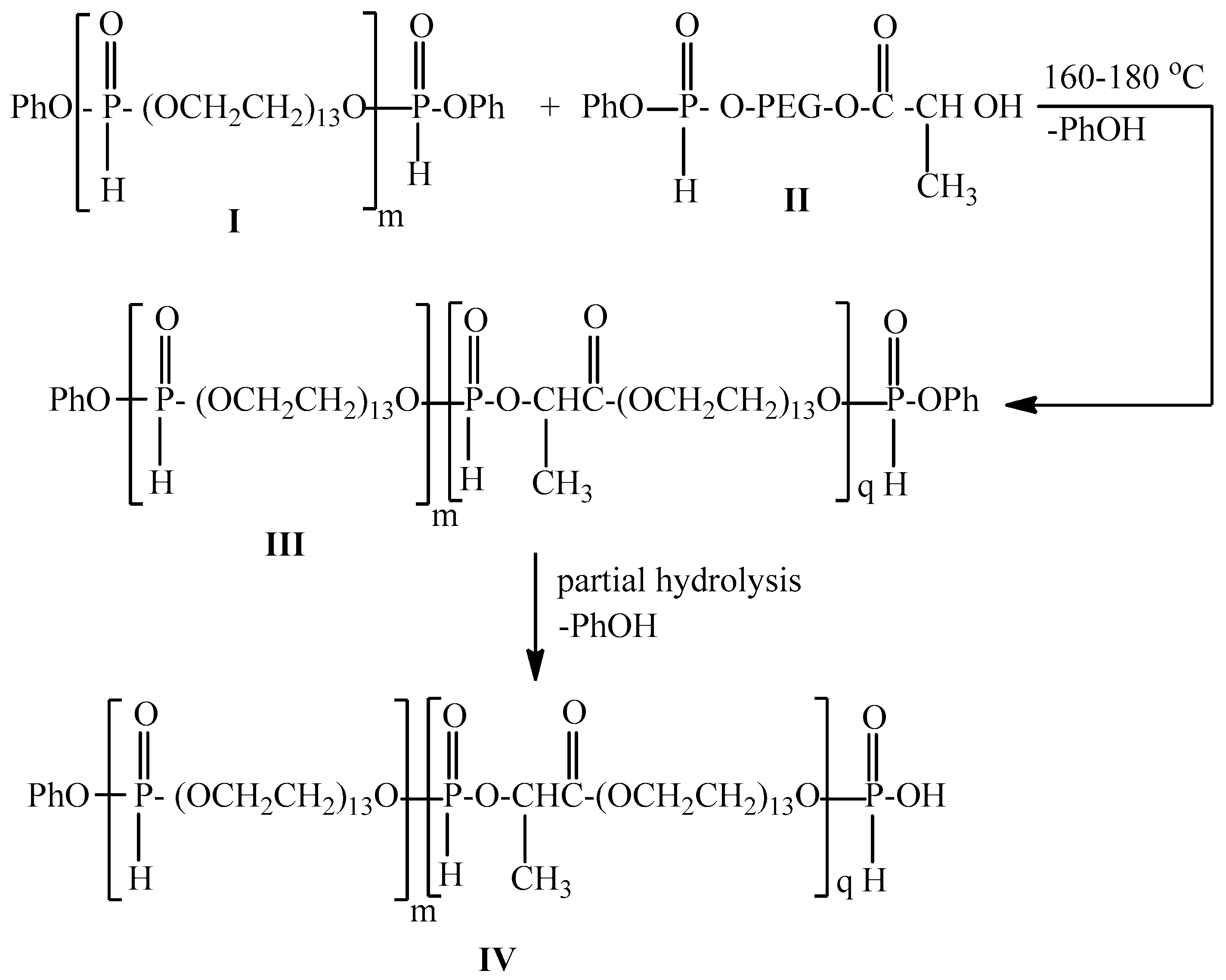

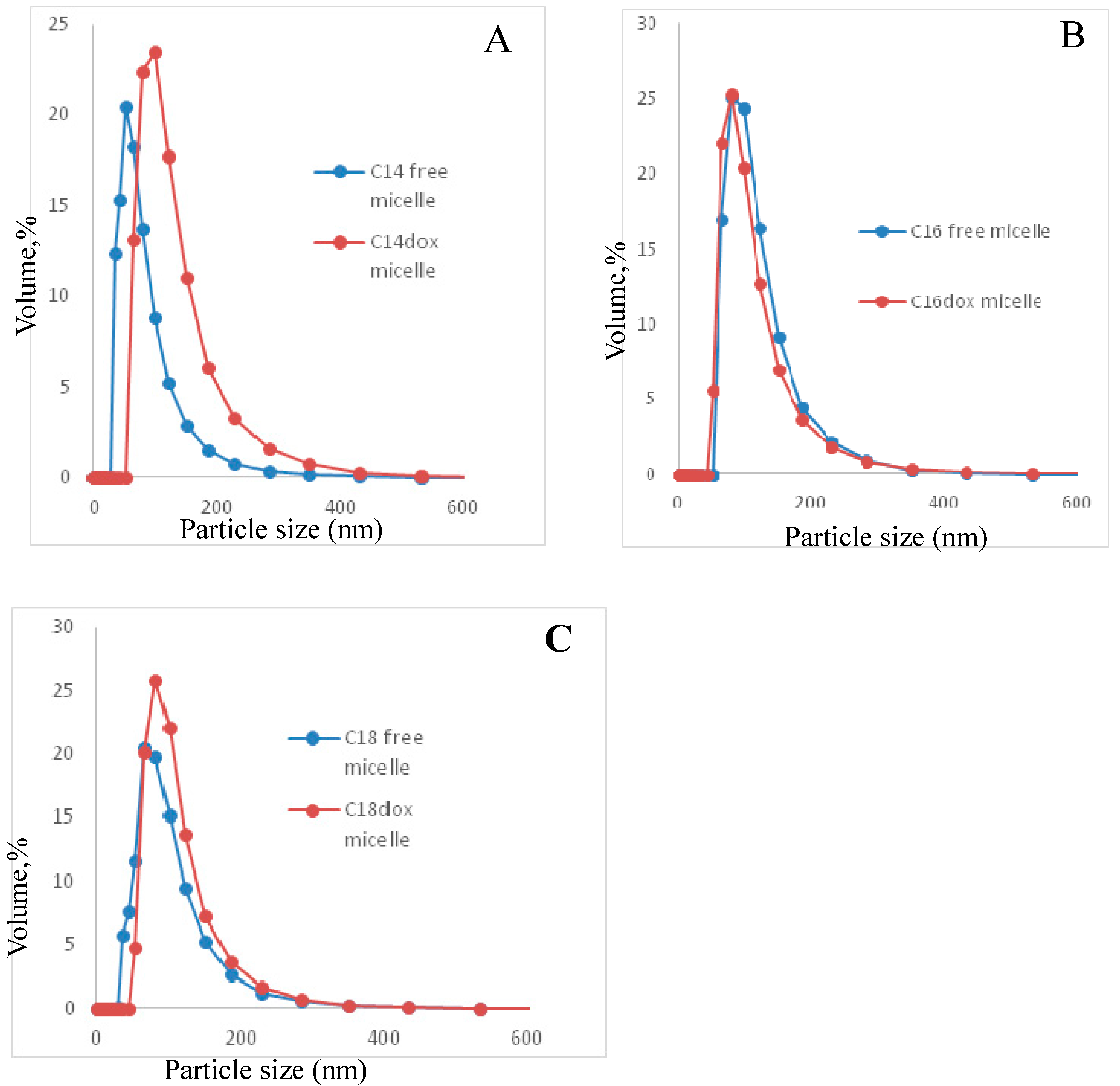

| Polymer | Micelle Size (nm) | DOX-Micelle Size (nm) | Micelle PDI | DOX-Micelle PDI |
|---|---|---|---|---|
| C14 | 71.0 ± 48.9 | 116.9 ± 53.8 | 0.257 ± 0.02 | 0.230 ± 0.02 |
| C16 | 105.3 ± 45.3 | 119.9 ± 47.6 | 0.182 ± 0.01 | 0.200 ± 0.01 |
| C18 | 86.4 ± 43.4 | 100.2 ± 41.1 | 0.231 ± 0.02 | 0.240 ± 0.002 |
| Polymer | Yield (%) | Drug Loading (%) | Encapsulation Efficiency (%) |
|---|---|---|---|
| C14 | 78.1 ± 5.9 | 1.5 ± 0.2 | 31.4 ± 2.0 |
| C16 | 89.8 ± 8.3 | 3.2 ± 0.3 | 57.4 ± 3.2 |
| C18 | 84.2 ± 7.0 | 2.4 ± 0.2 | 52.9 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mochizuki, K.; Mitova, V.; Makino, K.; Terada, H.; Takeuchi, I.; Troev, K. pH-Sensitive Amphiphilic Diblock Polyphosphoesters with Lactate Units: Synthesis and Application as Drug Carriers. Int. J. Mol. Sci. 2024, 25, 4518. https://doi.org/10.3390/ijms25084518
Mochizuki K, Mitova V, Makino K, Terada H, Takeuchi I, Troev K. pH-Sensitive Amphiphilic Diblock Polyphosphoesters with Lactate Units: Synthesis and Application as Drug Carriers. International Journal of Molecular Sciences. 2024; 25(8):4518. https://doi.org/10.3390/ijms25084518
Chicago/Turabian StyleMochizuki, Kasumi, Violeta Mitova, Kimiko Makino, Hiroshi Terada, Issei Takeuchi, and Kolio Troev. 2024. "pH-Sensitive Amphiphilic Diblock Polyphosphoesters with Lactate Units: Synthesis and Application as Drug Carriers" International Journal of Molecular Sciences 25, no. 8: 4518. https://doi.org/10.3390/ijms25084518